Abstract
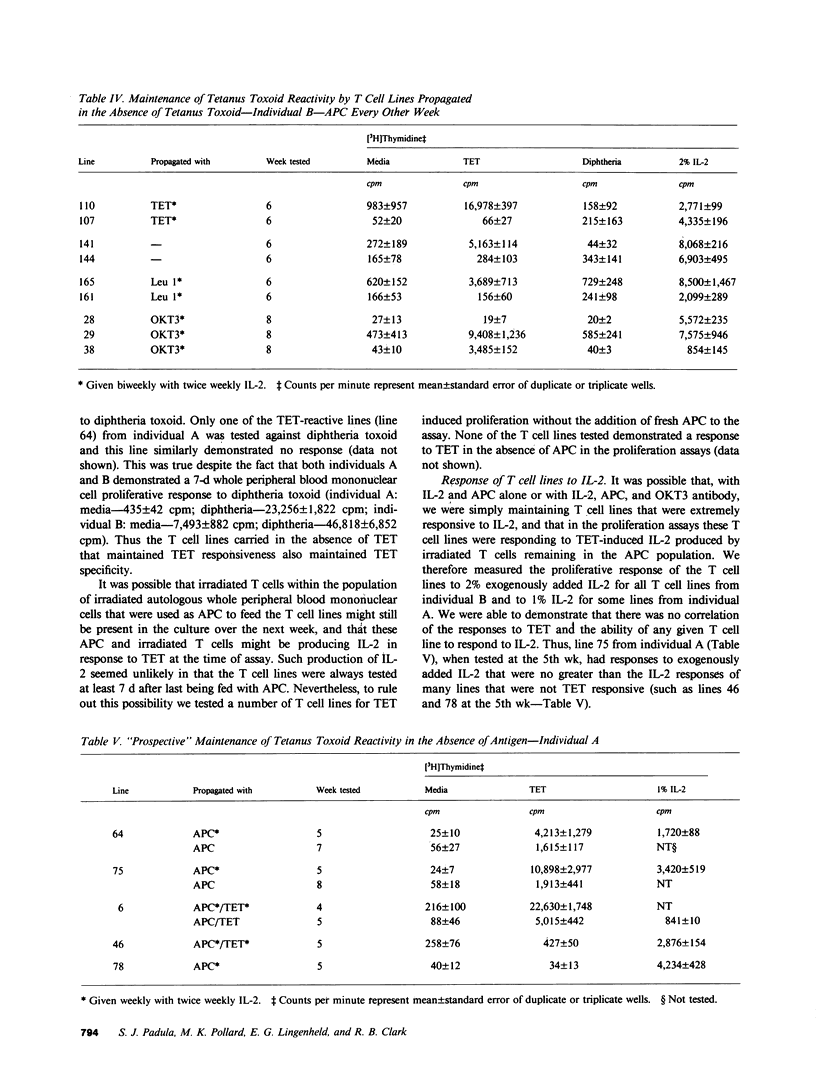
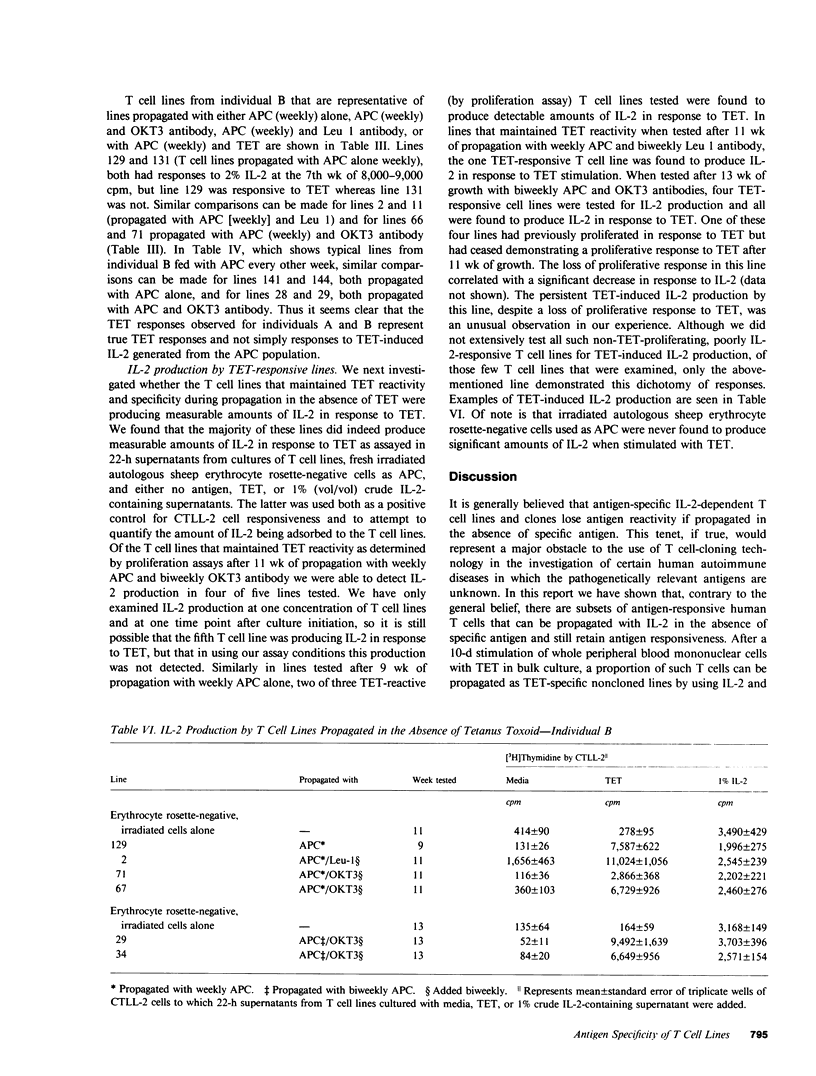
The in vitro growth of T cells obtained from localized anatomic sites of pathology may offer a new approach to the investigation of certain human autoimmune diseases. However, if interleukin-2-dependent T cell cloning is to be useful in helping to elucidate putative pathogenetic antigens in these diseases, the expansion of the small number of T cells obtainable from localized anatomic sites of pathology will often have to be accomplished in the absence of these, as yet undetermined, antigens. At present, it is a generally held belief that antigen-responsive, interleukin-2-dependent T cell lines and clones will lose antigen responsiveness if propagated in the absence of specific antigen. Thus, the use of T cell cloning might be viewed as being of limited usefulness in the investigation of certain human autoimmune diseases. In this report we demonstrate that, when propagated in the absence of antigen, human tetanus toxoid-specific, interleukin-2-dependent T cell lines will indeed lose antigen reactivity. However, if propagated in the absence of antigen but in the presence of antigen-presenting cells, the tetanus toxoid reactivity of a subset of such lines can be maintained. Moreover, the propagation with OKT3 antibody, in addition to antigen-presenting cells, may be even more effective in maintaining antigen reactivity. These results may suggest a new approach to the use of T cell cloning technology in the investigation of certain autoimmune diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak V., Fuks Z., Galilli N., Treves A. J. Selection and continuous growth of antigen-specific human T cells by antigen-treated monocytes. Eur J Immunol. 1983 Nov;13(11):952–956. doi: 10.1002/eji.1830131116. [DOI] [PubMed] [Google Scholar]
- Burns J. B., Zweiman B., Lisak R. P. Long-term growth in vitro of human cerebrospinal fluid T lymphocytes. J Clin Immunol. 1981 Jul;1(3):195–200. doi: 10.1007/BF00922763. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Dore-Duffy P., Donaldson J. O., Pollard M. K., Muirhead S. P. Generation of phenotypic helper/inducer and suppressor/cytotoxic T-cell lines from cerebrospinal fluid in multiple sclerosis. Cell Immunol. 1984 Apr 1;84(2):409–414. doi: 10.1016/0008-8749(84)90113-8. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Muirhead S. P., Pollard M. K. Generation of long-term T-cell lines from synovial fluid. Clin Immunol Immunopathol. 1984 Nov;33(2):287–292. doi: 10.1016/0090-1229(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman P. B., Moody C. E., Innes J. B., Gibbons J. J., Weksler M. E. Studies on the syngeneic mixed lymphocyte reaction. III. Development of a monoclonal antibody with specificity for autoreactive T cells. J Exp Med. 1983 Oct 1;158(4):1307–1318. doi: 10.1084/jem.158.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman P. B., Raff H. V., Gilbert R. C., Picker L. J., Stobo J. D. T cells and macrophages involved in the autologous mixed lymphocyte reaction are required for the response to conventional antigen. J Immunol. 1980 Sep;125(3):1374–1379. [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983 Dec 16;222(4629):1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff H., Carter C., Oppenheim J. J. Inhibition of concanavalin A-induced human lymphocyte mitogenic factor (Interleukin-2) production by suppressor T lymphocytes. J Immunol. 1980 Oct;125(4):1823–1828. [PubMed] [Google Scholar]
- Santoli D., Defreitas E. C., Sandberg-Wollheim M., Francis M. K., Koprowski H. Phenotypic and functional characterization of T cell clones derived from the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 1984 May;132(5):2386–2392. [PubMed] [Google Scholar]



