Abstract
The purpose of this study was to investigate the hypothesis that dietary supplementation with glutamic acid has beneficial effects on growth performance, antioxidant system, intestinal morphology, serum amino acid profile and the gene expression of intestinal amino acid transporters in growing swine fed mold-contaminated feed. Fifteen pigs (Landrace×Large White) with a mean body weight (BW) of 55 kg were randomly divided into control group (basal feed), mycotoxin group (contaminated feed) and glutamate group (2% glutamate+contaminated feed). Compared with control group, mold-contaminated feed decreased average daily gain (ADG) and increased feed conversion rate (FCR). Meanwhile, fed mold-contaminated feed impaired anti-oxidative system and intestinal morphology, as well as modified the serum amino acid profile in growing pigs. However, supplementation with glutamate exhibited potential positive effects on growth performance of pigs fed mold-contaminated feed, ameliorated the imbalance antioxidant system and abnormalities of intestinal structure caused by mycotoxins. In addition, dietary glutamate supplementation to some extent restored changed serum amino acid profile caused by mold-contaminated feed. In conclusion, glutamic acid may be act as a nutritional regulating factor to ameliorate the adverse effects induced by mycotoxins.
Introduction
Mycotoxins are a group of structurally diverse secondary metabolites produced by a wide variety of fungal species and are commonly detected in cereal crops and cereal-based food products in temperate regions [1]–[3]. According to the numerous well-designed experiments, mycotoxins are absorbed into the metabolic cycle by a paracellular pathway through the tight junctions [4], and then exert acute and chronic physiology and immune toxicity on animals and humans [5], [6]. The ingestion of mycotoxins-contaminated feed lowers animal growth performance and meat quality [7], simultaneously alters gene expression [8], [9] and decreases the activity of intestinal glucose transporters [10]. Meanwhile, mycotoxins inhibit the proliferation of intestinal cells by inducing oxidative DNA damage and G1-phase arrest [11], cause severe inflammatory reaction [12],and unbalance the antioxidant system [13], [14] which play important roles in protecting our body against reactive oxygen species (ROS) [15]. Intestinal barrier dysfunction caused by mycotoxins [16] allows exogenous pathogenic antigens invasion, such as natural toxins and additional mycotoxins, which compromise intestinal homeostasis [17]. In contrast to normal feed or single-toxin contaminated feed, mold-contaminated feed contains multiple mycotoxins, such as aflatoxin B1 (AFB1), deoxynivalenol (DON), α-zearalenone (α-ZEA), ochratoxins (OCH), toxin-2 [18], [19]. Such multi-mycotoxin-contaminated feed plausibly imposes more serious damage on animals than the consumption of any single mycotoxin alone. Thus, contamination of feed by mixed myxotoxins greatly affects the health and economic stability of many farm industries, including swine production.
Glutamate is an important functional amino acid because of its physiological and immune contributions [20]. Glutamate serves as a pivotal substrate for other biological active molecules, including glutamine, glutathione, proline, and arginine [21], [22]. Considerable information from ongoing investigations in young pigs, preterm infants, and adult humans has shown that dietary glutamate is extensively metabolized in the intestine [23], [24]. Oxidation of glutamate in enterocytes is a major metabolic fate to product major oxidative fuels [25], [26] which are indispensable for intestinal cells proliferation and intestinal integrity and function [27]–[29]. In addition, glutamate locates at the center of disposal of amino acid and protein metabolism [30]. Therefore, glutamate plays important roles in facilitating protein biosynthesis and turnover, regulating gene expression, and enhancing immunity [22]. Many studies have demonstrated that dietary supplementation with glutamate restores mucous circulation and metabolism of amino acids as well as prevents the apoptosis of enterocytes [31], [32]. Akiba et al. have reported that L-glutamate enhances mucosal defenses by preventing cellular injury in small intestine [33]. Therefore, given the immune and physiological functions of glutamate, dietary supplementation with glutamate is rationally served as a promising approach to protect animals and humans from toxins exposure. Although many studies have reported some methods to reduce the presence of mysotoxin like physical and chemical degradation and the use of adsorbents, little information is known about glutamic acid to protect animals from the mycotoxins. Therefore, the current study was investigated to test the hypothesis that dietary supplementation with glutamate could mitigate the cytotoxic effects of mycotoxins on growing pigs.
Materials and Methods
Experimental design
Fifteen swine (Landrace×Large White) (ZhengHong Co., China) with a mean body weight (BW) of 55 kg were randomly divided into three treatment groups (n = 5/group): 1) the control group received basal feed; 2) the mycotoxin group received contaminated feed; 3) the glutamate group received contaminated feed and dietary supplementation with 2% glutamate (purity >99%, Beijing Chemclin Biotech, Beijing, China). Contaminated feed was mildewed clearly under ambient conditions (temperature 23–28°C, humidity 68–85%) as described by Liu et al [34] and the mycotoxins were detected by liquid chromatography (Beijing Taileqi, Beijing, China) (Table 1). The basal diets were formulated to meet or exceed the nutritional needs of growing pigs as recommended by the NRC (1998) (Table 2). The content of glutamate and other amino acids in basal diet was measured and presented in previous papers [35]–[37]. In the present study, control experiments were performed with diet containing no added amino acid. Indeed, we did not use alanine supplementation as a classical isonitrogenous control since the amount of glutamate used represents a negligible amount of supplemental nitrogen [38]. All pigs were allowed free access to water throughout the experimental period. This study approved by Laboratory Animal welfare Commission of the Institute of Subtropical Agriculture, Chinese Academy of Sciences [38].
Table 1. Mycotoxin content of contaminated and non-contaminated feed mixtures.
| Catalogue | AFB1 (ppb) | ZEN (ppm) | OCH (ppb) | DON (ppm) | FB1 (ppm) | T-2 (ppm) |
| Limit of detection | 0.05 | 0.01 | 0.5 | 0.1 | 0.05 | 0.1 |
| Non-contaminatedfeed | undetected | 0.821 | 3.6 | 1 | 0.6 | undetected |
| Contaminated feed | 0.62 | 0.573 | 11.39 | 3 | 2 | undetected |
Samples were collected at every preparing feed in each group and then mixed their together respectively. Among these mycotoxins, AFB1 means Aflatoxin B1 (ppb); ZEN means zearalenone (ppm); OCH means ochratoxin (ppb); DON means deoxynivalenol (ppm); FB1 means Fusarium B1 (ppm) and T-2 means T-2 fungal toxin (ppm).
Table 2. Composition and nutrient level of the basal diet.
| Ingredients | Contents (%) | Nutrient Substance | Contents (%) |
| Corn | 67.22 | Crude protein | 16 |
| Soybean meal | 21.8 | Ca | 0.6 |
| Wheat bran | 7.95 | P | 0.5 |
| Limestone | 1.03 | CaHPO4 | 0.69 |
| Lys | 0.771 | Salt | 0.31 |
| Met+Cys | 0.584 | Additive premix | 1 |
Premix provided the following per kilogram of the diet: Sepiolite 8.072g; pig vitamin 750mg; FeSO4⋅H2O 317mg; CuSO4⋅5H2O 294mg; Antioxidants 200mg; MnSO4⋅H2O 172mg; ZnSO4⋅H2O 153mg; KI 24mg; Na2SeSO3 18mg.
Sample collection
All blood samples were collected through a jugular vein from all of the pigs. Serum was separated by centrifugation at 1,500 g for 10 min at 4°C and stored at −20°C until analysis [39]. At day 60, the pigs were sacrificed and two gut samples were taken from both the mid-jejunum and mid-ileum. One of the gut sample (3 cm) was kept in 4% neutral buffered formalin for the determination of histomorphology and the other one (approximately 2 g) was immediately frozen in liquid nitrogen and stored at −70°C for subsequent analyses of gene expression [40].
Average daily weight gain (ADG) and feed conversion rate (FCR)
Pigs were weighed individually at day 0 and 60, and the feed consumption per pig was also recorded per pig at the same time to calculate the average daily weight gain (ADG; kg/pig/day) and the average feed conversion rate (RCR) [37].
Determination of serum T-SOD, GSH-Px and D-lactate activities, and DAO level
Serum Total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) activities were measured using spectrophotometric kits in accordance with the manufacturer’s instructions (Nanjing Jiangcheng Biotechnology Institute, China) (PMID:21617969). Serum D-lactate was determined using an assay kit in accordance to the manufacturer’s instructions (Biovision Inc., USA). Diamine oxidase (DAO) level was measured according to Aarsen [41].
Determination of serum amino acids
Twenty-seven amino acids were detected in serum based on our previous study [42]–[43] via LC-MS/MS (HPLC Ultimate3000 and 3200 Q TRAP LC-MS/MS): L-arginine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-phenylalanine, L-methionine, L-threonine, L-tryptophan, L-valine, glycine, L-serine, L-tyrosine, L-asparagine, L-aspartic acid, L-citrulline, L-glutamic acid, L-glutamine, L-ornithine, L-cystine, L-homocystine, L-alanine, L-carnosine, hydroxy-L-proline, 1-methyl-L-histidine, 3-methyl-L-histidine, and L-proline.
Intestinal histomorphometry determination
The samples of the jejunum and ileum that had been kept in 4% neutral buffered formalin were processed using routine histological methods and mounted in paraffin blocks (PMID:22086211). Six-micrometer-thick sections were cut and stained with Masson’s trichrome. All specimens were examined under a light microscope (Nikon, Japan). Villus height and crypt depth were measured using an image-analysis system [44].
RNA extraction and cDNA synthesis
Total RNA was isolated from liquid nitrogen-pulverized tissues as described above using TRIzol regent (Invitrogen, USA) and treated with DNase I (Invitrogen, USA) according to the manufacturer’s instructions [45]. The quality of RNA was checked by 1% agarose gel electrophoresis after staining with 10 µg/ml ethidium bromide. The RNA had an OD260:OD280 ratio between 1.8 and 2.0. First-strand cDNA was synthesized with oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen, USA).
Quantification of mRNA by real-time PCR analysis
Primers were designed with Primer 5.0 based on the cDNA sequence of the pig to produce an amplification product (Table 3). GAPDH was used as a housekeeping gene to normalize target gene transcript levels. Real-time PCR was performed using SYBR Green PCR Mix, containing MgCl2, dNTP, and Hotstar Taq polymerase. Two µl of cDNA template was added to a total volume of 25 µl containing 12.5 µl SYBR Green mix and 1 µmol/l each of forward and reverse primers. We used the following protocol: (i) pre-denaturation (10 s at 95°C); (ii) amplification and quantification, repeated 40 cycles (5 s at 95°C, 20 s at 60°C); (iii) melting curve (60–99°C with a heating rate of 0.1°C S-1 and fluorescence measurement) (PMID:22086211).
Table 3. Primer pairs used in the RT-PCR reaction.
| Gene | Accession No. | Nucleotide sequence of primers (5′–3′) |
| β-Actin | NM_001172909.1 | F:CTGCGGCATCCACGAAACT |
| R:AGGGCCGTGATCTCCTTCTG | ||
| SLC7A1 | NM_001012613.1 | F:TGCCCATACTTCCCGTCC |
| R:GGTCCAGGTTACCGTCAG | ||
| SLC7A7 | NM_001253680.1 | F:TTTGTTATGCGGAACTGG |
| R:AAAGGTGATGGCAATGAC | ||
| SLC1A1 | NM_001164649.1 | F:ATAGAAGTTGAAGACTGGGAAAT |
| R:GTGTTGCTGAACTGGAGGAG | ||
| SLC5A1 | NM_001164021.1 | F:GGCTGGACGAAGTATGGTGT |
| R:ACAACCACCCAAATCAGAGC |
SLC7A1: solute carrier family 7 (cationic amino acid transporter, y+system), member 1; SLC7A7: solute carrier family 7 (amino acid transporter light chain, y+L system), member 7; SLC1A1: solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1; SLC5A1: solute carrier family 5 (sodium/glucose cotransporter), member 1. All these primer sequence was designed based on the sequence corresponding to the accession number described above.
Statistical Analysis
All statistical analyses were performed using SPSS17.0 software. The normality and constant variance for date were tested by levene’s test, and then dates were subjected to one-way analysis of variance followed by the Duncan (D) multiple comparisons test. Values with different superscript letters are significantly different (P<0.05), while values with the same or no superscript letters are not significantly different (P>0.05). Data are expressed as the mean ± standard error of the mean (46).
Result
Growth performance
In current study, the ADG and FCR were measured to evaluate the growth performance and feed efficiency of young pig respectively. The ADG in the mycotoxin group between days 1 and 60 (0.58±0.02 kg/d) was lower (P<0.05) than those in the control group (0.71±0.02 kg/d) (Figure 1). Meanwhile, mold-contaminated feed (3.66±0.04) significantly increased (P<0.05) the FCR compared with control group (3.22±0.11). However, dietary supplementation with glutamate had little effects on the ADG (0.62±0.01 kg/d) and the FCR (3.57±0.04) compared with mycotoxin group. The ADG in glutamate group was significant lower (P<0.05) than those in the control group.
Figure 1. ADG and FCR in growing pigs fed mycotoxin-contaminated diets.
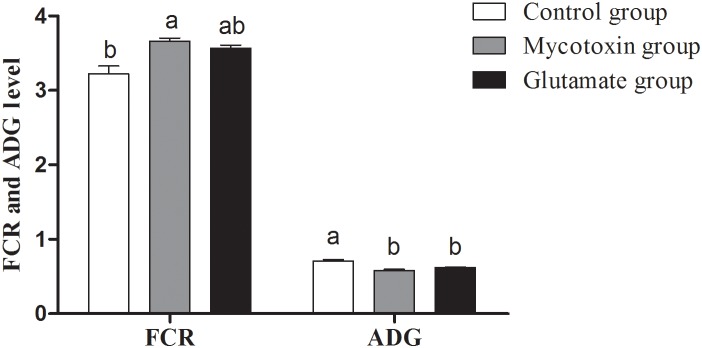
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. The data with different letters in the same factor differ significantly (P<0.05), and same letters means no significant difference (p>0.05).
Determination of serum T-SOD and GSH-Px activity
SOD and GSH-Px are two major antioxidant enzymes to scavenge the excessive internal reactive oxygen species (ROS) which exert radical-mediates damages to biological macromolecules (proteins, lipids and DNA) [42], therefore the activities of SOD and GSH-Px are plausibly regarded as a mark reflecting redox of organism [15]. In this study, compared with the control group, mold-contaminated feed significantly decreased T-SOD activity (P<0.05) and GSH-Px activity (P>0.05) (Figure 2). However, dietary supplementation with glutamic acid significantly augmented (P<0.05) serum T-SOD and GSH-Px activities compared with the mycotoxin group, and the serum T-SOD and GSH-Px activities in glutamate group were restored to parallel with the control group (Figure 2).
Figure 2. Serum GSH-Px and T-SOD activities in growing pigs fed mycotoxin-contaminated feed.
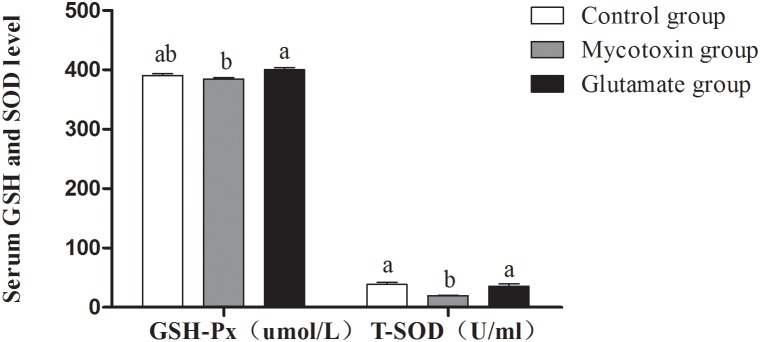
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. The data with different letters in the same factor differ significantly (P<0.05), and same letters means no significant difference (p>0.05).
Determination of serum D-lactate and DAO
In this study, serum D-lactate levels and DAO activity were measured to evaluate intestinal integrity. As shown in figure 3, after pigs exposed to contaminated feed, the D-lactate levels were significantly increased (P<0.05). However, the D-lactate levels in the glutamate group were significantly lowered (P<0.05) than those in the mycotoxin group. There was no significant difference in the level of DAO among all groups (Figure 3).
Figure 3. Serum D-Lactate and DAO activities of growing pigs fed mycotoxin-contaminated feed.
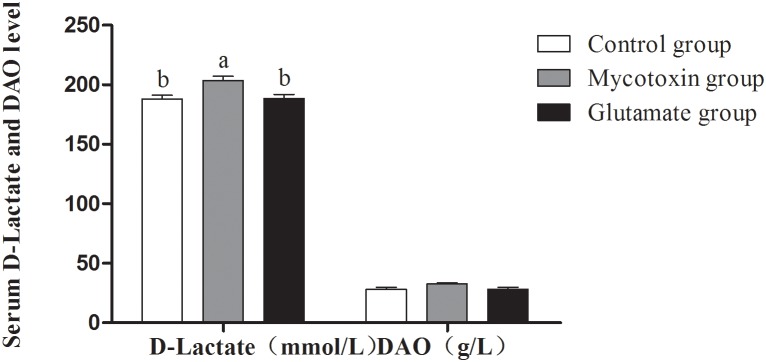
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. The data with different letters in the same factor differ significantly (P<0.05), and same letters means no significant difference (p>0.05).
Microscopic Evaluation
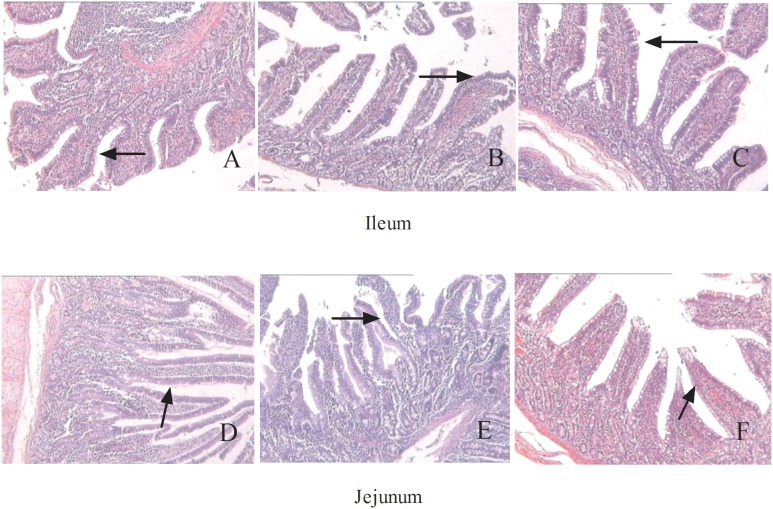
In this study, compared with control group, pigs with contaminated feed remarkably increased (P<0.05) in the villus height and crypt depth in the ileum and jejunum, while there was no difference in the ratio of villus height to crypt depth in the ileum and jejunum (Table 4). However, glutamate supplementation significantly decreased (P<0.05) ileum the crypt depth and jejunum villus height, but had no significant difference on the ratio of villus height to crypt depth in the ileum and jejunum (Table 4). As shown in Figure 4, no histological damage was observed in the ileum (Fig. 4A) and jejunum (Fig. 4D) in the control group. In the contaminated feed group, villi in the ileum (Fig. 4B) and jejunum (Fig. 4E) were scattered and desquamated. The glutamate group showed larger villi in both the ileum (Fig. 4C) and jejunum (Fig. 4F).
Table 4. Effect of dietary supplementation with glutamate on morphological characteristics in intestinal tissues in pigs fed experimental diets.
| Catalogue | Control group | Mycotoxin group | Glutamate group |
| Ileum villus height (µm) | 255.45±4.89b | 356.15±8.08a | 332.03±11.96a |
| Ileum crypt depth (µm) | 120.00±1.56b | 216.97±7.17a | 139.90±8.75b |
| Jejunum villus height (µm) | 295.08±6.39b | 343.30±16.04a | 298.65±7.05b |
| Jejunum crypt depth (µm) | 99.13±3.90b | 132.45±6.84a | 113.35±6.31ab |
| Ileum V/C | 2.97±0.18 | 2.48±0.20 | 2.68±0.22 |
| Jejunum V/C | 2.41±0.39 | 2.2±0.41 | 2.22±0.65 |
| Ileum goblet cells number | 32.00±1.92a | 23.25±3.38b | 25.00±2.47ab |
| Jejunum goblet cells number | 16.50±1.50a | 12.75±0.86b | 16.33±0.62a |
| Ileum lymphocyte number | 223.75±17.67 | 179.75±26.71 | 214.00±26.05 |
| Jejunum lymphocyte number | 286.00±27.25 | 248.20±18.83 | 226.80±11.38 |
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. Villus height and crypt depth were measured using an image-analysis system. Among these indexes, Ileum C/V means the ration of ileal villus height to crypt depth and Jejunum C/V means the ration of jejunal villus height to crypt depth. The data with different letters in the same row differ significantly (P<0.05), and same letters mean no significant difference (p>0.05).
Figure 4. Histological evaluation of intestinal tissues (HE×100) in growing pigs fed mould-contaminated feed.
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. Fig. 4A and D represented control group and Fig. 4B and D represented contaminated group and Fig. 4C and F represented glutamate group. There is no histological damage observed in the control group (Fig. 4A and D). In mycotoxin group, the villus was scattered and desquamated seriously in jejunum and ileum (Fig. 4B and D). A greater villus in jejunum and ileum was observed in glutamate group (Fig. 4C and F).
Serum amino acid parameters
As shown in Table 5, the consumption of contaminated feed resulted in decreases in the serum levels of some amino acids. Compared with control group, contaminated feed significantly decreased (P<0.05) the serum concentrations of L-glutamine, L-proline, 1-methyl-L-histidine, hydroxy-L-proline and L-tyrosine and simultaneously the concentrations of L-glutamic acid and amino acids related to its metabolism (L-ornithine) in mycotoxin group tended to be lower (P>0.05), while the level of L-citrulline was significantly higher than that in the control group (P<0.05). However, dietary supplementation with glutamate significantly restored (P<0.05) the serum levels of 1-methyl-L-histidine, hydroxy-L-proline, L-homocystine, and L-histidine, and the levels of L-homocystine and L-histidine were even higher than those in the control group. In addition, the concentrations of L-glutamic acid and L-proline in the glutamate group tended to be higher (P>0.05) than those in the mycotoxin group.
Table 5. Effect of dietary supplementation with glutamate on concentration of serum amino acid parameters in growing pigs fed mycotoxin-contaminated feed.
| Item | The molecularformula | Control group(ug/ml) | Mycotoxin group (ug/ml) | Glutamate group (ug/ml) |
| L-arginine | C6H14N4O2 | 23.34±0.32 | 20.79±2.82 | 20.43±0.32 |
| L-histidine | C11H17N3O4 | 32.69±3.53b | 32.76±2.70b | 45.42±5.61a |
| L-isoleucine | C6H13NO2 | 10.85±0.98 | 10.74±0.79 | 9.91±0.26 |
| L-leucine | C6H13NO2 | 22.48±0.21 | 22.61±0.89 | 22.05±0.64 |
| L-lysine | C6H14N2O2 | 16.69±1.41 | 16.03±0.80 | 14.41±1.56 |
| L-phenylalanine | C5H11O2NS | 12.16±0.87 | 11.03±1.52 | 10.63±0.24 |
| L-methionine | C9H11NO2 | 32.93±0.90b | 31.23±5.41b | 50.28±3.50a |
| L-threonine | C4H9NO3 | 10.17±0.34 | 10.08±0.94 | 9.57±0.94 |
| L-tryptophan | C11H12N2O2 | 6.87±0.36 | 6.31±0.45 | 6.15±0.89 |
| L-valine | C5H11NO2 | 20.30±1.81 | 22.17±1.73 | 22.39±2.61 |
| Glycine | C2H5NO2 | 87.83±3.25a | 67.02±3.11b | 74.33±2.37ab |
| L-serine | C3H7NO3 | 10.05±1.73 | 10.19±0.55 | 10.62±0.96 |
| L-tyrosine | C2H7NSO3 | 23.25±1.24a | 17.60±0.86b | 17.13±2.08b |
| L-asparagine | C9H11NO3 | 2.84±0.28 | 3.26±0.40 | 3.31±0.09 |
| L-aspartic acid | C4H8N2O3 | 1.72±0.28b | 1.67±0.09b | 2.76±0.48a |
| L-citrulline | C4H7NO4 | 10.13±0.91b | 17.70±1.09a | 13.15±0.83b |
| L-glutamic acid | C6H13N3O3 | 57.25±3.09 | 49.31±2.85 | 53.12±8.78 |
| L-glutamine | C5H9NO4 | 7.20±0.67a | 3.90±0.30b | 4.90±0.38b |
| L-ornithine | C5H10N2O3 | 13.09±0.92 | 11.02±1.21 | 12.05±2.14 |
| L-cystine | C5H12N2O2 | 0.49±0.12 | 0.32±0.06 | 0.43±0.10 |
| L-homocystine | C3H7NO2S | 0.18±0.01b | 0.15±0.02b | 0.25±0.02a |
| L-alanine | C4H9NO2S | 44.03±6.70 | 44.66±3.67 | 48.85±9.85 |
| L-carnosine | C3H7NO2 | 0.76±0.18 | 0.69±0.09 | 0.61±0.25 |
| Hydroxy-L-proline | C5H9NO3 | 80.85±5.76a | 29.31±4.09c | 53.18±4.70b |
| 1-methyl-L-histidine | C7H11N3O2 | 0.70±0.05a | 0.49±0.03b | 0.69±0.06a |
| 3-methyl-L-histidine | C7H11N3O2 | 1.64±0.10ab | 1.46±0.08b | 1.74±0.12a |
| L-proline | C5H9NO2 | 46.13±0.33a | 20.37±0.36b | 25.95±2.68b |
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. The data with different letters in the same row differ significantly (P<0.05), and same letters mean no significant difference (p>0.05).
Amino acid transporter gene expression
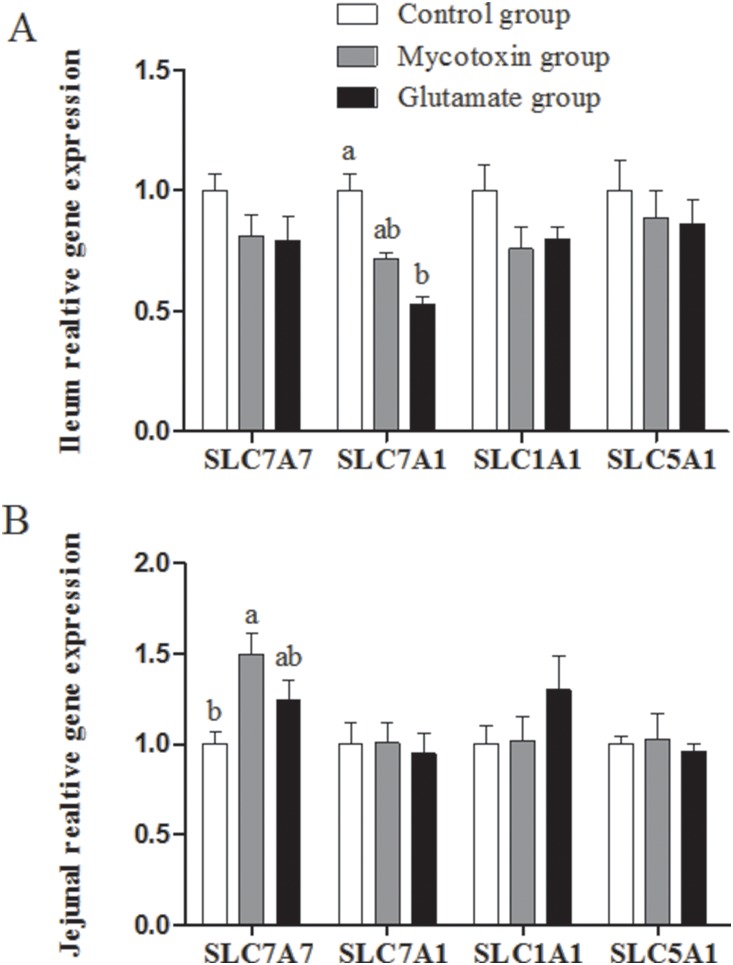
The ileal solute carrier family 7 (amino acid transporter light chain, y+L system), member 7 (SLC7A7), solute carrier family 7 (cationic amino acid transporter, y+system), member 1 (SLC7A1), solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 (SLC1A1), and solute carrier family 5 (sodium/glucose co-transporter), member 1 (SLC5A1) mRNA abundances in the mycotoxin group exhibited no significant difference compared to those in the control group (Figure 5A). Contaminated feed also exhibited no effects on mRNA abundances of these genes in the jejunum excepting significantly increased (P<0.05) mRNA abundance of SLC7A7 (Figure 5B). There was no difference about the mRNA expression of these transporters between mycotoxin group and glutamate group in the jejunum and ileum (P>0.05).
Figure 5. Effect of dietary supplementation with glutamate on elative mRNA abudances in ileum (A) and jejunum (A) of growing pigs fed mould-contaminated feed.
The treatments consisted of a control group (n = 5) receiving uncontaminated feed, a mycotoxin group (n = 5) receiving mould-contaminated diet, and the glutamate group (n = 5) receiving mould-contaminated diet and 2% glutamate. SLC7A1: solute carrier family 7 (cationic amino acid transporter, y+system), member 1; SLC7A7: solute carrier family 7 (amino acid transporter light chain, y+L system), member 7; SLC1A1: solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1; SLC5A1: solute carrier family 5 (sodium/glucose cotransporter), member 1. The data with different letters in the same row differ significantly (P<0.05), and same letters means no significant difference (p>0.05).
Discussion
Glutamate is an important versatile functional amino acid because of its nutritional and immune contributions. Many well-designed studies have shown that glutamate performs critical roles in regulating intestinal health and maintaining intestinal homeostasis by providing pivotal oxidative fuels that are indispensable for enterocytes proliferation and turnover and enhancing intestinal barrier function [21], [32]. Although dietary glutamate is crucial for intestinal health, little is known about its role in protecting the gut from toxin-induced injury. Thus, we conducted the experiment to explore whether dietary supplementation with glutamate could attenuate the cytotoxic effects of mycotoxins on growing pigs.
In current study, liquid chromatography determination showed that AFB1 (0.62 ppb), OCH (11.39 ppb), DON (3 ppm), and FB1 (2 ppm) were the main mycotoxins in moldy feed. Although the AFB1 and OCH levels were quite low (the concentrations were still higher than those in the control group), there is as yet no method suitable for determining whether their co-effects might produce more serious impairments than either compound alone. A growing number of studies have shown that consumption of mycotoxins decreases ADG, feed intake, and thus lower animal performance [46]. Indeed, in our study, contaminated feed lowered pig growth performance and feed efficiency with significantly decreasing ADG and increasing FCR respectively, which parallelized with previous report that DON significantly decreases pig growth performance [45]. According to previous studies, ingestion of mycotoxins can remarkably damage intestinal structure, gut barrier and intestinal immunity, leading to the compromise intestinal function [4], [12,]. Thus adverse effects of mycotoxins on intestinal function maybe account for the pig growth-suppression caused by contaminated feed in our study. However, supplementation with 2% glutamate failed to mitigate the growth-suppression caused by mycotoxins. Similarly, previous reports have shown that dietary supplementation with arginine and glutamine also fails to alleviate the growth-suppression induced by mycotoxins in growing pig. Thus, we speculate the reason may be that the supplemental glutamate concentration in current study is insufficient to overwhelm the mycotoxin-induced growth-suppression. As a result, to elucidate this point, further study need to be carried out.
Poor growth performance is relevant to injuries of intestinal absorption and intestinal function caused by mycotoxins. Many of studies have demonstrated that mycotoxins can damage intestinal structure, impair intestinal carrier function and unbalance antioxidant system [13], [45], [46]. Similar to the previous studies, fed contaminated feed impaired the intestinal structure, antioxidant system and intestinal barrier function in our experiment. Intestinal histological and morphological impairment and intestinal barrier dysfunction lead to poor nutrient absorption, and then lower animal performance and in this study growth-suppression induced by mycotoxins have indirectly demonstrated this point. Intriguingly, according to previous investigations, supplementation with amino acid and peptide preparations may counteract the toxic effects of mycotoxins in mice and pigs [47]. For example, supplementation with protein and amino acids overcomes the mycotoxicoses [47], [48] and the addition of proline exhibits a beneficial effect on the jejunum impairment induced by DON [49]. As expected, dietary supplementation with glutamate not only remarkably improved structure of the intestine (based on histological and morphological findings), but also restored intestinal barrier function and antioxidant system with decreased serum D-lactate level and increased serum SOD and GSH-Px levels which can scavenge excessive ROS. These results have demonstrated certain beneficial roles of glutamate restore impaired intestinal function in pigs after challenge with contaminated feed. In mammals, glutamate plays an important role in the synthesis and turnover of non-essential amino acids and protein in the gut [50] and also provides major oxidative fuels, which play critical roles in reducing experimental intestinal hyper-permeability and facilitating gut integrity and function [51]. Meanwhile, glutamate is a precursor for glutathione and N-acetylglutamate in enterocytes and glutathione is involved in intestinal redox state and in the detoxication process and simultaneously performs pivotal roles in regulating intestinal function [52]. Thus, considering the versatile beneficial function of glutamate in intestine, it is plausible that dietary supplementation with glutamate may to some extent protect the intestinal homeostasis from contaminated feed.
Serum amino acids are the substrate for nutritional anabolism and catabolism, playing important roles in immune response and growth performance. In current study, the contaminated feed decreased some of the serum amino acid concentration. In particular, the serum level of L-glutamine, L-proline, 1-methyl-L-histidine, hydroxy-L-proline, and L-tyrosine were significantly decreased and L-glutamic acid and amino acids related to its metabolism (L-ornithine) tended to be lower. Meloche et al. have reported that T 2 toxin reduces amino acid uptake as well as the plasma amino acid concentration [53], which is consistent with our present results. However, dietary supplementation with glutamate restores L-histidine, L-methionine, L-homolysine, 1-methyl-L-histidine and 3-methyl-L-histidine levels, but fails to restore serum L-glutamate, L-glutamine and L-citrulline levels. Similarly, Boutry et al have also reported glutamate supplementation fail to reverse decreased glutamate, glutamine and citrulline concentrations in plasma in endotoxemia. A possible explanation for these results is that both glutamate and other amino acids (glutamine, ornithine and proline) are accumulated in the intestinal mucosa and then preferentially are used for oxidative metabolism to produce ATP and protein biosynthesis in enterocytes [21] to repair injured intestinal function induced by mycotoxins, rather than are transferred to the bloodstream. The levels of serum amino acids are related to amino acid transporters because of amino acid-sensor and -carrier function of amino acid transporters [54]. Before used for metabolism, luminal amino acids must be transported into the bloodstream through amino acid transporters (e.g., SLC7A7, SLC7A1, SLC1A1, and SLC5A1) which are extensively located at the intestinal mucosa. In current study, contaminated feed exhibited no significant effects on the mRNA expression of intestinal amino acid transporters. However, previous studies have indicated that mycotoxins inhibit amino acid transporters expression [55]. The discrepancy with other studies may be growing pigs that highly adapt to contaminated feed. However, dietary supplementation with glutamate exhibits no benefits to amino acid transporters expression after contaminated feed challenge. Previous study have reported that dietary supplementation with arginine or N-carbamylglutamate up-regulates of SLC1A1 gene expression or vascular endothelial growth factor or and mTOR Signaling Activity [56]–[59]. However, our previous investigation has indicated that supplementation with arginine also fails to up-regulate intestinal amino acid transporters [37]. Thus, with respected to this contradictory results, we speculate that the reason may be due to animal model, duration of feeding and the concentration of glutamate supplementation. However, further studies should be carried out to elucidate this point in detail.
In conclusion, treatment of pigs with mold-contaminated feed has adverse effects on growth performance, structure of the intestine (histology, morphology and barrier function), and serum amino acid profile. Glutamate likes other functional amino acids can improve animal health [60]–[77]. Dietary supplementation with glutamate partially counteracts the impairments induced by mycotoxins. Therefore, glutamate may be useful as a nutritional regulating factor to alleviate the adverse effects of mycotoxins.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The present work was supported by grants from the National Natural Science Foundation of China (31110103909, 31330075, 31272463), and the Hunan Provincial Natural Science Foundation of China (No. 12JJ2014, 13JJ2034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beardall J, Miller JD (1994) Natural occurrence of mycotoxins other than aflatoxin in Africa, Asia and South America. Mycotoxin Res 10: 21–40. [DOI] [PubMed] [Google Scholar]
- 2. Xiao H, Wu MM, Tan BE, Yin YL, Li TJ, et al. (2013a) Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. Journal of Animal Science 91: 4772–4780. [DOI] [PubMed] [Google Scholar]
- 3. Mankeviciene A, Jablonskyte-Rasce D, Maiksteniene S (2014) Occurrence of mycotoxins in spelt and common wheat grain and their products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31: 132–138. [DOI] [PubMed] [Google Scholar]
- 4. Burkhardt B, Pfeiffer E, Metzler M (2009) Absorption and metabolism of the mycotoxins alternariol and alternariol-9-methyl ether in Caco-2 cells in vitro. Mycotoxin Res 25: 149–157. [DOI] [PubMed] [Google Scholar]
- 5. Coppock RW, Jacobsen BJ (2009) Mycotoxins in animal and human patients. Toxicol Ind Health 25: 637–655. [DOI] [PubMed] [Google Scholar]
- 6. Abbas HK, Yoshizawa T, Shier WT (2013) Cytotoxicity and phytotoxicity of trichothecene mycotoxins produced by Fusarium spp. Toxicon 74: 68–75. [DOI] [PubMed] [Google Scholar]
- 7. Andretta I, Kipper M, Lehnen CR, Hauschild L, Vale MM, et al. (2012) Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 6: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 8. Rustemeyer SM, Lamberson WR, Ledoux DR, Wells K, Austin KJ, et al. (2011) Effects of dietary aflatoxin on the hepatic expression of apoptosis genes in growing barrows. J Anim Sci 89: 916–925. [DOI] [PubMed] [Google Scholar]
- 9. Dietrich B, Neuenschwander S, Bucher B, Wenk C (2012) Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal 6: 278–291. [DOI] [PubMed] [Google Scholar]
- 10. Grenier B, Applegate TJ (2013) Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins (Basel) 5: 396–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleck SC, Burkhardt B, Pfeiffer E, Metzler M (2012) Alternaria toxins: Altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol Lett 214: 27–32. [DOI] [PubMed] [Google Scholar]
- 12. Becker C, Reiter M, Pfaffl MW, Meyer HH, Bauer J, et al. (2011) Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res 27: 287–293. [DOI] [PubMed] [Google Scholar]
- 13. Modra H, Blahova J, Marsalek P, Banoch T, Fictum P, et al. (2013) The effects of mycotoxin deoxynivalenol (DON) on haematological and biochemical parameters and selected parameters of oxidative stress in piglets. Neuro Endocrinol Lett 34: 84–89. [PubMed] [Google Scholar]
- 14. Hou YJ, Zhao YY, Xiong B, Cui XS, Kim NH, et al. (2013) Mycotoxin-containing diet causes oxidative stress in the mouse. PLoS One 8: e60374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin J, Ren W, Liu G, Duan J, Yang G, et al. (2013) Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res 47: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 16. Danicke S, Brosig B, Klunker LR, Kahlert S, Kluess J, et al. (2012) Systemic and local effects of the Fusarium toxin deoxynivalenol (DON) are not alleviated by dietary supplementation of humic substances (HS). Food Chem Toxicol 50: 979–988. [DOI] [PubMed] [Google Scholar]
- 17. Yunus AW, Blajet-Kosicka A, Kosicki R, Khan MZ, Rehman H, et al. (2012) Deoxynivalenol as a contaminant of broiler feed: intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult Sci 91: 852–861. [DOI] [PubMed] [Google Scholar]
- 18. Soleimany F, Jinap S, Rahmani A, Khatib A (2011) Simultaneous detection of 12 mycotoxins in cereals using RP-HPLC-PDA-FLD with PHRED and a post-column derivatization system. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28: 494–501. [DOI] [PubMed] [Google Scholar]
- 19. Makun HA, Dutton MF, Njobeh PB, Mwanza M, Kabiru AY (2011) Natural multi-occurrence of mycotoxins in rice from Niger State, Nigeria. Mycotoxin Res 27: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Yin YL, Shu X, Li TJ, Li FN, et al. (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 21. Chen G, Zhang J, Zhang Y, Liao P, Li TJ, et al. (2014) Oral MSG administration alters hepatic expression of genes for lipid and nitrogen metabolism in suckling piglets. Amino Acids 46: 245–250. [DOI] [PubMed] [Google Scholar]
- 22. Wu GY, Bazer FW, Davis TA, Kim SW, Li P, et al. (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janeczko MJ, Stoll B, Chang X, Guan X, Burrin DG (2007) Extensive gut metabolism limits the intestinal absorption of excessive supplemental dietary glutamate loads in infant pigs. J Nutr 137: 2384–2390. [DOI] [PubMed] [Google Scholar]
- 24. Feng Z, Zhou X, Wu F Yao K, Kong XF, et al. (2014) Both Dietary Supplementation with Monosodium L-Glutamate and Fat Modify Circulating and Tissue Amino Acid Pools in Growing Pigs, but with Little Interactive Effect. PLOS ONE 9: e84533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riedijk MA, De Gast-Bakker DAH, Wattimena JLD, Van Goudoever JB (2007) Splanchnic oxidation is the major metabolic fate of dietary glutamate in enterally fed preterm infants. Pediatric Research 62: 468–473. [DOI] [PubMed] [Google Scholar]
- 26. Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, et al. (1999) Substrate oxidation by the portal drained viscera of fed piglets. Am J Physiol 277: E168–175. [DOI] [PubMed] [Google Scholar]
- 27. Wu X, Zhang Y, Liu Z, Li TJ, Yin YL (2012) Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. J Anim Sci 90 Suppl 4: 337–339. [DOI] [PubMed] [Google Scholar]
- 28.Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, et al.. (2014) Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J. [DOI] [PMC free article] [PubMed]
- 29. Feng Y, Ralls MW, Xiao W, Miyasaka E, Herman RS, et al. (2012) Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann N Y Acad Sci 1258: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergen WG, Wu G (2009) Intestinal nitrogen recycling and utilization in health and disease. J Nutr 139: 821–825. [DOI] [PubMed] [Google Scholar]
- 31. Boutry C, Matsumoto H, Bos C, Moinard C, Cynober L, et al. (2012) Decreased glutamate, glutamine and citrulline concentrations in plasma and muscle in endotoxemia cannot be reversed by glutamate or glutamine supplementation: a primary intestinal defect? Amino Acids 43: 1485–1498. [DOI] [PubMed] [Google Scholar]
- 32. Blachier F, Boutry C, Bos C, Tome D (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90: 814S–821S. [DOI] [PubMed] [Google Scholar]
- 33. Akiba Y, Watanabe C, Mizumori M, Kaunitz JD (2009) Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol 297: G781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu YL, Meng GQ, Wang HR, Zhu HL, Hou YQ, et al. (2011) Effect of three mycotoxin adsorbents on growth performance, nutrient retention and meat quality in broilers fed on mould-contaminated feed. British Poultry Science 52: 255–263. [DOI] [PubMed] [Google Scholar]
- 35. Tan B, Yin Y, Liu Z, Li X, Xu H, et al. (2009) Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37: 169–175. [DOI] [PubMed] [Google Scholar]
- 36. Tan B, Yin Y, Liu Z, Tang W, Xu H, et al. (2011) Dietary L-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem 22: 441–445. [DOI] [PubMed] [Google Scholar]
- 37. Yin J, Ren W, Duan J, Wu L, Chen S, et al. (2014) Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids. 46: 883–892. [DOI] [PubMed] [Google Scholar]
- 38. Yin FG, Zhang ZZ, Huang J, Yin YL (2010) Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Brit J Nutr 103: 1404–1412. [DOI] [PubMed] [Google Scholar]
- 39. Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, et al. (2010) Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39: 1477–1486. [DOI] [PubMed] [Google Scholar]
- 40. Wu X, Yin YL, Li TJ, Huang RL, Zhang Z, et al. (2010) Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39: 831–839. [DOI] [PubMed] [Google Scholar]
- 41. Tan BE, Li XG, Kong XF, Yin YL, Li TJ, et al. (2010) Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37: 323–331. [DOI] [PubMed] [Google Scholar]
- 42. Ruan Z, Yang M, Zhou Y, et al. (2014) Metabolomic analysis of amino acid and energy metabolism in rats supplemented with chlorogenic acid. Amino Acids 46: 2219–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yin J, Ren WK, Wu XS, Yang G, Wang J, et al. (2013) Oxidative stress-mediated signaling pathways: A review. Journal of Food Agriculture & Environment 11: 132–139. [Google Scholar]
- 44. Ren WK, Chen S, Yin J, Duan J, Li T, et al. (2014) Dietary Arginine Supplementation of Mice Alters the Microbial Population and Activates Intestinal Innate Immunity. J Nutr 144: 568–579. [DOI] [PubMed] [Google Scholar]
- 45. Ren W, Yu R, Liu G, Yin J, Yin YL, et al. (2013) DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. Vaccine 31: 367–72. [DOI] [PubMed] [Google Scholar]
- 46. Wu L, Wang W, Yao K, Zhou T, Yin J, et al. (2013) Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One 8: e69502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Belokrylov GA, Popova O, Sorochinskaia EI (1999) [Detoxication of benzene and aflatoxin B1 by amino acid and peptide preparations in mice and chicken]. Biull Eksp Biol Med 128: 419–421. [PubMed] [Google Scholar]
- 48. Mezes M, Balogh K, Toth K (2010) Preventive and therapeutic methods against the toxic effects of mycotoxins - a review. Acta Vet Hung 58: 1–17. [DOI] [PubMed] [Google Scholar]
- 49. Awad WA, Rehman H, Bohm J, Razzazi-Fazeli E, Zentek J (2005) Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the jejunums of laying hens. Poult Sci 84: 928–932. [DOI] [PubMed] [Google Scholar]
- 50. Nakamura H, Kawamata Y, Kuwahara T, Torii K, Sakai R (2013) Nitrogen in dietary glutamate is utilized exclusively for the synthesis of amino acids in the rat intestine. Am J Physiol Endocrinol Metab 304: E100–108. [DOI] [PubMed] [Google Scholar]
- 51. Wu GY, Bazer FW, Davis TA, Johnson GA, Yin YL, et al. (2007) Important roles for arginine-family amino acids in swine nutrition and production. Livestock Science 122: 8–22. [Google Scholar]
- 52. Martensson J, Jain A, Meister A (1990) Glutathione Is Required for Intestinal Function. Proceedings of the National Academy of Sciences of the United States of America 87: 1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meloche JL, Smith TK (1995) Altered tissue amino acid metabolism in acute T-2 toxicosis. Proc Soc Exp Biol Med 210: 260–265. [DOI] [PubMed] [Google Scholar]
- 54. Taylor PM (2014) Role of amino acid transporters in amino acid sensing. Am J Clin Nutr 99: 223S–230S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Awad WA, Rehman H, Bohm J, Razzazi-Fazeli E, Zentek J (2005) Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the Jejunums of laying hens. Poultry Science 84: 928–932. [DOI] [PubMed] [Google Scholar]
- 56. Yang HS, Fu DZ, Kong XF, Wang WC, Yang XJ, et al. (2013) Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J Anim Sci 91: 2740–2748. [DOI] [PubMed] [Google Scholar]
- 57. Yao K, Yin YL, Chu WY, Liu ZQ, Dun D, et al. (2008) Dietary Arginine Supplementation Increases mTOR Signaling Activity in Skeletal Muscle of Neonatal Pigs. J Nutr 138: 867–872. [DOI] [PubMed] [Google Scholar]
- 58. Yao K, Wang L, Ding D, Li T, Yin YL, et al. (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. 2012; 42 (6): 2491–500. [DOI] [PubMed] [Google Scholar]
- 59. Yao K, Guan S, Li T, Huang R, Li T, et al. (2011) Dietary L-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr. 105: 703–709. [DOI] [PubMed] [Google Scholar]
- 60. Kang P, Zhang L, Hou Y, Ding D, Yi D, et al. (2014) Effects of L-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (CPS) –challenged pigs. Asian-Aust. J Anim Sci 27: 1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ren WK, Yin J, Wu M, Li TJ, Yin YL, et al. (2014) Serum Amino Acids Profile and the Beneficial Effects of L-Arginine or L-Glutamine Supplementation in Dextran Sulfate Sodium Colitis. PLOS One 2014 9: e88335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen S, Liu S, Zhang F, Li T, Yin YL, et al. (2014) Effects of dietary L-glutamine supplementation on specific and general defense responses in mice immunized with inactivated Pasteurella multocida vaccine. Amino Acids. Amino Acids 46: 2365–2375. [DOI] [PubMed] [Google Scholar]
- 63. Ren WK, Duan JL, Yin J, Li TJ, Yin YL, et al. (2014) Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. Amino Acids 46: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 64. Geng MM, Li TJ, Kong XF, Song XY, Chu WY, et al. (2011) Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids. 40: 1513–1522. [DOI] [PubMed] [Google Scholar]
- 65. He LQ, Yang HS, Hou YQ, Li TJ, Fang J, et al. (2013) Effects of dietary L-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids. 45: 383–391. [DOI] [PubMed] [Google Scholar]
- 66. Wu X, Shu XG, Xie CY, Li J, Hu J, et al. (2013) The Acute and Chronic Effects of Monosodium L-Glutamate on Serum Iron and Total Iron-Binding Capacity in the Jugular Artery and Vein of Pigs. Biol Trace Elem Res 153: 191–195. [DOI] [PubMed] [Google Scholar]
- 67. Tan BE, Yin YL, Kong XF, Li T, Yin YL, et al. (2010) L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yin YL, Tan BE (2010) Manipulation of dietary nitrogen, amino acids and phosphorus to reduce environmental impact of swine production and enhance animal health. Journal of Food, Agriculture & Environment. 8: 447–462. [Google Scholar]
- 69. Yao K, Fang J, Yin YL, Feng ZM, Li T, et al. (2011) Tryptophan metabolism in animals: important roles in nutrition and health. Frontiers in Bioscience S3: 286–297. [DOI] [PubMed] [Google Scholar]
- 70. Li FN, Yin YL, Tan BE, Li H, Duan Y, et al. (2011) Leucine nutrition in animals and humans: mTOR signaling 3 and beyond. Amino Acids 41: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 71. He QH, Tang HR, Ren PP, Li TJ, Yin YL, et al. (2011) Dietary Supplementation with L -Arginine Partially Counteracts Serum Metabonome Induced by Weaning Stress in Piglets. Journal of Proteome Research. 10: 5214–5221. [DOI] [PubMed] [Google Scholar]
- 72. Tan BE, Li XG, Yin YL, Li TJ, Kong XF, et al. (2012) Regulatory roles for L-arginine in reducing white adipose tissue. Frontiers in Bioscience. 17: 2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong XF, Yin YL, Wu GY (2012) Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Journal of Nutritional Biochemistry 23 1178–1183. [DOI] [PubMed]
- 74. Liu XD, Wu X, Yin YL, Li T, Huang RL, et al. (2012) Effects of dietary L-arginineor N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222,VEGFAandeNOSexpression in umbilicalvein. Amino Acids 42: 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X, Wu L, Yin YL, Liu YQ, et al.. (2012) Effect of dietary arginine and N-carbamoylglutamate supplementation reproduction and gene expression of eNOS, VEGFA and PlGF1 in on in late pregnancy of sows placenta. Animal Reproduction Science. 132 187–192. [DOI] [PubMed]
- 75. Tan BE, Li XG, Wu GY, Yin YL, et al. (2012) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of L-arginine. Amino Acids 43: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 76. Wu GY, Wu ZL, Dai ZL, Wang JJ, Yin YL, et al. (2013) Dietary requirements of nutritionally non-essentialaminoacids by animals and humans Amino Acids 2013. 44: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 77. Wu X, Xie CY, Yin YL, Li TJ, Huang RL, et al. (2013) Effect of L-arginine on HSP70 expression in liver in weanling piglets. BMC Veterinary Research 9: 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.