Abstract
Gene therapy approaches using recombinant adeno-associated virus serotype 2 (rAAV2) and serotype 8 (rAAV8) have achieved significant clinical benefits. The generation of rAAV Reference Standard Materials (RSM) is key to providing points of reference for particle titer, vector genome titer, and infectious titer for gene transfer vectors. Following the example of the rAAV2RSM, here we have generated and characterized a novel RSM based on rAAV serotype 8. The rAAV8RSM was produced using transient transfection, and the purification was based on density gradient ultracentrifugation. The rAAV8RSM was distributed for characterization along with standard assay protocols to 16 laboratories worldwide. Mean titers and 95% confidence intervals were determined for capsid particles (mean, 5.50×1011 pt/ml; CI, 4.26×1011 to 6.75×1011 pt/ml), vector genomes (mean, 5.75×1011 vg/ml; CI, 3.05×1011 to 1.09×1012 vg/ml), and infectious units (mean, 1.26×109 IU/ml; CI, 6.46×108 to 2.51×109 IU/ml). Notably, there was a significant degree of variation between institutions for each assay despite the relatively tight correlation of assay results within an institution. This outcome emphasizes the need to use RSMs to calibrate the titers of rAAV vectors in preclinical and clinical studies at a time when the field is maturing rapidly. The rAAV8RSM has been deposited at the American Type Culture Collection (VR-1816) and is available to the scientific community.
Introduction
Remarkable clinical successes have been achieved in humans using recombinant adeno-associated viral (rAAV) vectors for hemophilia B, Leber congenital amaurosis,, and lipoprotein lipase deficiency (LPLD), among other diseases (Manno et al., 2006; Bainbridge et al., 2008; Maguire et al., 2008; Nathwani et al., 2011; Gaudet et al., 2013). AAV serotype 2 is so far the best characterized and most used serotype for gene transfer studies, but other AAV serotypes with more efficient gene delivery profiles for specific tissues are currently in human trials and their use will likely increase. One example is the clinical trial for hemophilia B using rAAV serotype 8 that has shown long-term expression of the transgene by the liver with significant clinical benefit (Nathwani et al., 2011). The market authorization by the European Medicine Agency in 2012 of Glybera, an rAAV1 vector for the treatment of LPLD patients, was a milestone in the field of gene therapy and prompted many pharmaceutical companies to move into this field (Bryant et al., 2013).
As the first reference standard initiative in the viral vector gene therapy field, an Adenovirus Reference Material Working Group was established and generated a human adenovirus 5 Reference Standard Material (RSM; ATCC VR-1516) in 2002 (Hutchins, 2002). A similar need was recognized associated with the use of rAAV relating to the inability to calibrate vector doses administered by different investigators to animals and humans. The need for RSMs for AAV vectors was recognized by the research community, and an AAVRSM Working Group was established and committed to the development of an rAAV2 Reference Standard Material (rAAV2RSM) (Snyder et al., 2002). The AAVRSM Working Group is a volunteer organization and comprises members from both industry and universities, under the guidance of the FDA and NIH (Moullier et al., 2008, 2012). The AAVRSM Working Group decided in 2002 that the first AAV RSM had to be based on the prototypical AAV serotype 2. Thus, the rAAV2RSM was produced by cotransfection of HEK293 cells with an AAV2 genome/hGFP transgene plasmid and a second plasmid encoding the AAV2 replication and capsid proteins, and was purified by sequential rounds of column chromatography (Potter et al., 2008).
The rAAV2RSM was characterized by 16 laboratories worldwide, the data were published, and this material is available for the scientific community through the American Tissue Culture Collection (ATCC; VR-1616) (Lock et al., 2010). Following this example, another effort was initiated by the AAVRSM Working Group in 2008 to generate a novel RSM based on serotype 8 (Gao et al., 2002). The rAAV8RSM Working Group included members who participated in the AAV2RSM effort from industry and academia, from Europe, United States, and Japan (Moullier et al., 2008). The rAAV8RSM was initiated in the framework of the European Clinigene Network of Excellence. The rAAV8RSM was manufactured in Europe by two laboratories (Atlantic Gene Therapies at Nantes, France, and the Center of Animal Biotechnology and Gene Therapy at the Universitat Autonoma de Barcelona, Spain), and the characterization of the material involved 16 laboratories worldwide (coordinated by the University of Florida and the University of Pennsylvania). The rAAV8RSM is a pseudotyped AAV2 genome vector with capsid serotype 8 (Gao et al., 2002) expressing the GFP protein. To harmonize the two reference standards, the rAAV8RSM contains the same vector genome that was used for the rAAV2RSM. The vector was also produced by cotransfection of HEK293 cells, but the purification step differed from the rAAV2RSM, as it was purified by density ultracentrifugation using an optimized cesium chloride (CsCl) gradient-based protocol (Ayuso et al., 2012) and formulated in dPBS at a target concentration of >1011 vg/ml in cryovials frozen at EFS-Atlantic Bio-GMP.
The protocols for the characterization were based on the previously developed consensual protocols for the rAAV2RSM but adapted to serotype 8 (ATCC Virus Reference Materials). The rAAV8RSM characterization assays performed by the 16 laboratories included (1) capsid titer by enzyme-linked immunosorbent assay (ELISA); (2) vector genome titer by quantitative polymerase chain reaction (qPCR); (3) infectious titer by medium tissue culture infective dose (TCID50) with qPCR readout; and (4) purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The AAV8RSM was also tested for sterility, endotoxin, and mycoplasma. Altogether, this international effort led to the generation of more than 4040 vials of an rAAV8RSM that is stored at ATCC (No. VR-1816) and is available to the community.
Finally, to cross-validate the use of the AAVRSMs, we conducted a side-by-side comparison of rAAV2RSM versus rAAV8RSM in terms of vector genome titration and capsid content using non-serotype-dependent techniques. The data generated by four laboratories within the AAV8RSM Working Group were in agreement with the mean values reported by the community for the rAAV2RSM (Lock et al., 2010) and the rAAV8RSM (data from this article).
Materials and Methods
Reference standard material manufacturing
Production and purification of the rAAV8RSM were carried out at Atlantic Gene Therapies–UMR 1089 in Nantes (France) and the Center of Animal Biotechnology and Gene Therapy (CBATEG) at the Universitat Autonoma de Barcelona (Spain).
Production was initiated by co-transfection of a certified master cell bank of HEK293 cells (ABG-EFS) in five-chamber Corning Cellstacks (at Nantes) or Corning Roller Bottles with plasmid pTR-UF-11 (ATCC # MBA-331), containing the vector genome and hGFP expression cassette (Burger et al., 2004) and the pDP8 helper plasmid harboring the rep gene from AAV2, the cap gene from AAV8, the adenovirus helper genes E2A, E4, and VA-RNA, and the ampicillin resistance gene (No. PF478; Plasmidfactory) using a calcium phosphate precipitation method. Same lots of FBS (Hyclone-Thermo scientific), trypsin (PAA Laboratories GmbH), DMEM (PAA), Pen/Strep (PAA), PBS (PAA), and dPBS (PAA) were split between the two manufacturing sites.
For the transfection, the complete medium (DMEM, 10% FBS, 1% Pen/Strep) was removed and replaced by a transfection medium (DMEM, 2% FBS, 1% Pen/Strep) including the transfection mixture. After 6–15 hr at 37°C and 5% CO2, the transfection medium was then removed and replaced by a fresh exchange medium (DMEM, 1% Pen/Strep) before 3 days of incubation at 37°C and 5% CO2. The cells were harvested and centrifuged at 1500 g for 10 min at 4°C. The cell pellet was discarded and the supernatant was precipitated with PEG at a final concentration of 8% for a period of 15 hr to 3 days at 5±3°C. Samples of these cell pellets from Nantes and Barcelona were tested for mycoplasma and found to be negative by PCR using the Mycotrace kit (PAA, ref: Q052-20). The PEG-precipitated supernatant was then centrifuged at 5000 g for 45 min at 4°C. The supernatant was discarded and the PEG-pellet was resuspended in tris buffer saline before benzonase digestion at 37°C for 30 min.
Following benzonase digestion, the viral suspension was centrifuged at 10,000 g for 10 min at 4°C and the vector-containing supernatant was loaded on a step density CsCl gradient (1.5 g/cm3 at the bottom and 1.3 g/cm3 on top) in UltraClear tube for SW28 rotor (Beckman Coulter). The gradient was centrifuged at 28,000 rpm for 24 hr at 15°C. The full particles band was collected with a 10 ml syringe and transferred to a new UltraClear tube for SW41 rotor filled with 1.375 g/cm3 density CsCl. The second gradient was centrifuged at 38,000 rpm for 48 hr at 15°C. The enriched full particle band was then collected with a 10 ml syringe (the volume recovered for each run is indicated in Supplementary Tables S1 and S2; Supplementary Data are available online at www.liebertpub.com/hum). The viral suspension was then subjected to four successive rounds of dialysis under slight stirring in a Slide-a-Lyzer cassette (Pierce) against dPBS (containing Ca and Mg). No other additives were included in the formulation buffer (dPBS).
Each purified vector sublot was finally collected, sampled for vg titer and purity assay, and stored at less than −70°C in polypropylene low-binding cryovials.
The specifications of the dPBS (PAA Laboratories) used as a formulation buffer were as follows:
pH 7.0–7.5
Osmolality 240–320 mOsmol/kg
Endotoxin <1 EU/ml
Composition: KCL 0.2 g/l; KH2PO4 0.2 g/l; NaCl 8.00 g/l; Na2HPO4 anhyd. 1.15 g/l; CaCl2–2H2O 0.132 g/l; MgCl2–2H2O 0.1 g/l.
rAAV8RSM fill finish and final quality control
Fill finish and final quality control (QC) were carried out at EFS-ABG, a European pharmaceutical site dedicated to ATMPs manufacturing. All of the sublots produced at Nantes and Barcelona (except two lots, as described in the Results section) were combined and diluted to 200 ml in dPBS (see specifications and composition above). This purified bulk was subsequently tested for endotoxin (EP 2.6.14), mycoplasma (EP 2.6.7), and bioburden (EP 2.6.12) by Vitrology Ltd., and the vector genome titer was determined by qPCR at Atlantic Gene Therapies, before final formulation, sterile filtration, and filling in an ISO5 cleanroom at EFS-ABG with environmental monitoring. The purified bulk was diluted with 325 ml of dPBS and was then sterile-filtered using a 0.2 μm PES filter (Sartorius). The filtered formulated bulk was then vialed in 14 sterile 50 ml conical tubes (Corning; polypropylene 50 ml tubes, USP class VI) stored at less than −70°C. The sterile bulk was then filled into 4088 cryovials (Corning; 1.2 ml polypropylene cryogenic vial, USP class VI) with a volume of 0.125 ml/vial using an electronic adjustable repeating pipette AutoRep (Rainin). Before storage at less than −70°C, the vials were labeled according to ATCC requirements as follows: ATCC, VR-1816, rAAV8-RSS—Reference Material, ABG[date] −0.125 ml, Store at less than −70°C—For research use only. Lot #03112010SP2pcg.
Due to the number of vials (>4000 vials), seven fill days were required. Each fill date is indicated on the label. At each filling session, the first and the last cryovials filled were transferred to QC for further sterility assay. Thus, a total of 28 cryovials were submitted to sterility assay at Vitrology Ltd. The final product was submitted to preliminary tests for vector genome titer (qPCR SV40 and dot blot hybridization using a GFP probe), transducing titer, and purity/identity (silver-stained SDS-PAGE gel). A summary of QC performed on the purified bulk and on the final product is shown in Table 1. The SDS-PAGE silver stain purity assay is shown in Supplementary Fig. S1 and suggests that the AAV8RSM purity is similar to the control AAV2RSM, one which was determined at 94% using Sypro Ruby staining following SDS-PAGE (Lock et al., 2010).
Table 1.
Quality Control of Intermediate and Final Product
| Test item | Tests | Method | Laboratory | Results |
|---|---|---|---|---|
| Purified bulk | Vector genome (vg/ml) | qPCR | UMR1089 | 5.7×1012 vg/ml |
| Bioburden | EP 2.6.12 | SGS-Vitrology | 0 cfu/ml | |
| Endotoxin | EP 2.6.14 | SGS-Vitrology | <0.03 EU/ml | |
| Mycoplasma | EP 2.6.7 | SGS-Vitrology | Negative | |
| Final product | Vector genome (vg/ml) | qPCR | UMR1089 | 6.4×1011 vg/ml |
| Vector genome (vg/ml) | Dot blot | UMR1089 | 6.3×1011 vg/ml | |
| Transducing units (TU/ml) | Transduction of cells; GFP read out | UMR1089 | 1.5×108 TU/ml | |
| Sterility | EP 2.6.1 | SGS-Vitrology | Sterile |
qPCR, quantitative polymerase chain reaction.
rAAV8 RSM handling
For rAAV8RSM characterization, each testing laboratory received two vials from ATCC on dry ice. Upon receipt, both vials were stored frozen at −70°C to −90°C. One vial was thawed at room temperature while mixing gently and then kept on wet ice. Within 1 hr of thawing, the infectious titer was conducted. The remainder of the thawed vial was stored at 4°C and mixed gently upon use. Within 5 days of vial thaw, the particle titer, vector genome titer, and purity/identity assays were performed. These steps were repeated for the second vial starting on a different calendar day.
rAAV8RSM characterization assays
Brief descriptions of each characterization assay follow. For those wishing to reproduce these assays, detailed protocols can be requested directly from M. Lock or R. Snyder.
Particle titer
The particle concentration was determined by each laboratory, using four separate dilution series from a single vial in the Progen AAV8 Titration ELISA (Progen Biotechnik GMBH; Article number PRAAV8), against a standard curve prepared from a previously titered rAAV8 preparation (WL217S). The particle content of WL217S was determined by first spiking the preparation with the adenoviral reference material (ARM ATCC VR-1516, with a known particle concentration) and then obtaining images of the particles by electron microscopy. Because of the size difference between AAV and adenovirus, the particles of each can be distinguished and counted on electron micrographs. Several fields were counted, and the average ratio of AAV particles to the internal ARM standard was used to compute the particle titer for WL217S.
Vector genome titer
The vector genome concentration was determined in duplicate, testing one replicate from each of two vials, by qPCR of serial dilutions of rAAV8RSM against a standard curve of plasmid pTR-UF-11 (ATCC MBA-331) (Burger et al., 2004). Some labs performed more than two tests for vector genome titrations (Table 3).
Table 3.
rAAV8 Reference Standard Material Raw Characterization Data
| Laboratory | Replicate | Particle titer (ELISA) (pt/ml) | Genome titer (qPCR) (vg/ml) | Infectious titer (TCID50) (IU/ml) |
|---|---|---|---|---|
| A | 1 | 2.68×1011 | 3.60×1011 | 1.65×1011 |
| 2 | 2.08×1011 | 4.58×1011 | 1.36×1011 | |
| 3 | 2.46×1011 | 1.36×1011 | ||
| B | 1 | 5.39×1013 | 3.47×1012 | |
| 2 | 9.11×1013 | 4.73×1012 | ||
| C | 1 | 8.25×1011 | 5.53×1011 | 9.28×109 |
| 2 | 5.68×1011 | 3.39×1011 | 9.28×109 | |
| 3 | 3.42×1011 | 1.12×1010 | ||
| D | 1 | 6.03×1011 | 5.19×1011 | 2.00×109 |
| 2 | 8.90×1011 | 8.50×1011 | 1.65×109 | |
| E | 1 | 4.88×1011 | 6.32×1011 | 1.65×109 |
| 2 | 5.53×1011 | 3.00×1011 | 1.82×109 | |
| F | 1 | 4.57×1011 | 7.71×1010 | 5.22×109 |
| 2 | 3.81×1011 | 7.58×1010 | 4.74×109 | |
| 3 | 8.14×1010 | |||
| G | 1 | 8.08×1011 | 1.18×1012 | 3.23×109 |
| 2 | 4.73×1011 | 1.02×1012 | 5.22×109 | |
| 3 | 9.60×1011 | 4.74×109 | ||
| H | 1 | 3.10×1012 | 6.32×108 | |
| 2 | 2.64×1012 | 2.42×108 | ||
| I | 1 | 6.26×1011 | 9.56×1011 | 6.32×108 |
| 2 | 7.40×1011 | 8.25×1011 | 4.31×108 | |
| 3 | 7.19×1011 | |||
| 4 | 4.86×1011 | |||
| 5 | 5.39×1011 | |||
| J | 1 | 2.24×1012 | ||
| 2 | 2.40×1012 | |||
| K | 1 | 4.77×1011 | 7.02×1010 | |
| 2 | 5.52×1011 | 4.65×1010 | ||
| L | 1 | 4.35×1011 | 7.45×1011 | 5.22×108 |
| 2 | 4.13×1011 | 3.29×1011 | ||
| 3 | 8.79×1011 | |||
| 4 | 4.20×1011 | |||
| M | 1 | 3.17×1011 | 7.06×1011 | 1.65×109 |
| 2 | 2.45×1011 | 8.58×1011 | 9.28×108 | |
| 3 | 5.28×1011 | |||
| 4 | 2.26×1011 | |||
| N | 1 | 1.09×1012 | 1.55×1011 | 1.12×109 |
| 2 | 8.73×1011 | 1.57×1011 | 4.31×108 | |
| O | 1 | 6.22×1011 | 2.61×1012 | 5.75×108 |
| 2 | 6.22×1011 | 3.53×1012 | 3.56×108 | |
| P | 1 | 3.19×1011 | 2.59×1011 | 6.32×108 |
| 2 | 4.51×1011 | 3.73×1011 | 2.67×108 | |
| 3 | 3.48×1011 | 9.28×108 | ||
| Mean | 5.50×1011 | 9.62×1011 | 2.67×109 | |
| SD | 2.20×1011 | 1.10×1012 | 3.32×109 |
Numbers in bold were considered outlyers.
Infectious titer
Serial 10-fold dilutions of rAAV8RSM were made on HeLa RC32 cells (ATCC CRL-2972) (Tessier et al., 2001) and co-infected with adenovirus type 5 (ATCC VR-1516). Seventy-two hours after infection, total cell DNA was extracted and analyzed for AAV vector genome copies by qPCR. Input vector genomes were subtracted and TCID50 titers calculated according to the method of Spearman–Karber.
Purity and identity
The purity and identity of the rAAV8RSM were evaluated by SDS-PAGE, using Sypro Ruby (Invitrogen) or silver staining (SilverXpress; Invitrogen). The AAV8 VP1, VP2, and VP3 capsid protein bands were evaluated for their stoichiometry and size. Purity relative to nonvector impurities visible on stained gels was determined.
Results
rAAV8RSM manufacturing and predistribution QC
The manufacturing of the rAAV8RSM was carried out by two laboratories that harmonized their production protocols: Atlantic Gene Therapies–UMR 1089 in Nantes (France) and the Center of Animal Biotechnology and Gene Therapy (CBATEG) at the Universitat Autonoma de Barcelona (Spain).
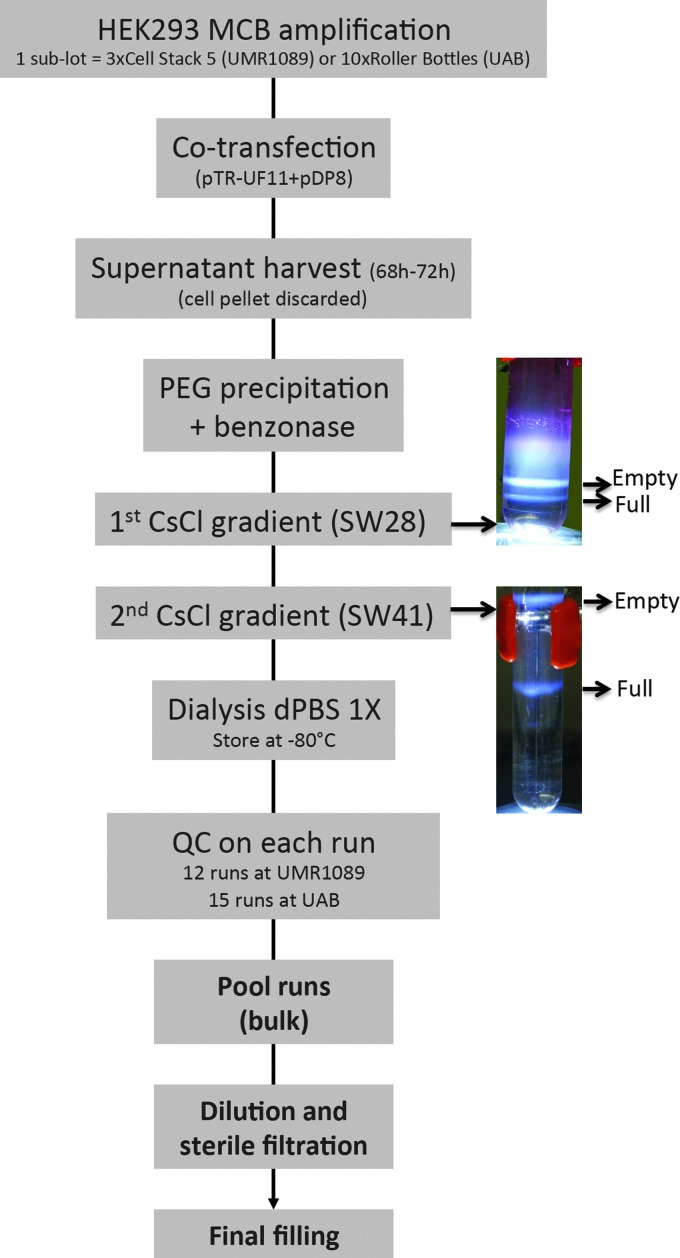
The rAAV8RSM is an rAAV vector serotype 8 (Gao et al., 2002) produced using a HEK293 transfection-based protocol. HEK293 cells were amplified from a cGMP master cell bank and transfected by the vector plasmid pTR-UF11 (Burger et al., 2004), the same plasmid used to generate the rAAV2RSM (Potter et al., 2008; Lock et al., 2010), and the pDP8 helper plasmid (see Materials and Methods section: Reference standard material manufacturing). Twelve sublots of rAAV8RSM were produced and purified in France and 15 sublots in Spain. The protocol followed was previously described (Ayuso et al., 2010). Since it was known that up to 80% of functional rAAVs from serotype 8 are released into cell culture supernatant (Vandenberghe et al., 2010), the cell pellet was discarded and the supernatant was processed by PEG precipitation and subsequently purified by double-cesium-chloride (CsCl) gradient ultracentrifugations followed by a dialysis step against phosphate buffered saline containing calcium and magnesium (dPBS) (Fig. 1).
FIG. 1.
Flow chart showing the steps of rAAV8RSM manufacturing. Note the pictures on the right showing the empty and full vector particle bands at the first and second CsCl gradient step. MCB, master cell bank; PEG, polyethylene glycol; QC, quality control; rAAV8, recombinant adeno-associated virus serotype 8; RSM, Reference Standard Materials.
In-process QC testing was performed on each sublot for purity, identity, and vector genome titer to meet predetermined minimum specifications: (1) >85% purity and the correct AAV capsid protein banding pattern and (2) vector genome titer >1012 vg/ml. Silver staining of SDS-PAGE gel of each sublot is shown in Supplementary Fig. S2. The sublot BCN7 was discarded because of the presence of significant impurities (Supplementary Fig. S2B). Identity of the VP proteins was verified on a few sublots by Western blot analysis using anti-capB1 or anti-AAV2 polyclonal antibodies (Supplementary Fig. S2C). The Western blot analysis using a polyclonal anti-AAV2 antibody suggests that the extra-band migrating below VP3 revealed by silver stain (Supplementary Fig. S2A) is a cleavage product of the capsid proteins (Van Vliet et al., 2006). The vector genome titer was determined in each sublot by dot-blot and/or qPCR using the rAAV2RSM as a control (Supplementary Tables S1 and S2).
The sublot BCN4 was discarded because of the vector genome titer being below the target concentration. Accordingly, a total of 25 selected sublots were then combined and diluted with dPBS to generate the purified bulk. The purified bulk was then tested for vector genome titer, bioburden, mycoplasma, and endotoxin before fill and finish (Table 1). A total of 4088 vials containing 0.125 ml were subsequently filled into 1.2 ml polypropylene low-binding cryovials. Vector genome and infectious titers (transducing units) as well as microbiological tests were performed on the final product before shipment to the American Type Culture Collection (catalog no. VR-1816) (Table 1). In summary, we generated about 500 ml of rAAV8RSM with an estimated titer of 6×1011 vg/ml, resulting in a total vector amount of >3×1014 vg.
Characterization of the rAAV8RSM
For the characterization of the rAAV8RSM, we took advantage of the experience previously gained when the community characterized the rAAV2RSM, and therefore we used similar protocols and reagents adapted to the AAV8 serotype. The assays chosen for the rAAV8RSM characterization included (1) confirmation of the serotype and capsid particle titer by an AAV8-specific ELISA (Progen) (Sonntag et al., 2011); (2) vector genome titer by qPCR; (3) infectious titer by tissue culture infective dose (TCID50) with qPCR readout; (4) evaluation of the purity, capsid subunit stoichiometry, and chemical integrity of the capsid by SDS-PAGE.
Key reagents used for the characterization of the rAAV8RSM were already used for the rAAV2RSM characterization and are available from ATCC. For both genome and infectious titers assays, a qPCR primer–probe set directed to the SV40 polyA sequence and a dilution series specific to the RSM were selected. For the purity and identity assay, commercially available assay reagents with the highest sensitivity were suggested. Using these reagents, the protocols were adapted for rAAV8 vectors and were beta-tested at the University of Pennsylvania Gene Therapy Program against both in-house standards and the rAAV8RSM itself. The finalized protocols were posted on the ATCC website.
The rAAV8RSM (ATCC VR-1816) was distributed along with the required reagents and handling instructions to 16 laboratories worldwide (Table 2), which volunteered to conduct one or more of the characterization assays. The characterization phase of the rAAV8RSM proceeded from 2011 to 2013. Upon receipt, the RSM was evaluated by the 16 testing laboratories according to the posted protocols, and the data were recorded on the assay spread sheets provided, which contained the necessary calculations for titer determination.
Table 2.
rAAV8 Reference Standard Material Testing Laboratories
| Telethon Institute of Genetics and Medicine, TIGEM, Italy |
| Universitat Autonoma de Barcelona, Spain |
| Research Institute at Nationwide Children's Hospital, USA |
| University of Florida, USA |
| Atlantic Gene Therapies, France |
| Genethon, France |
| International Center for Genetic Engineering and Biotechnology (ICGEB), Italy |
| German Cancer Research Center (DKFZ), Germany |
| University of Pennsylvania, USA |
| Jichi Medical University, Japan |
| Sangamo BioSciences, USA |
| University of Massachusetts Medical School, USA |
| Universite Libre de Bruxelles, Belgium |
| uniQure, The Netherlands |
| Children's Hospital of Philadelphia, USA |
| Lausanne University Hospital, Switzerland |
rAAV8, recombinant adeno-associated virus serotype 8.
The raw data that emerged from the testing laboratories for the three quantitative titer assays (Table 3) were statistically analyzed to determine true mean titer values and 95% confidence intervals. The distribution was first visualized as histograms (Supplementary Fig. S3), and it was noted that the data did not appear to be normally distributed. Since valid estimation of the mean and confidence interval relies on an underlying normal distribution, it was clear that some form of transformation was warranted. Common statistical transformation methods employed are square root, natural log, and log base 10. Therefore, means and intervals were calculated on the transformed data, and the results were then back-transformed to the original measurement scale; the data for each assay were processed in this way using all three transformations. Analysis of the mean and median (for normally distributed data, these two values are the same) and skewness and kurtosis estimates confirmed that the transformations were performing as expected and were similar to those obtained for the AAV2RSM (Supplementary Table S3).
Supplementary Fig. S4 shows the results of the most successful transformations for each assay as quantile–quantile plots. In this analysis, the quantiles (i.e., 5%, 10%, etc.) obtained from the transformed data were compared with the quantiles that would be expected for a normal distribution. A line that extends through the 25th and 75th quantile is shown on the plots, and the nearer the points are to this line, the more normal are the data distribution. The capsid particle titer assay data appeared normally distributed without the need for transformation (Supplementary Fig. S4a). For the vector genome titer and for the infectious titer, a log base 10 transformation appeared the most appropriate (Supplementary Fig. S4b and c). The transformed data are summarized in Supplementary Table S4. For each assay, two and three standard deviation limits were calculated corresponding to nominal 95% and 99.7% confidence bounds on individual values. Any test result lying outside of the three standard deviations was considered to be an outlier. Using this criterion, only one test result from the particle titer assay and one test result from infectious titer were determined to be outliers and were removed from the analysis (Table 3, in bold); the values reported in Supplementary Table S4 exclude these data points.
An assumption we made when calculating the mean values and confidence intervals is that each test result is independent of another; however, this assumption does not take into account that all institutions submitted duplicate (and in some cases triplicate) test results for the assays. To assess the degree of correlation between samples, Pearson coefficients were determined for the first two duplicate samples per institution, and the coefficients obtained were 0.71 for vector particle titer, 0.96 for vector genome titer, and 0.97 for infectious units titer. These results indicated that there was a significant correlation within institutions and that the assumption that each result is independent was violated. To account for this correlation, the transformed data were modeled, using a linear random effect modeling approach (Littell et al., 2006). This allows for a unique component associated with each institution to be included in the model, under the assumption that these institutional random effects have a mean of zero. When the correlation within an institution is accounted for, the precision of the mean estimate as illustrated by the width of the 95% confidence interval is decreased (Table 4). Taking the transformed, modeled data as the true estimate of the mean, we reached the following determinations for the rAAV8RSM: the mean particle titer is 5.5×1011 pt/ml, with 95% confidence that the true value lies in the range 4.26×1011 to 6.75×1011 pt/ml; the mean vector genome titer is 5.75×1011 vg/ml, with 95% confidence that the true value lies in the range 3.05×1011 to 1.09×1012 vg/ml; and the mean infectious titer is 1.26×109 TCID50 IU/ml, with 95% confidence that the true value lies in the range 6.46×108 to 2.51×109 TCID50 IU/ml (Table 4).
Table 4.
Final rAAV8 Reference Standard Material Titer Estimates After Transformation and Modeling
| Titer units | Transformation | Mean | Lower 95% confidence limit for the mean | Upper 95% confidence limit for the mean | ±2 SD |
|---|---|---|---|---|---|
| Particles (pt)/ml | None | 5.5×1011 | 4.26×1011 | 6.75×1011 | 1.06×1011 to 9.94×1011 |
| Vector genomes (vg)/ml | Log10 | 5.75×1011 | 3.05×1011 | 1.09×1012 | 4.57×1010 to 7.24×1012 |
| Infectious units (IU)/ml | Log10 | 1.26×109 | 6.46×108 | 2.51×109 | 1.32×108 to 1.20×1010 |
Interestingly, the vector genome titer was very similar to the vector particle titer, indicating that the AAV8RSM is virtually devoid of empty particles. This finding is consistent with the CsCl gradient purification protocol used in the production of the rAAV8RSM that efficiently separates empty versus full particles (Ayuso et al., 2010).
The DNA content of the AAV8RSM was extracted and native agarose gel electrophoresis was used to confirm the homogeneity and the size of the vector genome (expected genome size of 4.3 kb) (Supplementary Fig. S5).
The purity of the rAAV8RSM was assessed by SDS-PAGE analysis. The RSM was examined under both denaturating and nondenaturating conditions using Sypro Ruby and silver stains (Fig. 2). Under denaturating conditions, all proteins including the denatured AAV8 capsids are expected to enter the gel, and impurities would be detected as protein bands other than the capsid proteins VP1, VP2, and VP3. Under nondenaturating conditions, the capsid would remain intact and would not be expected to enter the resolving gel, whereas impurities would enter the gel; proteins, which previously comigrated with the capsid proteins on denaturating gels, would thus be detected. Silver nitrate staining was included since it is capable of detecting DNA, lipid, and carbohydrate impurities as well as nanogram levels of protein (Weiss et al., 2009). Sypro Ruby is a protein-specific fluorescent dye that has sensitivity close to that of silver stain (Rabilloud et al., 2001; Weiss et al., 2009). In each case, the rAAV8RSM was analyzed alongside an internal laboratory-standard AAV vector. The consensus data from the 14 testing laboratories that carried out the purity test estimated that the rAAV8RSM was greater than 99% pure and confirmed that VP1, VP2, and VP3 co-migrated with the AAV capsid proteins of the internal vector standards (Fig. 2 and data not shown).
FIG. 2.
The rAAV8RSM was run on SDS-PAGE gels under both denaturating and native conditions and then stained with (a) silver stain and (b) Sypro Ruby. In-house rAAV standard was run as a positive control and buffer as a negative control. The lanes for each gel are (1) benchmark ladder (unstained or prestained)—reduced; (2) negative control—reduced; (3) AAV8 reference material—reduced; (4) empty; (5) positive control—reduced; (6) benchmark ladder (unstained or prestained)—native; (7) negative control—native; (8) AAV reference material—native; (9) empty; and (10) positive control—native. SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Comparison of rAAV2RSM versus rAAV8RSM
Having an increasing repertoire of AAV serotypes entering the clinical arena is encouraging for the field of AAV-mediated gene transfer. However, the characterization of the AAV preparations and the comparison of data between studies will become more complex without the appropriate use of common standards. As an example, here we have compared the vector genome titers and the capsid content of rAAV2RSM and rAAV8RSM using non-serotype-dependent techniques. This comparative study was performed in four institutions independently within the AAV8 RSM Working Group. The vector genome titer was measured by qPCR according to the SOPs written for the rAAV8RSM and using the primers and probe targeting the SV40 polyA. Of note, these experiments were performed before the collection of the data from the 16 laboratories and the statistical analysis shown in Table 4.
The vector genome titers of the rAAV8RSM obtained by the four laboratories were within the 95% confidence limits shown in Table 4; however, while the titers for the rAAV2RSM were within the confident limits in two laboratories, in two other institutions they were slightly lower than the lower confidence limit for the rAAV2RSM (2.7×1010 to 4.75×1010 vg/ml). Interestingly, the use of the rAAV2RSM as a control evidenced a significant deviation of the titration in the first assay conducted in Institution C (Table 5, in bold), and therefore the experiment was repeated. Altogether, these data indicate that the same protocol for vector titration by qPCR could be used independently of the serotype; however, the inclusion of one or more RSMs was crucial to obtain confident titers and avoid significant deviations.
Table 5.
Titration by qPCR AAV2RSM Versus AAV8RSM
| Laboratory | Replicate | Genome titer AAV2RSM (qPCR) (vg/ml) | Genome titer AAV8RSM (qPCR) (vg/ml) |
|---|---|---|---|
| A | 1 | 2.37×1010 | 7.91×1011 |
| 2 | 1.37×1010 | 4.86×1011 | |
| 3 | 2.83×1010 | 5.39×1011 | |
| Mean | 2.19×1010 | 6.05×1011 | |
| B | 1 | 3.14×1010 | 5.28×1011 |
| 2 | 2.03×1010 | 2.26×1011 | |
| Mean | 2.58×1010 | 3.77×1011 | |
| C | 1 | 1.56×1011 | 4.55×1012 |
| 2 | 2.60×1011 | 6.20×1012 | |
| C′ | 1′ | 3.18×1010 | 5.76×1011 |
| 2′ | 3.11×1010 | 5.58×1011 | |
| 3′ | 3.40×1010 | 5.60×1011 | |
| Mean | 3.23×1010 | 5.64×1011 | |
| D | 1 | 2.00×1010 | 4.10×1011 |
| 2 | 3.80×1010 | 4.20×1011 | |
| 3 | 4.00×1010 | 9.10×1011 | |
| mean | 3.26×1010 | 5.80×1011 |
qPCR, quantitative polymerase chain reaction; RSM, Reference Standard Materials.
According to Lock et al. (2010), AAV2RSM qPCR titer with 95% confidence limits is 2.70×1010 vg/ml to 4.75×1010 vg/ml.
According to Table 4 in this article, AAV8RSM qPCR titer with 95% confidence is 3.05×1011 vg/ml to 1.09×1012 vg/ml.
Although the vector genome titer is the most widely used for dosing rAAV in clinical trials, it is also believed that total capsid content plays a critical role in the immune responses against the therapeutic vector (Manno et al., 2006; Mingozzi et al., 2007, 2009, 2013). Capsid titer can be measured by serotype-specific ELISA, as shown for rAAV2 (Grimm et al., 1999; Lock et al., 2010) and here for rAAV8; however, this assay is only available for several other serotypes, for example, AAV9, AAV1/6, AAV4, and AAV5 (Kuck et al., 2007; Sonntag et al., 2011), and it could be difficult to develop specific antibodies for hybrid/chimeric serotypes. Here, we have used SDS-PAGE gel and Sypro staining to semiquantitate the capsid content of two different serotypes of AAV, in this case the rAAV2RSM and the rAAV8RSM. The same four institutions mentioned above performed this experiment. The same AAV8 vector stock used for the standard curve of the ELISA kit (WL217S) was used here as a control, with the exception that here the vector was used in its native form while the ELISA standard is supplied lyophilized. Notably, this experiment was performed before the collection of the data from the 16 laboratories and the statistical analysis shown in Table 4; thus, the rAAV2RSM sample was loaded on the gel according to the previously published capsid titer of 9.1×1011 pt/ml (Lock et al., 2010), but the rAAV8RSM was loaded based on the capsid titer obtained in each institution using the specific AAV8 ELISA kit.
The amount of capsid of the rAAV2RSM was in agreement with the dose of WL217S used as standard, and, importantly, the amount of capsids loaded on the gel of the rAAV8RSM was similar to the rAAV2RSM (Fig. 3, and data not shown). Of note, the amount of vector genomes loaded on the SDS-PAGE gel was much higher for the rAAV8RSM than for the rAAV2RSM, since the rAAV2RSM had a much higher amount of empty capsids (Lock et al., 2010). These data indicate that both ELISAs (AAV2 and the AAV8) generate comparable titers in terms of capsids/ml. These data also indicate that SDS-PAGE, a non-serotype-dependent technique, is useful to estimate the capsid content of rAAV vectors when specific antibodies (and therefore an ELISA) are not available.
FIG. 3.
The rAAV2RSM and rAAV8RSM were run on SDS-PAGE gels under denaturating conditions and then stained with Sypro Ruby. Several decreasing amounts of vector particles (pt) of a reference rAAV8 vector (WL217S) were loaded as standard curve (lanes 2–6). Different amounts (1010 pt or 5×109 pt, lanes 7 and 8 or 9 and 10, respectively) of rAAV2RSM (lane 7 and 9) and rAAV8RSM (lane 8 and 10) were loaded on a gel according to a particle titer of 9.1×1011 pt/ml for rAAV2RSM (mean titer published by Lock et al., 2010) and 4.2×1011 pt/ml for the rAAV8RSM (titer calculated by this lab using the ELISA AAV8).
Discussion
More than 100 clinical trials using different serotypes of rAAV have been registered up to 2014. Recently, a gene therapy product based on rAAV (Glybera) has been granted with the marketing authorization from the European Medicine Agency opening new dynamics and business models for rAAV-based therapeutics (Bryant et al., 2013). Despite the extensive use of rAAV vectors, there has been a lack of standardization and inability to compare titer values for vectors made and tested within the same laboratory and within different ones. For this reason, an AAV2 Reference Standard Working group was formed in 2002 with the mission to generate and characterize an rAAV2RSM (Snyder et al., 2002; Moullier et al., 2008; Potter et al., 2008). Sixteen laboratories participated in the characterization, and the consensus titers (and other characterization data) of rAAV2RSM were compiled and published (Lock et al., 2010). However, vector systems based on novel rAAV serotypes (Gao et al., 2002, 2004) are being developed rapidly and the scientific community raised the question of whether other RSM will be needed.
An AAV8RSM Working Group including industry and academic institutions from 10 countries and 16 laboratories was established in 2008 to generate and characterize an AAV8RSM (Moullier et al., 2008, 2012; Ayuso et al., 2012). Most of the members had participated in the AAV2RSM Working Group, which facilitated the effort and maintained continuity. Here, we have described the manufacturing and the characterization of a novel reference for the field, namely, the rAAV8RSM. The upstream process for the rAAV8RSM was based on cotransfection of HEK293 cells, as for the rAAV2RSM; but the downstream process was based on density gradient ultracentrifugation, which differs from the rAAV2RSM that was purified by chromatography (Potter et al., 2008). Using the gradient purification protocol, we obtained an rAAV8RSM that is virtually free of empty capsids, which is a major difference compared with rAAV2RSM that contains more than 95% empty particles (Lock et al., 2010). Another significant difference between the two standards is the ratio of vector genomes/infectious particles; this ratio is 7.5 for the rAAV2RSM but >400 for rAAV8RSM. We hypothesize that this is because of the poor ability for rAAV8 to transduce cells in vitro, as already described (Gao et al., 2002; Mohiuddin et al., 2005). Identifying cell lines that can be efficiently infected with different AAV serotypes and helper viruses is greatly needed, as this impacts not only the infectious titer values but also the particle to infectious ratio and transgene expression assays. However, as long as similar vector genome/infectious particle ratios are obtained for different lots of the same serotype, thus demonstrating lot-to-lot product consistency, and the ratios can be correlated to vector potency, then the vector lots can be considered suitable for use.
The characterization of the rAAV2RSM demonstrated a high degree of variation between institutions for each assay despite the relatively tight correlation of assay results within an institution. Here, we have observed exactly the same outcome during the characterization of the rAAV8RSM. This poor degree of interlaboratory precision and accuracy is very illuminating, in light of the major effort to standardize the assays by providing detailed protocols and common reagents. In addition, several laboratories had already performed the same assays in the frame of the rAAV2RSM project and the AAV2RSM was made available to the community before launching the characterization campaign of the rAAV8RSM.
Vector genome titer is the most commonly used titer to dose patients in clinical trials. Here, we observed a very significant variation of the vector genome titer by qPCR of the rAAV8RSM, with the lowest titer being 4.6×1010 vg/ml and the highest titer 4.7×1012 vg/ml (Table 3). Having a two-log difference in the titer of the same rAAV material is a cause for concern considering that most of the clinical trials use doses within one-log variation (Manno et al., 2006; Nathwani et al., 2011), and may reflect not only the qPCR variability itself (different PCR machines, different sources of PCR primers and probes, storage and handling of PCR reagents, preparation, and spectrophotometry of plasmid standard curves), but also rAAV sample preparation and recovery. qPCR has become the method of choice for AAV genome titration because of its simplicity and relative robustness. However, as we note here, the assay is not without sources of variability. As the field progresses, new and improved assays with greater degrees of precision are likely to emerge. In this regard, an AAV vector genome quantitation method based upon droplet digital PCR has recently been described, which yields improved intra- and interassay precision in comparison with qPCR, as well as eliminating the need for a plasmid standard curve (Lock et al., 2014). Adoption of such techniques by the AAV gene therapy community may help to reduce the type of interlaboratory variation observed in this study.
The data presented here stress, more than ever, the necessity to work toward the calibration of vector titers and to standardize dosage units within the field. Now, two different rAAVRSMs are available to help validate in-house quantitative assays at a time where the field is maturing rapidly. As an example, four independent laboratories within the AAV8RSM working group performed an experiment consisting of titrating simultaneously the rAAV2RSM and the rAAV8RSM using the same protocols and reagents. The utilization of rAAV2RSM as a control permitted one laboratory to discard one of the tests that produced significant biased results with the overall result that the final titer of the rAAV8RSM obtained fell within the confidence limits for all 16 laboratories.
Interestingly, the capsid titer for both rAAV2RSM and rAAV8RSM determined by a commercial ELISA was the parameter with the least variation, perhaps because the kit provided the reagents and internal controls. Also, the capsid titers determined by the ELISA for serotype 2 and for the serotype 8 are comparable as showed by a non-serotype-dependent methodology (Table 5). Quantification of total capsid content is critical for clinical trials, since immune responses are clearly dependent on the vector capsid (Manno et al., 2006; Nathwani et al., 2011). The availability of ELISA methods and RSMs will help the field to better characterize vector preparations used in clinical trials. Even if an ELISA is not available for a specific serotype or chimeric AAV vector, it is possible to use other methods to measure total capsids, such as electronic microscopy (see Materials and Methods section: rAAVSRSM characterization assays) (Grimm et al., 1999), optical density (Sommer et al., 2003), or SDS-PAGE gel staining (Fig. 3). rAAV2RSM and rAAV8RSM could be used to evaluate total capsid load for those alternative AAV serotypes.
A stability monitoring program that tracks physical and infectious titers over time is ongoing for the AAV2RSM generated in 2006, and data from several years have been collected (data not shown). Similarly, the AAV8RSM Working Group will follow the stability of the AAV8RSM at regular intervals.
Regulatory European (European Directorate for the Quality of Medicines [EDQM]) and U.S. (Food and Drug Administration [FDA], Center for Biologies Evaluation and Research [CBER], Office of Cellular, Tissue and Gene Therapies [OCTGT], and Division of Cellular and Gene Therapies [DCGT]) bodies have encouraged the AAV2RSM and AAV8RSM Working Group to generate and characterize reference materials and recommended the use of these standards to the sponsors of rAAV vector INDs and IMPDs.
In summary, this article describes the manufacturing and the characterization of a novel rAAV8RSM material deposited at ATCC under the reference VR-1816 that represents an important tool to better determine vector dosing units. Having validated and calibrated methods to determine rAAV titers of preclinical and clinical vectors will accelerate the development of rAAV-based medicines and will protect patients participating in future trials.
Supplementary Material
Acknowledgments
This work was supported by the Clinigene network of excellence (European Commission FP6, Clinigene, LSHB-CT-2006-018933). We thank Odile Cohen-Haguenauer for conceiving, writing, and submitting the Clinigene European Network of Excellence. We also thank the support of several partners of the Clinigene AAV platform to the AAV8RSM project: Robin Ali, Muriel Audit, George Dickson, Anne Douar, Amit Nathwani, Amos Panet, Thierry Vandendriessche, and Bas Blits.
We are also indebted to Liz Kerrigan at ATCC for coordinating the distribution of materials to testing laboratories, and Keith Carson (ISBiotech) and Denise Gavin (FDA) for useful discussions and guidance. We acknowledge the generosity of members of the AAV RSM Working Group who participated in the production and characterization of the rAAV8RSM.
We acknowledge the technical help of the following during the manufacturing and testing phase: Maria Molas, Jose Piedra, Katell Konan, Armelle Cassard, Solene Volant, Linda Poiraudeau, Frederic Broucque, Emilie Lecomte, Achille François, Antonella Ferrara, Diana Desgue, Marina Dapas, Michela Zotti, Rachida Siamari, Saskia Haast, Wienman Philips, and Albertine de Jong.
We acknowledge the generosity of PROGEN, ATCC, PlasmidFactory, Corning, PAA, SGS-Vitrology, Labclinics, and Clean Cells for providing free access to materials and reagents required for the production and characterization of the AAV8RSM.
Author Disclosure Statement
Richard Snyder owns equity in a gene therapy company that is commercializing AAV for gene therapy applications. J. Fraser Wright is scientific cofounder of and consultant to Spark Therapeutics, a gene therapy company. All other authors declare no conflict of interest.
References
- ATCC Virus Reference Materials. Available at: www.lgcstandards-atcc.org/Standards/Standards_Programs/ATCC_Virus_Reference_Materials (accessed Oct. 24, 2014)
- Ayuso E., Mingozzi F., Montane J., et al. (2010). High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 17, 503–510 [DOI] [PubMed] [Google Scholar]
- Ayuso E., Blouin V., Darmon C., et al. (2012). Reference materials for the characterization of adeno-associated viral vectors. In The Clinibook: Clinical Gene Transfer State of the Art. Cohen-haguenauer O., ed. (EDK, Paris: ) pp.83–90 [Google Scholar]
- Bainbridge J.W., Smith A.J., Barker S.S., et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- Bryant L.M., Christopher D.M., Giles A.R., et al. (2013). Lessons learned from the clinical development and market authorization of Glybera. Hum. Gene Ther. Clin. Dev. 24, 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C., Gorbatyuk O.S., Velardo M.J., et al. (2004). Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 10, 302–317 [DOI] [PubMed] [Google Scholar]
- Gao G.P., Alvira M.R., Wang L., et al. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Vandenberghe L.H., Alvira M.R., et al. (2004). Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet D., Methot J., Dery S., et al. (2013). Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 20, 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Kern A., Pawlita M., et al. (1999). Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 6, 1322–1330 [DOI] [PubMed] [Google Scholar]
- Hutchins B. (2002). Development of a reference material for characterizing adenovirus vectors. BioProcess J. 1, 25–29 [Google Scholar]
- Kuck D., Kern A., and Kleinschmidt J.A. (2007). Development of AAV serotype-specific ELISAs using novel monoclonal antibodies. J. Virol. Methods 140, 17–24 [DOI] [PubMed] [Google Scholar]
- Littell R.C., Milliken G.A., Stroup W.W., et al. (2006). SAS for Mixed Models, 2nd edition. (SAS Institute, Inc., Cary, NC: ) [Google Scholar]
- Lock M., McGorray S., Auricchio A., et al. (2010). Characterization of a recombinant adeno-associated virus type 2 Reference Standard Material. Hum. Gene Ther. 21, 1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M., Alvira M.R., Chen S.J., et al. (2014). Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Hum. Gene Ther. Methods 25, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S., Arruda V.R., Pierce G.F., et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 [DOI] [PubMed] [Google Scholar]
- Mingozzi F., Maus M.V., Hui D.J., et al. (2007). CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13, 419–422 [DOI] [PubMed] [Google Scholar]
- Mingozzi F., Meulenberg J.J., Hui D.J., et al. (2009). AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 114, 2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., Anguela X.M., Pavani G., et al. (2013). Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 5, 194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin I., Loiler S., Zolotukhin I., et al. (2005). Herpesvirus-based infectious titering of recombinant adeno-associated viral vectors. Mol. Ther. 11, 320–326 [DOI] [PubMed] [Google Scholar]
- Moullier P., and Snyder R.O. (2008). International efforts for recombinant adeno-associated viral vector reference standards. Mol. Ther. 16, 1185–1188 [DOI] [PubMed] [Google Scholar]
- Moullier P., and Snyder R.O. (2012). Recombinant adeno-associated viral vector reference standards. Methods Enzymol. 507, 297–311 [DOI] [PubMed] [Google Scholar]
- Nathwani A.C., Tuddenham E.G., Rangarajan S., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Phillipsber G., Phillipsberg T., et al. (2008). Manufacture and stability study of the recombinant adeno-associated virus serotype 2 reference standard. Bioprocessing J. 7, 8–14 [Google Scholar]
- Rabilloud T., Strub J.M., Luche S., et al. (2001). A comparison between Sypro Ruby and ruthenium II tris (bathophenanthroline disulfonate) as fluorescent stains for protein detection in gels. Proteomics 1, 699–704 [DOI] [PubMed] [Google Scholar]
- Snyder R.O., and Flotte T.R. (2002). Production of clinical-grade recombinant adeno-associated virus vectors. Curr. Opin. Biotechnol. 13, 418–423 [DOI] [PubMed] [Google Scholar]
- Sommer J.M., Smith P.H., Parthasarathy S., et al. (2003). Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 7, 122–128 [DOI] [PubMed] [Google Scholar]
- Sonntag F., Kother K., Schmidt K., et al. (2011). The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J. Virol. 85, 12686–12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier J., Chadeuf G., Nony P., et al. (2001). Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 75, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H., Xiao R., Lock M., et al. (2010). Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum. Gene Ther. 21, 1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet K., Blouin V., Agbandje-McKenna M., et al. (2006). Proteolytic mapping of the adeno-associated virus capsid. Mol. Ther. 14, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W., Weiland F., and Gorg A. (2009). Protein detection and quantitation technologies for gel-based proteome analysis. Methods Mol. Biol. 564, 59–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.