Abstract
Background: Insomnia and sleep-disordered breathing (SDB) are the most common sleep disorders among midlife women. Although promoting sleep hygiene behaviors may be a useful behavioral approach for the management of insomnia or SDB, the frequency with which women engage in these behaviors is unclear.
Methods: Participants were from the Study of Women's Health Across the Nation (SWAN) Sleep Study (N=321; age range=48–58 years). Out of the full sample, 10.3% (n=33) met Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition diagnostic criteria for insomnia, 15.3% (n=49) had clinically significant SDB (apnea–hypopnea index ≥15), and 4.7% (n=15) met criteria for both insomnia and SDB, resulting in an overall prevalence of 15.0% (n=48) for insomnia and 19.9% (n=64) for SDB. Participants provided diary-based assessments of sleep hygiene behaviors for 14–35 days. Two positive behaviors (sufficient exercise, regular morning out-of-bed time) and four negative behaviors (taking long daytime naps, caffeine consumption near bedtime, alcohol consumption near bedtime, smoking) were examined. These behaviors were compared between women with and without insomnia or SDB following adjustment for sociodemographic factors and mental and physical health indices.
Results: Women with insomnia engaged in significantly fewer negative sleep hygiene behaviors than women without insomnia (1.61±0.15 vs. 2.09±0.09 behaviors; p<0.01); specifically, women with insomnia were less likely to take long naps (odds ratio [OR]=0.30, 95% confidence interval [CI]: 0.12–0.74) or consume caffeine near bedtime (OR=0.44, 95% CI: 0.20–0.98). In contrast, women with SDB were less likely to be physically active than women without SDB (OR=0.52, 95% CI: 0.27–0.98), but no other differences in sleep hygiene behaviors were observed.
Conclusions: These data suggest that insomnia in midlife women is not associated with poor sleep hygiene. Increasing physical activity may be a valuable recommendation for midlife women with SDB.
Introduction
Sleep is an essential component of health in midlife women.1 However, sleep disorders are common in this population, as 15%–25% of midlife women meet diagnostic criteria for insomnia2,3 and up to 20% have at least moderate-severity sleep-disordered breathing (SDB).4 The high prevalence of these sleep disorders in midlife women has significant public health implications, as insomnia and SDB are associated with daytime impairment5 and cardiovascular,6 metabolic,7 and psychiatric morbidity8 in this population.
Poor sleep hygiene behaviors may contribute to or exacerbate the sleep disturbances in midlife women with insomnia or SDB (Table 1). Sleep hygiene recommendations involve the promotion of specific behaviors that help sleep (e.g., regular exercise, maintaining a regular out-of-bed time) and the avoidance of specific behaviors that interfere with sleep (e.g., smoking, evening alcohol, evening caffeine, daytime napping);9 the associations of each of these behaviors with sleep are well documented.10–14 Sleep hygiene recommendations are commonly integrated into behavioral treatments for insomnia;15 however, despite being emphasized less frequently, sleep hygiene behaviors are also important for SDB.16,17 For instance, in those with existing SDB, alcohol use, or smoking may exacerbate SDB severity by depressing upper airway dilator tone or causing upper airway inflammation, respectively.18,19 Moreover, exercising, maintaining a regular out-of-bed time, refraining from evening caffeine, and/or minimizing daytime napping could facilitate better sleep quality (e.g., more slow-wave sleep, less fragmentation), which is protective against SDB.20,21
Table 1.
Common Sleep Hygiene Behaviors
| Positive sleep hygiene behaviors: behaviors that promote better sleep |
| Exercise regularly |
| Get out of bed at the same time every day |
| Negative sleep hygiene behaviors: behaviors that interfere with sleep |
| Long daytime naps |
| Caffeine consumption near bedtime |
| Alcohol consumption near bedtime |
| Smoking |
Because of the possible utility of sleep hygiene education for the behavioral management of insomnia or SDB, determining the frequency of these behaviors could inform future interventions to improve sleep in midlife women. However, it is not clear how frequently those with insomnia or SDB engage in sleep hygiene behaviors,22,23 especially among midlife women. In fact, the only research to specifically examine sleep hygiene behaviors in midlife women found that women with insomnia had a more stable bedtime and were less likely to consume caffeine or alcohol compared to women without insomnia.24 The purpose of the present study was to compare the frequency of six self-reported sleep hygiene behaviors among midlife women with insomnia or SDB against those without insomnia or SDB. Diary-based assessments of exercise frequency, morning out-of-bed time regularity, caffeine timing, alcohol timing, smoking, and daytime napping were obtained daily across 14–35 days in 321 midlife women.
Materials and Methods
Participants
The Study of Women's Health Across the Nation (SWAN) Sleep Study is a cross-sectional study of sleep in a multiethnic sample of midlife women. The SWAN Sleep Study is an ancillary study of the longitudinal SWAN cohort (N=3302), a study of the menopausal transition and its consequences on health and functioning, being conducted at 7 clinical sites across the United States.25 For the SWAN Sleep Study, 370 participants were recruited from four of the seven core SWAN sites (Chicago, IL, Detroit area, MI, Oakland, CA, and Pittsburgh, PA) during a period of time between their fifth and seventh annual core SWAN visits. Exclusions for the Sleep Study included postmenopausal status, current hormone replacement therapy use, current treatment for cancer, permanent or rotating night shift employment, regular consumption of more than four alcoholic drinks per day, and noncompliance with core SWAN procedures (e.g., >50% of annual visits missed). During the final year of recruitment, exclusion criteria were revised to allow postmenopausal women not currently using hormone replacement therapy.26 Written informed consent was obtained by all participants in accordance with approved protocols and guidelines of the institutional review board of each participating institution.
SWAN Sleep Study participants were excluded from the present analyses if data required for insomnia or SDB determination were unavailable (n=30), covariate data were missing (n=12), or <14 days of sleep hygiene behavior data were available (n=7), resulting in a final sample of 321 women. Compared with women whose data were retained for analysis, excluded participants were more likely to smoke (23% vs. 11%; chi-squared=5.01, p=0.03) and be of African American race (53% vs. 35%; chi-squared=6.00, p=0.01). Excluded participants did not differ from those included in analyses on the prevalence of insomnia or SDB or any other sociodemographic characteristics or sleep hygiene behaviors.
Procedure
The SWAN Sleep Study was conducted across a complete menstrual cycle or 35 days, whichever was shorter. In participants with regular menstrual cycles, the protocol was initiated within 7 days of the start of menstrual bleeding. Women who had irregular menstrual cycles or were noncycling were scheduled at their convenience.
Insomnia assessment
The 13-item Insomnia Symptom Questionnaire (ISQ)27 was administered on the last day of the Sleep Study protocol. The ISQ assesses the presence, frequency, and severity of various insomnia-related complaints, with determination of the presence or absence of insomnia based upon Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV) criteria for primary insomnia.28 The ISQ does not determine whether insomnia-related complaints occur exclusive of another sleep disorder, mental disorder, medication, or general medical condition (criteria C–E for DSM-IV diagnostic criteria for primary insomnia [307.42]). Thus, the ISQ did not differentiate between “primary” or “secondary” insomnia, which is more consistent with DSM-5 and International Classification of Sleep Disorders-Third Edition (ICSD-3) criteria for insomnia disorder.29,30 The ISQ was validated in the SWAN Sleep Study sample; internal consistency was high (α=0.89), with 42% sensitivity and 92% specificity against diary-based research criteria for insomnia (sleep onset latency ≥31 minutes, wakefulness after sleep onset ≥31 minutes, or sleep efficiency <85% on ≥3 days/week).27
SDB Assessment
Polysomnography (PSG; Vitaport-3, Temec Instruments, Kerkrade, Netherlands) was conducted in participants' homes according to their habitual sleep/wake schedule over three consecutive nights at the beginning of the Sleep Study, with the first night used for assessment of SDB. In addition to a standard PSG montage, additional monitors used for determination of SDB included nasal pressure cannula, oronasal thermistor, respiratory inductance plethysmography, and fingertip oximetry. Respiratory events were evaluated using American Academy of Sleep Medicine definitions,31 and visual sleep stage scoring was conducted in 20-second epochs using standard criteria.32 We categorized an apnea–hypopnea index (AHI) ≥15 as clinically significant SDB.
Sleep hygiene behaviors
Participants used daily diaries to record sleep hygiene behaviors (mean 29.3±6.1 days per participant; range: 14–35 days). At bedtime each day, participants indicated (1) the number of minutes they napped during the day; (2) the category of the most vigorous exercise they performed on that day [0: none, 1: light (e.g., walking), 2: moderate (e.g., jogging); or 3: vigorous (e.g., running)]; (3) the number of caffeinated beverages and time the last beverage was consumed; (4) the number of alcoholic drinks consumed and time the last drink was consumed; and (5) the number of cigarettes that were smoked that day. For caffeine and alcohol, the time of final consumption was expressed relative to self-reported bedtime for that night. In the morning, among other queries, participants indicated the time they got out of bed.
Sleep hygiene behaviors were classified as “positive” or “negative” based upon whether they are known to promote or interfere with sleep, respectively (Table 1). Positive sleep hygiene behaviors included (1) sufficient exercise, and (2) maintaining a consistent morning out-of-bed time. A woman was considered a regular exerciser if she averaged ≥3 days/week of light-, moderate-, or vigorous-intensity exercise across observation. Although avoidance of exercise close to bedtime is sometimes included in sleep hygiene recommendations, we did not examine late-night exercise as a negative sleep hygiene behavior since empirical and epidemiological evidence does not support this recommendation33,34 and only 4% of the present sample regularly exercised within 2 hours of bedtime. We classified women whose within-person standard deviation of diary-based out-of-bed time was <60 minutes as having a “regular” morning out-of-bed time. Standard sleep hygiene guidelines recommend maintaining a consistent out-of-bed time,9 with a typical goal of keeping the out-of-bed time within a 1-hour window regardless of weekday or weekend.35–37
Negative sleep hygiene behaviors included (1) taking long daytime naps; (2) consuming caffeine near bedtime; (3) consuming alcohol close to bedtime; and (4) smoking. We classified women with daytime napping if they reported any daytime naps >30 minutes during the observation period, as long daytime naps may interfere with subsequent nighttime sleep.14,38 We classified women as consuming caffeine near bedtime if they reported any caffeine use within 5 hours prior to bedtime, since the half-life of caffeine is 4–6 hours39 and most sleep hygiene recommendations advise avoiding caffeine 4–6 hours prior to bedtime.9 We classified women as consuming alcohol close to bedtime if they reported any alcohol consumption within 4 hours of bedtime. Sleep hygiene recommendations suggest abstaining from alcohol near bedtime,9 and alcohol consumption up to 4 hours prior to bedtime significantly impacts sleep architecture.40,41 We classified women as smokers if they reported any smoking during observation, as even mild nicotine use impairs subjective and objective indices of sleep.42
Covariates
Sociodemographic factors and indices of mental and physical health were included as covariates due to their possible relationship with insomnia, SDB, and/or sleep hygiene behaviors. Age was calculated at the time of the sleep study, and race/ethnicity was self-identified as Caucasian, African American, or Chinese. Education (less than a college degree, college degree, or more) was assessed during the core SWAN baseline interview. Marital status, body mass index (BMI), menopausal status, and the number of chronic health conditions were assessed from a physical examination and structured interview at the closest core SWAN visit prior to the Sleep Study. Marital status was classified as married/living as married or single/widowed/divorced/separated. Body mass index was based upon in-person measurements and calculated as kg/m2. As determined by bleeding patterns, menopausal status was categorized as being pre- or early perimenopausal, late perimenopausal, or postmenopausal. The number of chronic health conditions was calculated as the sum of the self-reported presence of the following conditions: diabetes, hypertension, hypercholesterolemia, prior stroke/myocardial infarction, angina, arthritis/osteoarthritis, hyper- or hypoactive thyroid, chronic migraines, or prior cancer (excluding skin).
Nocturnal vasomotor symptoms and use of medications that affect sleep were assessed by daily diary entries throughout the Sleep Study. Medications, including both prescription and over-the-counter medicines, were coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification system (www.whocc.no/atcddd). Medications with the following ATC codes were considered to affect sleep: N02A (opioids), N03A (antiepileptics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants), and R06A (antihistamines). Medication use was dichotomized as <3 nights/week or ≥3 nights/week. Nocturnal vasomotor symptoms (VMS) were assessed each morning, as participants were asked to report the number of cold sweats, hot flashes, and night sweats they experienced during the previous night's sleep. Nocturnal VMS were categorized as being present if at least one VMS was reported that night, and participants were categorized based upon experiencing nocturnal VMS <3 nights/week or ≥3 nights/week.
Depressive symptoms and PSG total sleep time were assessed during the Sleep Study. The Quick Inventory of Depressive Symptomatology-Self Report43 was used to assess depressive symptoms during days 4, 14, and the last day of the Sleep Study. Scores were calculated minus the sleep items and averaged across the three time points, with higher scores indicating greater depressive symptoms. Polysomnographic total sleep time (TST) was based upon up to 2 nights of PSG recording following the initial night of SDB assessment; TST was calculated as the total minutes of any sleep stage following sleep onset, averaged across both recording nights when 2 nights of data were available.
Statistical analyses
Binary logistic regression analyses were used to examine the likelihood of engaging in individual positive or negative sleep hygiene behaviors given the presence or absence of insomnia or SDB. Each model included age, race/ethnicity, education, marital status, BMI, menopausal status, number of chronic health conditions, nocturnal VMS, use of medication that affects sleep, depressive symptoms and PSG TST as covariates. Since it was possible that women could meet both insomnia and SDB criteria, preliminary analyses included an insomnia/SDB interaction in each model. As each of these interaction terms were found to be nonsignificant (p>0.15 each), they were removed from the final models.
Because health behaviors tend to cluster together,44 we also summed the individual positive sleep hygiene behaviors (range: 0–2) and negative sleep hygiene behaviors (range: 0–4) for each participant. Analysis of covariance (ANCOVA) was used to determine whether the cumulative number of positive and negative sleep hygiene behaviors differed between those with and without insomnia or SDB following adjustment for the previously described covariates. Insomnia/SDB interaction terms were nonsignificant (p>0.14) and removed from the final ANCOVA models.
Analyses were conducted using SAS v. 9.3 (SAS Institute; Cary, NC). All statistical tests were two-tailed, with statistical significance set at p<0.05.
Results
Participant characteristics
Participant characteristics are summarized in Table 2. Of the 321 women, the mean (±SD) age was 52.1±2.1 years; 48% of the women were Caucasian, 35% were African American, and 17% were Chinese. The majority of the women were married or living as married (64%), had a college degree or greater education (52%), and were pre- or early perimenopausal (63%). Approximately 10% (n=33) of the sample met diagnostic criteria for insomnia only, 15% (n=49) were classified as having SDB only, and 4.7% (n=15) had both insomnia and SDB. Among the women with insomnia, there was considerable heterogeneity in the type(s) of insomnia complaint endorsed. Out of the 33 women with insomnia only, 15 endorsed difficulty initiating sleep, 23 indicated difficulty maintaining sleep, and 21 reported unrefreshing sleep; 17 of the women endorsed at least 2 different types of insomnia complaints. Likewise, of the 15 women with insomnia and SDB, 8 endorsed difficulty initiating sleep, 10 indicated difficulty maintaining sleep, and 11 reported unrefreshing sleep; 10 of the women endorsed 2 or more types of insomnia complaints.
Table 2.
SWAN Sleep Study Participant Characteristics
| Insomnia | SDB | ||||
|---|---|---|---|---|---|
| Characteristic | All(N=321) | Absent(n=273) | Present(n=48) | Absent(n=257) | Present(n=64) |
| Age, years | 52.1 (2.1) | 52.1 (2.2) | 52.4 (1.9) | 52.0 (2.0) | 52.8 (2.4)** |
| Race/ethnicity, n (%) | |||||
| African American | 112 (34.9) | 90 (33.0) | 22 (45.8) | 85 (33.1) | 27 (42.2) |
| Caucasian | 155 (48.3) | 132 (48.3) | 23 (47.9) | 128 (49.8) | 27 (42.2) |
| Chinese | 54 (16.8) | 51 (18.7) | 3 (6.3) | 44 (17.1) | 10 (15.6) |
| Marital status, n (%) | |||||
| Single/widowed/divorced | 114 (35.5) | 95 (34.8) | 19 (39.6) | 87 (33.9) | 27 (42.2) |
| Married/living as married | 207 (64.5) | 178 (65.2) | 29 (60.4) | 170 (66.1) | 37 (57.8) |
| Education, n (%) | |||||
| Less than college degree | 153 (47.7) | 128 (46.9) | 25 (52.1) | 122 (47.5) | 31 (48.4) |
| College degree or more | 168 (52.3) | 145 (53.1) | 23 (47.9) | 135 (52.5) | 33 (51.6) |
| Menopausal status, n (%) | |||||
| Pre-/early perimenopause | 202 (62.9) | 179 (65.6) | 23 (47.9) | 174 (67.7) | 28 (43.8) |
| Late perimenopause | 60 (18.7) | 51 (18.7) | 9 (18.8) | 41 (16.0) | 19 (29.7)* |
| Postmenopause/surgical | 59 (18.4) | 43 (15.8) | 16 (33.3)** | 42 (16.3) | 17 (26.5) |
| Nocturnal VMS, n (%) | |||||
| No/intermittent occurrence | 215 (67.0) | 192 (70.3) | 23 (47.9) | 174 (67.7) | 41 (54.1) |
| At least 3 nights/week | 106 (33.0) | 81 (29.7) | 25 (52.1)** | 83 (32.3) | 23 (35.9) |
| Body mass index, kg/m2 | 30.0 (7.8) | 29.2 (7.3) | 34.1 (9.4)** | 28.4 (6.4) | 36.4 (9.5)** |
| Chronic health conditions, sum | 0.8 (1.0) | 0.7 (1.0) | 1.2 (1.2)** | 0.6 (0.9) | 1.3 (1.3)** |
| IDS (sleep items removed), 0–24 | 2.8 (2.7) | 2.3 (2.2) | 5.5 (3.3)** | 2.6 (2.5) | 3.5 (3.2)* |
| Polysomnographic TST, minutes | 381.7 (60.8) | 384.5 (58.9) | 365.7 (69.2)* | 383.1 (61.1) | 375.9 (59.8) |
| Use of sleep-affecting medication, n (%) | |||||
| No/intermittent use | 245 (76.3) | 215 (78.8) | 30 (62.5) | 196 (76.3) | 49 (76.6) |
| At least 3 nights/week | 76 (23.7) | 58 (21.2) | 18 (37.5)* | 61 (23.7) | 15 (23.4) |
Data are presented as mean (standard deviation) or n (%), as appropriate. Comparisons of participant characteristics were performed separately according to the presence/absence of insomnia and the presence/absence of SDB using analysis of variance or chi-squared tests, as appropriate. *p<0.05; **p<0.01.
IDS, Inventory of Depressive Symptomatology; SDB, sleep-disordered breathing; TST, total sleep time; VMS, vasomotor symptoms.
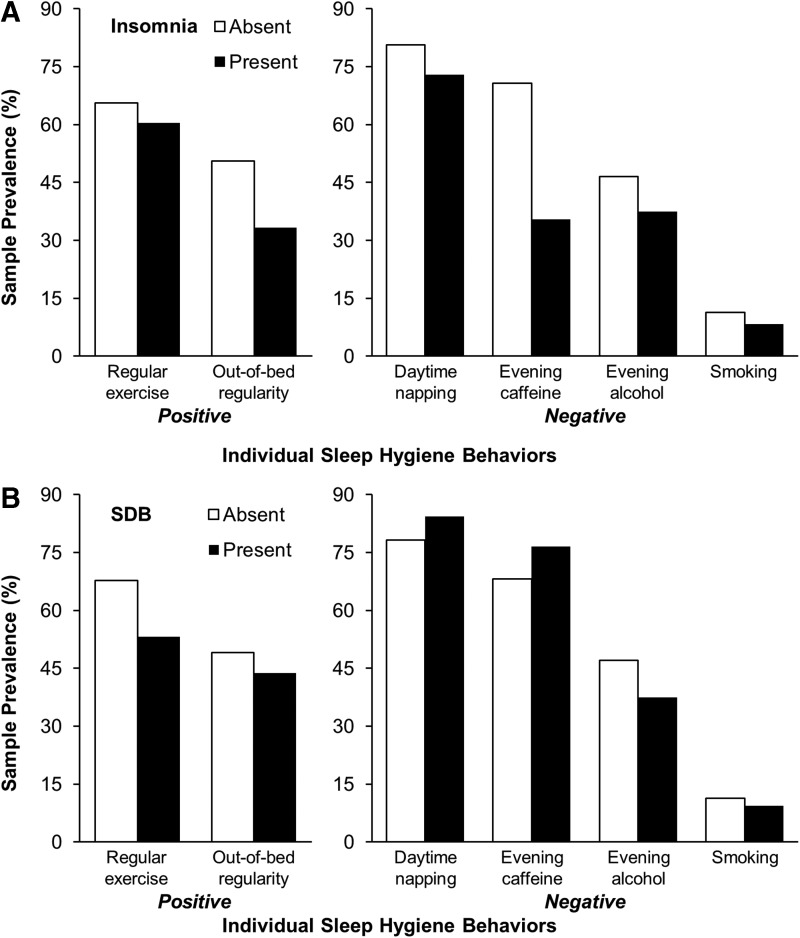
Differences in sociodemographic characteristics and indices of physical and mental health between women with and without insomnia and between women with and without SDB are displayed in Table 2. Women with insomnia had a higher BMI, more chronic health conditions, greater depressive symptoms, and shorter PSG TST than women without insomnia. In addition, women with insomnia were more likely to be postmenopausal, use sleep-related medication ≥3 days/week, and report nocturnal VMS ≥3 days/week than women without insomnia. Compared to women without SDB, women with SDB were older, had a higher BMI, more chronic health conditions, greater depressive symptoms, and were more likely to be late perimenopausal. A summary of the frequency of sleep hygiene behaviors according to the presence or absence of insomnia or SDB is provided in Fig. 1.
FIG. 1.
Frequency of individual sleep hygiene behaviors among women with and without insomnia or sleep-disordered breathing. (A) Frequency of individual sleep hygiene behaviors according to the presence or absence of insomnia. (B) Frequency of individual sleep hygiene behaviors according to the presence or absence of sleep-disordered breathing. SDB, sleep-disordered breathing.
Individual sleep hygiene behaviors and insomnia or SDB
Table 3 displays the results of logistic regression analyses that examined the likelihood of engaging in individual sleep hygiene behaviors given the presence of insomnia or SDB. The odds of engaging in positive sleep hygiene behaviors did not differ between women with and without insomnia. Compared to those without insomnia, women with insomnia were significantly less likely to take naps >30 minutes (odds ratio [OR]=0.30, 95% confidence interval [CI]: 0.12–0.74) or consume caffeine within 5 hours of bedtime (OR=0.44, 95% CI: 0.20–0.98). Compared to those without SDB, women with SDB were significantly less likely to be physically active (OR=0.52; 95% CI: 0.27–0.98). The odds of engaging in any individual negative sleep hygiene behaviors did not differ between women with or without SDB. Results were unchanged when those with both insomnia and SDB were excluded from analyses.
Table 3.
Odds of Engaging in Specific Sleep Hygiene Behaviors Among Women with Insomnia or Sleep-Disordered Breathing
| Sleep hygiene behavior | InsomniaOR (95% CI) | SDBOR (95% CI) |
|---|---|---|
| Positive behaviors | ||
| Sufficient exercise (≥3 days/week of exercise) | 1.01 (0.47,2.15) | 0.52 (0.27,0.98)* |
| Regular out-of-bed timing (SD <60 minutes) | 0.70 (0.33,1.48) | 1.18 (0.61,2.27) |
| Negative behaviors | ||
| Daytime napping (>30 minutes) | 0.30 (0.12,0.74)** | 0.66 (0.27,1.60) |
| Caffeine consumption within 5 hours of bedtime | 0.44 (0.20,0.98)* | 0.98 (0.46,2.11) |
| Alcohol consumption within 4 hours of bedtime | 0.67 (0.30,1.51) | 1.36 (0.66,2.82) |
| Smoking | 0.48 (0.13,1.69) | 1.04 (0.34,3.16) |
Data indicate the odds of engaging in a specific sleep hygiene behavior in those meeting criteria for insomnia or moderate-severity sleep-disordered breathing compared with those without the respective sleep disorder. Analyses adjusted for age, race, marital status, education, menopausal status, vasomotor symptoms, body mass index, use of medications that affect sleep, number of chronic health conditions, depressive symptoms, and polysomnographic total sleep time. *p<0.05; **p<0.01.
CI, confidence interval; OR, odds ratio; SD, standard deviation;
Cumulative sleep hygiene behaviors and insomnia or SDB
The number of positive sleep hygiene behaviors did not differ between women with and without insomnia [0.94±0.12 vs. 1.02±0.07 (adjusted least squares mean±standard error); F1,305=0.37, p=0.55] or between women with and without SDB (1.03±0.08 vs. 0.92±0.11; F1,305=0.85, p=0.36). Women with insomnia engaged in significantly fewer negative sleep hygiene behaviors than women without insomnia (1.61±0.15 vs. 2.09±0.09; F1,305=9.44, p<0.01), whereas the number of negative sleep hygiene behaviors did not differ between women with and without SDB (1.84±0.14 vs. 1.86±0.09; F1,305=0.03, p=0.85). Results were unchanged when those with insomnia and SDB were excluded from analyses.
Discussion
Sleep hygiene behaviors are commonly recommended to improve sleep quality in individuals with insomnia and, to a lesser extent, SDB.15,16 The guidelines vary from study to study,9 but common components include obtaining regular exercise, maintaining a regular out-of-bed time, avoiding daytime napping, minimizing caffeine and alcohol consumption near bedtime, and not smoking. Although poor sleep hygiene is unlikely to be the primary cause of sleep disturbance, engaging in poor sleep hygiene behaviors may perpetuate or exacerbate the severity of existing insomnia or SDB. Therefore, knowing the frequency with which these sleep hygiene behaviors are engaged in among those with significant sleep disturbances may help to guide future efforts at modifying such behaviors to control sleep-related problems.
We found that midlife women who met DSM-IV diagnostic criteria for insomnia were, overall, less likely to report engaging in negative sleep hygiene behaviors than women without insomnia. Specifically, women with insomnia were less likely to nap or consume caffeine near bedtime compared with women without insomnia. These findings might seem counterintuitive, since prior studies have found adults with insomnia were more likely to exhibit poor sleep hygiene behaviors,23,45,46 including consuming excessive alcohol,22,23 having greater sleep timing variability,23,47 being physically inactive,22 smoking,23 and frequent napping.23,48 However, other studies have failed to observe differences in sleep hygiene behaviors between those with and without insomnia.49–51 Interestingly, our data are consistent with the findings of Cheek and colleagues,24 who found that midlife women with insomnia consumed less caffeine and were more likely to abstain from alcohol compared with women without insomnia. It is possible that midlife women suffering from insomnia are more mindful of the detrimental effects of poor sleep hygiene and, as a result, are more likely to follow positive sleep hygiene behaviors and avoid negative sleep hygiene behaviors. However, because we did not interview our participants regarding the reasons for engaging in sleep hygiene behaviors, we cannot determine whether participants deliberately engaged in these behaviors to self-treat their insomnia. Regardless of the reason, our data do not support an association between self-reported sleep hygiene behaviors and insomnia in midlife women.
We also found that women with SDB were less likely to engage in regular exercise compared to their non-SDB counterparts, even with adjustment for BMI and depressive symptoms. These results are in agreement with studies that have found physical activity levels to be progressively lower with increasing severity of SDB.52,53 Although physical inactivity among adults with SDB may be the result of SDB-related fatigue and sleepiness,54 activity levels do not increase following continuous positive airway pressure (CPAP) therapy and resolution of sleepiness.55 Because the benefits of physical activity in those with SDB include reduced SDB severity along with improved sleep quality and daytime functioning,56,57 efforts to incorporate physical activity into standard SDB treatment (i.e., CPAP, oral appliances) seems warranted.
Women with SDB did not differ from those without SDB on any other individual sleep hygiene behaviors or on the cumulative number of positive or negative sleep hygiene behaviors. In contrast, other studies have found that napping frequency58 and heavy caffeine consumption59,60 are higher in those with SDB, and that CPAP therapy does not reduce caffeine consumption.61 Moreover, prior studies provided conflicting evidence as to whether smoking and alcohol consumption differ between those with and without SDB.59,62–64 As these previous studies focused upon clinical or population-based samples, our data are the first to focus upon sleep hygiene behaviors in a community-based sample of midlife women with SDB.
Sleep hygiene interventions have traditionally been restricted to individuals with poor sleep quality or insomnia. Although modest improvements in sleep and/or daytime function have typically been found following sleep hygiene treatment,65–67 the prevailing opinion is that sleep hygiene is not efficacious as a stand-alone treatment for insomnia.15 However, multiple behaviors comprise sleep hygiene recommendations and both the specific recommendations and their mode of implementation vary widely among studies. Therefore, evaluation of sleep hygiene as a stand-alone treatment is difficult.9 Whereas implementing a comprehensive but untargeted sleep hygiene intervention could minimize the potential importance of the most salient behaviors, targeting one behavior at a time or behavior(s) that are prevalent and germane to the sample could optimize its efficacy.68,69
Although not a primary aim of the current study, an interesting finding was the high prevalence of comorbid insomnia and SDB in our sample of midlife women. Indeed, 31% of those who met criteria for insomnia also had at least moderate-severity SDB and 23% of those with SDB also met criteria for insomnia, with this insomnia/SDB combination comprising 4.7% of the entire sample. These prevalence estimates are in line with other recent studies, which have found insomnia to be present in 21%–39% of those with insomnia and for SDB to co-occur in 7%–69% of those with insomnia.70 Although difficulty maintaining sleep is the most frequent insomnia complaint among adults with comorbid insomnia and SDB, other insomnia complaints are common;71 in our sample, we found a wide variety of insomnia complaints among the women with SDB and insomnia. The causal pathway for the insomnia/SDB combination is likely bidirectional: although the frequent awakenings from SDB events may induce insomnia,72 the fragmented and “light” sleep that is characteristic of insomnia may predispose to SDB.21 Our sample did not have adequate statistical power to examine the sleep hygiene behaviors of women with both insomnia and SDB. Future research should endeavor to characterize the sleep hygiene behaviors of this unique sleep disorder combination, as there is initial evidence that the combination of insomnia and SDB may be difficult to treat.70
The present study has multiple strengths, most notably the consideration of multiple sleep hygiene behaviors in a community sample with significant sleep disruption. Other notable strengths include the large sample and daily assessment of sleep hygiene behaviors over 14–35 days. Because health behaviors tend to cluster,44 an additional advantage was the consideration of both individual and cumulative positive and negative sleep hygiene behaviors. The study was also aided by the use of validated methods to ascertain the presence of insomnia and SDB. Another significant strength of the study was inclusion of PSG total sleep time as a covariate, as short sleep duration has typically been associated with poor health behaviors.73–75 Because prior studies that focused on short sleep did not account for sleep complaints or disorders, poor sleep hygiene behaviors may have been associated with unmeasured insomnia or SDB in these studies.
There are also several limitations to the current study. One such limitation was the reliance upon brief self-reports of sleep hygiene behaviors. The brevity of these assessments did not allow for additional detail into the various behaviors, such as the source of caffeine (e.g., tea, coffee, soda) or duration and type of exercise, and it is unknown how well these self-reported behaviors correlate with objective assessment in this sample. However, the brief self-report assessments allowed for daily reports of these behaviors and helped to minimize participant burden. An additional limitation was that we did not assess sleep hygiene recommendations related to the sleep environment (e.g., minimize noise, darken the room). The cross-sectional nature of the analyses represents another limitation. Because categorization of SDB and insomnia were determined concurrently with the assessment of sleep hygiene behaviors, we cannot determine whether poor sleep hygiene behaviors led to insomnia or SDB or vice versa. Additional limitations involve our assessments of SDB and insomnia. Because our SDB assessment was restricted to a single night, there is a possibility of SDB misclassification due to inter-night variability in AHI.76 The ISQ, used to define the presence of insomnia, is unable to distinguish between primary and secondary insomnia. Moreover, the ISQ has high specificity but low sensitivity relative to diary-assessed indicators of difficulty initiating and maintaining sleep,27 so some women may have been misclassified as not having insomnia. However, conducting clinical interviews for insomnia diagnosis was not feasible, and any possible ISQ misclassification would have biased the odds ratios toward the null. Finally, we were unable to examine the association between different types of insomnia complaints (e.g., difficulty initiating sleep, difficulty maintaining sleep) and sleep hygiene behaviors due to the high heterogeneity of insomnia complaints in our sample. Some sleep hygiene behaviors may impact only specific types of insomnia complaints (e.g., alcohol hastens sleep onset yet disrupts sleep maintenance40), and it would have been useful to examine these associations among midlife women.
Conclusions
In summary, we examined the frequency of self-reported individual positive and negative sleep hygiene behaviors in midlife women with insomnia or SDB, and we found several intriguing associations. Specifically, women with insomnia were less likely to report engaging in negative sleep hygiene behaviors than women without insomnia and, in particular, were less likely to report napping or consuming caffeine near bedtime. In contrast, women with SDB were less likely to be physically active than women without SDB, but no other differences in positive or negative sleep hygiene behaviors were observed. These data provide important insight on the sleep hygiene behaviors of midlife women with insomnia or SDB, and suggest that these behaviors should be considered when examining the health outcomes associated with these disorders. Future work should attempt to disentangle whether these sleep hygiene behaviors contribute to improved or worsened sleep quality among women with insomnia or SDB, and whether treatments that target individual sleep hygiene behaviors provide an efficient first-line treatment approach for improving sleep quality in adults with insomnia or SDB.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). The SWAN Sleep Study was funded by the NIH (Grants R01AG019360, R01AG019361, R01AG019362, and R01AG019363). Sleep data were processed with the support of UL1RR024153. Additional NIH grant support for Dr. Kline was provided by T32 HL082610 and K23 HL118318. Additional NIH grant support for Dr. Irish was provided by T32 MH019986. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical centers. University of Michigan, Ann Arbor: Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA: Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL: Howard Kravitz, PI 2009–present, Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser: Ellen Gold, PI; University of California, Los Angeles: Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY: Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark, NJ: Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI.
NIH program office. National Institute on Aging, Bethesda, MD: Winifred Rossi 2012: present; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD:– program officers.
Central laboratory. University of Michigan, Ann Arbor: Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating center. University of Pittsburgh, Pittsburgh, PA: Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA: Sonja McKinlay, PI 1995–2001.
Steering committee. Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
We thank the study staff at each site and all the women who participated in SWAN.
Author Disclosure Statement
Dr. Buysse has served as a paid consultant on scientific advisory boards for the following companies: Eisai, GlaxoSmithKline, Merck, Philips Respironics, Purdue Pharma, Sanofi-Aventis, and Takeda. He has also spoken at single-sponsored educational meetings for Sanofi-Aventis and Servier. Total fees from each of these sources was less than $10,000 per year. No competing financial interests exist for any of the other authors.
References
- 1.Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: A Society for Women's Health Research report. J Womens Health 2014;23:553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res 2000;9:35–42 [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med 2006;166:1262–1268 [DOI] [PubMed] [Google Scholar]
- 4.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J 2013;41:610–615 [DOI] [PubMed] [Google Scholar]
- 5.Chasens ER, Twerski SR, Yang K, Umlauf MG. Sleepiness and health in midlife women: Results of the National Sleep Foundation's 2007 Sleep in America poll. Behav Sleep Med 2010;8:157–171 [DOI] [PubMed] [Google Scholar]
- 6.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: The MONICA/KORA Augsburg cohort study. Sleep 2007;30:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall MH, Okun ML, Sowers MF, et al. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: The SWAN Sleep Study. Sleep 2012;35:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terauchi M, Hiramitsu S, Akiyoshi M, et al. Associations between anxiety, depression and insomnia in peri- and post-menopausal women. Maturitas 2012;72:61–65 [DOI] [PubMed] [Google Scholar]
- 9.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev 2003;7:215–225 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Samet J, Caffo B, Bankman I, Punjabi NM. Power spectral analysis of EEG activity during sleep in cigarette smokers. Chest 2008;133:427–432 [DOI] [PubMed] [Google Scholar]
- 11.Kline CE, Irish LA, Krafty RT, et al. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: The SWAN Sleep Study. Sleep 2013;36:1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landolt HP, Retey JV, Tonz K, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology 2004;29:1933–1939 [DOI] [PubMed] [Google Scholar]
- 13.Landolt HP, Roth C, Dijk DJ, Borbely AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. J Clin Psychopharmacol 1996;16:428–436 [DOI] [PubMed] [Google Scholar]
- 14.Owens JF, Buysse DJ, Hall M, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. J Clin Sleep Med 2010;6:330–335 [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia: An American Academy of Sleep Medicine review. Sleep 1999;22:1134–1156 [DOI] [PubMed] [Google Scholar]
- 16.Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev 2001;CD002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, III, Kupfer DJ, et al. Effects of diagnosis on treatment recommendations in chronic insomnia—a report from the APA/NIMH DSM-IV field trial. Sleep 1997;20:542–552 [DOI] [PubMed] [Google Scholar]
- 18.Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J 2000;16:909–913 [DOI] [PubMed] [Google Scholar]
- 19.Kim KS, Kim JH, Park SY, et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med 2012;8:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 2009;5:519–524 [PMC free article] [PubMed] [Google Scholar]
- 21.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med 1994;150:481–485 [DOI] [PubMed] [Google Scholar]
- 22.Haario P, Rahkonen O, Laaksonen M, Lahelma E, Lallukka T. Bidirectional associations between insomnia symptoms and unhealthy behaviours. J Sleep Res 2013;22:89–95 [DOI] [PubMed] [Google Scholar]
- 23.Jefferson CD, Drake CL, Scofield HM, et al. Sleep hygiene practices in a population-based sample of insomniacs. Sleep 2005;28:611–615 [DOI] [PubMed] [Google Scholar]
- 24.Cheek RE, Shaver JL, Lentz MJ. Variations in sleep hygiene practices of women with and without insomnia. Res Nurs Health 2004;27:225–236 [DOI] [PubMed] [Google Scholar]
- 25.Sowers MF, Crawford S, Sternfeld B, et al. SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and pathobiology. San Diego: Academic Press, 2000;175–188 [Google Scholar]
- 26.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN Sleep Study. Sleep 2009;32:73–82 [PMC free article] [PubMed] [Google Scholar]
- 27.Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med 2009;5:41–51 [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- 29.American Psychiatric Association Diagnostic and statistical manual of mental disorders (DSM-5), 5th ed. Washington, DC: American Psychiatric Association, 2013 [Google Scholar]
- 30.American Academy of Sleep Medicine International classification of sleep disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014 [Google Scholar]
- 31.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667–689 [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects (NIH Publication 204). Washington, DC: U.S. Government Printing Office, 1968 [Google Scholar]
- 33.Brand S, Kalak N, Gerber M, Kirov R, Puhse U, Holsboer-Trachsler E. High self-perceived exercise exertion before bedtime is associated with greater objectively assessed sleep efficiency. Sleep Med 15:1031–1036 [DOI] [PubMed] [Google Scholar]
- 34.Buman MP, Phillips B, Youngstedt SD, Kline CE, Hirshkowitz M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Med 2014;15:755–761 [DOI] [PubMed] [Google Scholar]
- 35.Manber R, Bootzin RR, Acebo C, Carskadon MA. The effects of regularizing sleep-wake schedules on daytime sleepiness. Sleep 1996;19:432–441 [DOI] [PubMed] [Google Scholar]
- 36.Lacks P. Behavioral treatment for persistent insomnia. New York: Pergamon Press, 1987 [Google Scholar]
- 37.Bootzin RR, Rider SP. Behavioral techniques and biofeedback for insomnia. In: Pressman MR, Orr WC, eds. Understanding sleep: The evaluation and treatment of sleep disorders. Washington, DC: American Psychological Association; 1997:315–338 [Google Scholar]
- 38.Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol 1996;271:R501–R510 [DOI] [PubMed] [Google Scholar]
- 39.Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med 1990;41:277–288 [DOI] [PubMed] [Google Scholar]
- 40.Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res 2013;37:539–549 [DOI] [PubMed] [Google Scholar]
- 41.Yules RB, Lippman ME, Freedman DX. Alcohol administration prior to sleep. The effect on EEG sleep stages. Arch Gen Psychiatry 1967;16:94–97 [DOI] [PubMed] [Google Scholar]
- 42.Jaehne A, Loessl B, Barkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev 2009;13:363–377 [DOI] [PubMed] [Google Scholar]
- 43.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–583 [DOI] [PubMed] [Google Scholar]
- 44.Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors. 2001 National Health Interview Survey. Am J Prev Med 2004;27:18–24 [DOI] [PubMed] [Google Scholar]
- 45.Lacks P, Rotert M. Knowledge and practice of sleep hygiene techniques in insomniacs and good sleepers. Behav Res Ther 1986;24:365–368 [DOI] [PubMed] [Google Scholar]
- 46.Kohn L, Espie CA. Sensitivity and specificity of measures of the insomnia experience: A comparative study of psychophysiologic insomnia, insomnia associated with mental disorder and good sleepers. Sleep 2005;28:104–112 [DOI] [PubMed] [Google Scholar]
- 47.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med 2010;11:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCrae CS, Rowe MA, Dautovich ND, et al. Sleep hygiene practices in two community dwelling samples of older adults. Sleep 2006;29:1551–1560 [DOI] [PubMed] [Google Scholar]
- 49.Harvey AG. Sleep hygiene and sleep-onset insomnia. J Nerv Ment Dis 2000;188:53–55 [DOI] [PubMed] [Google Scholar]
- 50.Yang CM, Lin SC, Hsu SC, Cheng CP. Maladaptive sleep hygiene practices in good sleepers and patients with insomnia. J Health Psychol 2010;15:147–155 [DOI] [PubMed] [Google Scholar]
- 51.Youngberg MR, Karpov IO, Begley A, Pollock BG, Buysse DJ. Clinical and physiological correlates of caffeine and caffeine metabolites in primary insomnia. J Clin Sleep Med 2011;7:196–203 [PMC free article] [PubMed] [Google Scholar]
- 52.Verwimp J, Ameye L, Bruyneel M. Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath 2013;17:1039–1046 [DOI] [PubMed] [Google Scholar]
- 53.Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res 2011;2011:751819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc 2003;35:1088–1092 [DOI] [PubMed] [Google Scholar]
- 55.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomised controlled trial. Sleep Med 2009;10:1056–1058 [DOI] [PubMed] [Google Scholar]
- 56.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 2011;34:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kline CE, Ewing GB, Burch JB, et al. Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. J Clin Sleep Med 2012;8:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenthal L, Bishop C, Guido P, et al. The sleep/wake habits of patients diagnosed as having obstructive sleep apnea. Chest 1997;111:1494–1499 [DOI] [PubMed] [Google Scholar]
- 59.Aurora RN, Crainiceanu C, Caffo B, Punjabi NM. Sleep-disordered breathing and caffeine consumption: results of a community-based study. Chest 2012;142:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bardwell WA, Ziegler MG, Ancoli-Israel S, et al. Does caffeine confound relationships among adrenergic tone, blood pressure and sleep apnoea? J Sleep Res 2000;9:269–272 [DOI] [PubMed] [Google Scholar]
- 61.Robinson GV, Pepperell JC, Davies RJ, Stradling JR. Caffeine levels following treatment of obstructive sleep apnoea. Thorax 2003;58:801–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath 2001;5:167–172 [DOI] [PubMed] [Google Scholar]
- 63.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 64.Theorell-Haglow J, Berne C, Janson C, Lindberg E. The role of obstructive sleep apnea in metabolic syndrome: a population-based study in women. Sleep Med 2011;12:329–334 [DOI] [PubMed] [Google Scholar]
- 65.Kakinuma M, Takahashi M, Kato N, et al. Effect of brief sleep hygiene education for workers of an information technology company. Ind Health 2010;48:758–765 [DOI] [PubMed] [Google Scholar]
- 66.Chen PH, Kuo HY, Chueh KH. Sleep hygiene education: Efficacy on sleep quality in working women. J Nurs Res 2010;18:283–289 [DOI] [PubMed] [Google Scholar]
- 67.Schoicket SL, Bertelson AD, Lacks P. Is sleep hygiene a sufficient treatment for sleep-maintenance insomnia? Behav Ther 1988;19:183–190 [Google Scholar]
- 68.Morita E, Miyazaki S, Okawa M. Pilot study on the effects of a 1-day sleep education program: influence on sleep of stopping alcohol intake at bedtime. Nagoya J Med Sci 2012;74:359–365 [PMC free article] [PubMed] [Google Scholar]
- 69.Hauri PJ. Consulting about insomnia: A method and some preliminary data. Sleep 1993;16:344–350 [DOI] [PubMed] [Google Scholar]
- 70.Ong JC, Crawford MR. Insomnia and obstructive sleep apnea. Sleep Med Clin 2013;8:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev 2009;13:287–293 [DOI] [PubMed] [Google Scholar]
- 72.Krakow B, Romero E, Ulibarri VA, Kikta S. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: A pilot study of subjective and objective causes. Sleep 2012;35:1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoenborn CA, Adams PF. Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United States, 2004–2006. NCHS Health E-Stats 2008. Available at: www.cdc.gov/nchs/data/hestat/sleep04-06/sleep04-06.htm Accessed August22, 2012
- 74.Strine TW, Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med 2005;6:23–27 [DOI] [PubMed] [Google Scholar]
- 75.Chaput JP, McNeil J, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with greater alcohol consumption in adults. Appetite 2012;59:650–655 [DOI] [PubMed] [Google Scholar]
- 76.Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath 2009;13:221–226 [DOI] [PubMed] [Google Scholar]