Abstract
The use of genomic profiling in acute myeloid leukemia (AML) has led to an improved understanding of disease pathogenesis. Genomic profiling has given rise to fundamental observations about the biology of AML and has served to better define clinical outcomes for patients based on somatic mutational status. As additional mutations are identified with a known or postulated role in AML pathogenesis, the challenge ahead will be learning how to integrate these findings into clinical practice in such a way that they have a meaningful impact on patient care, and ultimately on patient outcomes. Potential goals include utilizing genomic information for refined risk-stratification and clinical decision-making, and to identify genetic lesions that guide the use of molecularly-targeted therapies. The development of next-generation sequencing technologies has made genomic profiling a viable option for use in clinical practice, as it can provide robust, high-coverage sequencing data for multiple genes in one assay, within a clinically reasonable timeframe. Here, we discuss recent candidate gene sequencing studies, the development of prognostic models based on these studies, and the current and potential future uses of next-generation sequencing technologies in making treatment decisions for patients with AML.
INTRODUCTION
The standard treatment for patients with acute myeloid leukemia (AML) has not changed substantively over the past 40 years, with the notably exception of stem-cell transplantation. The use of induction regimens comprised of seven-day continuous infusion cytarabine and three-day daunorubicin was first described in 1973 [1]. This initial regimen remained largely unchanged until 2009 when a survival benefit was demonstrated for patients under 60 treated with dose- intensified daunorubicin [2]. In this context, it is not surprising that patient outcomes have improved only marginally, much of which can be attributed to improved supportive care [3]. However, the strategies for assignment of AML patients to specific sub-types at diagnosis and stratification to prognostic sub-groups are rapidly evolving. In addition, numerous recurrent genetic lesions have been described over the past 20 years, some of which may be amenable to pharmacologic inhibition with emerging therapeutic modalities.
The advent of cytogenetics, and subsequently the development of DNA resequencing technologies, have provided seminal insights into the biology of AML, and led to evolution in our understanding of AML pathogenesis. Initial genetic insights led to the development of the “two hit” model of leukemogenesis, which postulated that the genetic events causative of AML occur in two broad classes of genes with distinct roles in leukemic transformation. These include mutations in type I genes such as FLT3, JAK2 and RAS, which encode signaling effectors that confer a proliferation and survival advantage to leukemic cells. The second class of mutations are in type II genes (RUNX1, CEBPA, PML/RARA), and mutations in these genes are thought to impair the normal myeloid differentiation cascade from stem cell to mature granulocytes [4]. Recent candidate gene sequencing efforts have challenged this concept by revealing that not all patients with AML have mutations in type I or type II genes, suggesting that additional pathways are involved in AML pathogenesis. For example, alterations in genes involved in epigenetic modifications have been identified in AML [5], and indeed subsequent work has revealed distinct patterns of epigenomic alteration in AML subtypes with specific somatic mutational spectra [6].
It has also long been recognized that the outcome of patients with AML is heterogeneous, and that some patients derive benefit from more intensive therapy, including allogeneic stem cell transplant, while others do not. Indeed, many studies have shown that cytogenetic alterations and a subset of somatic mutations can be used to predict overall patient outcomes, as well as to guide decisions regarding consolidation therapy. With the advent of next generation sequencing (NGS) technologies, more disease–related alleles have been revealed in AML patients. Recent efforts have focused are integrating these findings into clinically-useful models to guide therapy and to predict prognosis.
CONVENTIONAL CYTOGENETICS AND MOLECULAR GENETICS
The prognostication of AML has historically been based on cytogenetic data, which can generally separate patients into favorable, intermediate and unfavorable risk groups. Patients with t(8;21) or inv(16) are classified as having good risk disease, whereas patients with monosomy of chromosomes 5, 7 or complex karyotype are considered to have poor risk disease [7], although there is a lack of consensus regarding the full spectrum of cytogenetic alterations that confer an adverse prognosis [3]. Allogeneic stem cell transplant has been historically reserved for unfavorable and a subset of intermediate risk patients [8]. However, only 45% of patients have a clonal cytogenetic abnormality, making the choice of optimal consolidation therapy for intermediate risk patients, most of whom have a normal karyotype, a continuing challenge.
Our understanding of prognostication in intermediate risk AML changed substantively after a landmark study by Schlenk and colleagues, who sought to further refine prognosis for patients with normal karyotype AML (NK-AML) through somatic mutational analysis. The investigators compared the outcome of 800 patients with NK-AML less than 60 years old who had been treated with allogeneic stem cell transplantation (SCT) or with consolidation chemotherapy. The authors sequenced five genes involved in leukemogenesis: FMS related tyrosine kinase 3 (FLT3), Nucleophosmin 1 (NPM1), CCAAT/enhancer binding protein alpha (CEBPA), the neuroblastoma RAS viral oncogene homolog (NRAS) and the runt-related transcription factor 1 gene (RUNX1). Two groups of patients were identified with a more favorable outcome: patients with mutations in CEBPA and patients with NPM1 mutations without a concurrent FLT3-ITD mutation. In addition, they demonstrated that allogeneic SCT improved outcome only in patients with FLT3-ITD mutations and in patients with the combination of unmutated NPM1, FLT3 and CEBPA [9]. This was the first report of molecular genetic abnormalities being used to risk-strategy AML patients and has served as the paradigm for subsequent studies in the field.
IDENTIFICATION OF NOVEL ALLELES IN AML
The advent of whole exome and whole genome sequencing has allowed for the discovery of novel recurrent mutations in AML. Ley, et al, in 2008 reported the whole genome sequence of a patient with NK-AML, which uncovered eight new mutations in addition to NPM1 and FLT3-ITD. Notably, these mutations were present in both diagnostic and relapse samples. This study served as proof-of-principle that whole genome sequencing could be used to interrogate the mutational spectrum of AML and, in a follow-up study, to evaluate clonal evolution of the disease [10] [11].
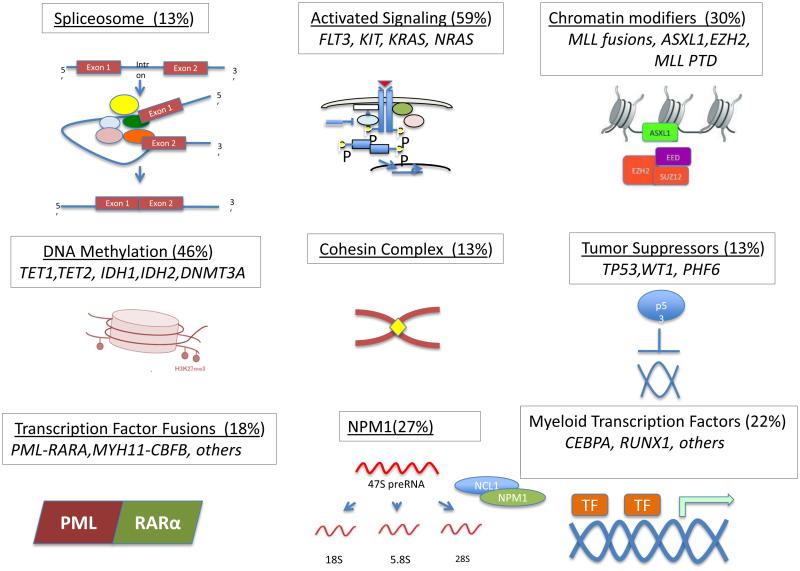
Subsequent work using similar approaches led to the discovery of recurrent IDH1 [12], and DNMT3A mutations in AML [5, 13], which have been biologically validated. More recent efforts from the Cancer Genome Atlas Research Network utilizing whole-genome/exome sequencing of 200 AML patients demonstrated 23 genes with a higher than expected mutational prevalence. Among these were genes known to have a role AML, such as NPM1 and FLT3, as well as genes encoding factors in novel pathways, including RNA splicing (U2AF1), epigenetic modification (EZH2) and members of the cohesin complex (SMC1A, SMC3), the roles of which are still being evaluated in AML pathogenesis (Figure 1).
Figure 1.
Panoramic view of genetic event events involved in the pathogenesis of AML. Nine different functional categories have been identified for each mutated pathway. Representative recurrently mutated genes in each pathway are depicted. The frequency of mutations detected in AML samples for each pathway is provided.
Candidate gene sequencing studies have also identified recurrent somatic mutations, some of which were first described in other hematologic malignancies. Mutations in PHF6, a Plant Homeodomain (PHD) finger-containing protein, have been reported in 3% of AML patients [14]. ASXL1, an epigenetic regulator, is mutated in 5-15% of AML patients [15-18]. TET2 mutations, initially described in MDS and MPNs [15] [19], have been found to occur in approximately 10% of AML patients [17].
These studies facilitated the discovery of new genes involved in leukemia pathogenesis and can also be used to refine prognosis. Importantly, compared to solid tumors, the mutational burden in AML is significantly lower, with an average of 13 somatic coding mutations per AML genome. Investigation into the relative contributions of mutations (“driver mutations” and “passenger mutations”) remains an important area of investigation. In this regard, Welch and colleagues [20] reported on whole genome sequencing efforts comparing FAB M1 with M3 AML in an effort to uncover potential driver mutations. This study revealed that many mutations present in AML genomes are likely ancestral to leukemia initiation (i.e, present in the hematopoietic cell that ultimately gives rise to the leukemic clone) and do not contribute directly to AML pathogenesis. Thus, the mutations which are rate-limiting in leukemic transformation may in fact be quite limited in scope. Further delineation of the relative contributions to pathogenesis of variant alleles will require validation in animal models (described by Attar in this issue) and is an ongoing endeavor.
PROGNOSTICATION USING INTEGRATED MOLECULAR GENETICS
As the number of new recurrent mutations reported in the literature has increased over the last few years, the challenge of how to incorporate these findings into the framework established by Schlenk, et al., has become increasingly important. Several groups have performed either single or multi-gene prognostic analyses, sometimes with conflicting results. Such analyses are often confounded by variability in patient characteristics and treatments and the lack of a validation cohort. Further, many mutations occur at reported frequencies of less than 10%. As such, each mutation requires large cohort sizes to assess independent prognostic relevance.
In an attempt to integrate genomic findings with clinical variables and cytogenetics to determine the prognostic relevance of multiple genes in a large homogeneously-treated and well clinically-annotated cohort of AML patients, Patel, et al., performed mutational profiling of 398 patients treated on the ECOG 1900 study [20]. ECOG 1900 was a phase III study which randomized newly diagnosed AML patients between 17 and 60 years of age to induction with daunorubicin at a dose of either 45mg/m2 or 90mg/m2, both with seven days of cytarabine [22]. They performed resequencing of 18 genes known to be recurrently mutated in AML in this cohort of patients who were randomized to induction chemotherapy with standard dose or intensified dose daunorubicin. The study demonstrated that in patients with unfavorable cytogenetics and in patients with favorable cytogenetics, prognosis was not impacted by the presence or absence of specific mutations, suggesting that the driver fusion genes govern overall prognosis in favorable risk and adverse risk AML.
Consistent with previous observations, 63% of the patient population had intermediate risk AML as defined by cytogenetics, and this patient subset demonstrated substantial genetic heterogeneity with nine different genotypes. Mutations in ASXL1 and PHF6, as well as FLT3- ITD and partial tandem duplication of MLL (MLL-PTD) mutations, were associated with reduced survival. Conversely, the presence of IDH2 R140Q mutations was associated with improved survival. This study refined the previously reported outcomes for intermediate-risk patients with NPM1 mutations who lacked FLT3-ITD mutations: specifically, patients with concurrent NPM1 and IDH2/IDH1 mutations had an overall survival of greater than 80% at 2 years, suggesting that this subgroup of patients will have a good outcome with high dose chemotherapy and without stem cell transplantation. Patients with NPM1 mutations but without IDH or FLT3-ITD mutations had an intermediate prognosis, suggesting that these patients may still need to be considered for more intense consolidative approaches, such as allogeneic SCT.
Most importantly, this study defined two subsets of patients with adverse outcomes based on mutational profiling. FLT3-ITD negative patients who had mutant TET2, ASXL1, PHF6 or MLL-PTD had a very poor outcome, consistent with previous reports from single gene studies. In addition to the relatively poor outcome of FLT3-ITD mutant patients, FLT3-ITD mutant patients with concurrent mutations in TET2, DNMT3A, an MLL-PTD, or trisomy 8 had worse outcome (3-year OS of 14.5%), compared to patients with FLT3-ITD mutations without these concurrent mutations (3-year OS of 35.2%). Patients with FLT3-ITD and CEBPA mutations had a better outcome, with a 3-year survival of 42%. These data suggest that although FLT3-ITD mutations predict adverse outcome, the prognostic relevance can be further refined with additional mutational data.
These results informed the development of a model based on which treatment decisions can be made (Table 1). Indeed, although there is convergent data on the prognostic implications of many of the mutations described in this study, such as FLT3-ITD [23] and DNMT3A [22], divergent outcomes have been reported for patients with IDH mutations. Several groups have reported that IDH1/2 mutations confer a worse prognosis in patients with NPM1 mutations without concomitant FLT3-ITD [23-25], whereas others have reported a favorable prognostic outcome of IDH2 mutations, particularly in the presence of concurrent NPM1 mutations [26]. It is important to note that treatment type, intensity, and age are likely confounding variables from the different studies; most studies in younger adults treated with aggressive therapy show that NPM1/IDH-mutant disease is chemosensitive and favorable, whereas studies in older adults suggest a worse prognosis in the elderly setting. As such, further validation in different prospective patient cohorts is needed to confirm these findings.
Table 1.
Revised AML risk stratification in patients and treatment recommendations based on integrated mutational profiling [20].
|
| ||||
|
Cytogenetic
Classification |
Mutations | Risk Profile |
Consolidation
Therapy |
|
|
| ||||
| Favorable | Any | Consider post remission chemotherapy alone |
||
|
|
Favorable | |||
| Normal karyotype or intermediate risk cytogenetic |
FLT3-ITD- negative |
Mutant NPM1 and IDH1 or IDH2 |
||
|
FLT3-ITD- negative |
Wild type ASXL1, MLL- PTD, PHF6, and TET2 |
Intermediate | Consider allo HSCT |
|
|
FLT3-ITD- negative or positive |
Mutant CEBPA | |||
| FLT3-ITD-positive | Wild type MLL-PTD, TET2
and DNMT3A |
|||
|
FLT3-ITD- negative |
Mutant TET2, MLL-PTD, ASXL1, or PHF6 |
|||
| FLT3-ITD-positive | Mutant TET2, MLL-PTD, or DNMT3A without mutant CEBPA |
Unfavorable | Consider allo HSCT |
|
|
|
||||
| Unfavorable | Any | |||
|
| ||||
Several other groups have generated prognostic models by integrating cytogenetic and molecular data. Grossman, et al., [27] developed a hierarchical model that identified five distinct prognostic subgroups: very favorable (PML-RARA, CEBPA double mutation), favorable (t(8;21), inv(16), NPM1 mutant without FLT3-ITD), intermediate (no mutations present in other categories), unfavorable (MLL-PTD, RUNX1 mutant, ASXL1 mutant), and very unfavorable (TP53 mutant). The European LeukemiaNet has published treatment recommendations that take into account molecular and cytogenetic data [28]. Four prognostic groups were identified by this analysis (Table 2). Subsequent work has validated the prognostic significance of these subgroups, but has also demonstrated that the percentage of younger (defined as age <60) versus older patients differ amongst these subgroups and may represent a confounding variable [29]. These studies raise the question of how patient age should be incorporated into prognostic models. Recent work by Pastore and colleagues sought to develop a prognostic model for patients with NK-AML for both overall and relapse-free survival using clinical data, patient characteristics (including age and performance status) and mutational status (CEBPA, NPM1, FLT3-ITD). This work established a scoring system that divides patients with NK-AML into three prognostic groups based on the above factors and can be applied to patients of all ages [32].
Table 2.
| Genetic Group |
Cytogenetics | Molecular Genetics |
Age <60 | Age ≥ 60 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CR | DFS (years) |
OS (years) |
CR | DFS (years) |
OS (years) |
|||
| Favorable | t(8;21) inv(16) t(16;16) |
96% | 5.5 | 11.5 | 83% | 1.1 | 1.6 | |
|
| ||||||||
| Normal karyotype |
Mutated NPM1
without FLT3-ITD or mutated CEBPA |
|||||||
|
| ||||||||
| Intermediate- I |
Normal karyotype |
Mutated NPM1 and FLT3-ITD; or, Wildtype NPM1 and FLT3-ITD; or, Wildtype NPM1 without FLT3-ITD |
76% | 0.8 | 1.2 | 61% | 0.6 | 0.9 |
|
| ||||||||
| Intermediate- II |
t(9;11) Cytogenetic abnormalities not classified as favorable or adverse |
79% | 1.2 | 2.1 | 63% | 0.7 | 0.9 | |
|
| ||||||||
| Adverse | Inv(3) or t(3;3) t(6;9) t(v;11) −5 or del(5q) −7 Abn(17p) Complex karyotype |
50% | 0.6 | 0.8 | 39% | 0.5 | 0.5 | |
While it is clear that substantial progress in the ability to predict outcome and to use this information to guide therapy has been achieved over the last 20 years, reconciling the different prognostic models from different studies into a clinically robust approach applicable to patients in routine clinical practice remains a significant challenge. For example, recurrent mutations in splicing factors [33, 34], epigenetic modifiers [35], RAS pathway components [36, 37], and the cohesin complex [20, 38] have been described in secondary AML, de novo AML [39], and are currently not integrated into the prognostic models described above (Table 3). This is further complicated by the continued identification of novel pathogenic mutations of uncertain prognostic significance, heterogeneity in patient treatment across cohorts (which may be expected to increase as more clinical trial data are reported), intra- and inter-patient heterogeneity, and variability in sequencing technologies employed in different studies. Meta-analyses in which robust data from different cohorts are assessed en bloc are needed to develop more robust, universally applicable prognostic models.
Table 3.
Recurrent alterations in AML requiring integration into large-scale prognostic models
USING MOLECULAR GENETICS TO GUIDE THERAPY
To date, molecular and cytogenetic data have largely been used to define prognosis and to guide the use of allogeneic SCT as a consolidative strategy. However, molecular data are increasingly being used to gauge response to therapy and, most importantly, to guide novel targeted therapy. The initial report from the ECOG 1900 study demonstrated that dose-intensified daunorubicin improved outcomes overall in younger adults with AML, but did not define specific subsets that benefit vs. once that do not benefit from more intensified induction therapy. However, post hoc genomic analysis of the ECOG 1900 trial determined that patients who had mutations in NPM1, DNMT3A or MLL fusions derived benefit from daunorubicin dose- intensification, whereas patients with other genotypes did not. This suggests that molecular evaluation at baseline might be useful to identify patients most likely to benefit from dose-intensification and to identify patients who are unlikely to derive benefit so that they can be spared the risks of cardiotoxicity and increased myelosuppression.
The challenge will be to extend this to other AML therapies. The hypomethylating agents azacytidine and decitabine are widely used, particularly for older patients or patients deemed to be unfit for standard induction chemotherapy. Anecdotal reports have indicated that DNMT3A mutations and TET2 mutations may affect response to decitabine [40] and azacytidine, respectively [41]. Although these findings will need to be confirmed in randomized, prospective studies, they highlight the potential to use genomic profiling to guide initial therapies for patients with AML. The identification and characterization of novel recurrent mutations in AML provide an opportunity to use this information to design rationally-targeted therapies for AML. To date, this has been most pronounced with the development of FLT3 inhibitors, several of which have been evaluated in early phase trials [42] [43-46]. Novel inhibitors of MLL (NCT01684150), IDH (NCT01915498), and BET bromodomains (NCT01943851), as well as inhibitors of downstream mediators of the RAS pathway (NCT01449058), are currently in clinical trials for patients with AML. It will be important to ascertain whether the presence or absence of additional mutations affects the response to these targeted therapies, and if complex genotypes can be used to inform combinational therapies for AML patients moving forward.
IMPLEMENTATION OF MASSIVELY PARALLEL SEQUENCING IN THE CLINICAL SETTING
Testing for mutations in NPM1, CEBPA, KIT and FLT3-ITD has been incorporated into daily clinical practice for risk stratification and is largely considered standard of care. The implementation of analysis of larger gene sets has been limited thus far by: 1) Lack of studies validating the use of the newer mutations in large numbers of uniformly-treated patients; 2) The lack of incorporation of other clinical variables or biomarkers into molecular-based risk models, 3) The limited number of studies which demonstrate that biomarkers impact therapeutic decisions, including the use of SCT or chemotherapy, and 4) the lack of availability of fast and simple assays to apply genomic profiling for daily clinical use [47].
As discussed above, prognostic models continue to evolve and the ideal set of molecular markers needed for accurate prognostication in the clinic remains uncertain. The implementation of newer sequencing technologies in the clinical setting also remains a challenge (reviewed by Graubert and Stone in this issue). Use of high throughput technologies allows the identification of large numbers of genetic alterations that can help predict outcome and serve as potential drug targets. However, the bioinformatics infrastructure and expertise needed to rapidly analyze sequencing data limits the general applicability of whole genome sequencing in the clinical setting at the current time. Another constraint is the precision and specificity of clinical NGS assays in development. For example, gene size, and “GC” content can hinder the applicability of the assay, and sequencing depth needs to be high enough to ensure that mutations are not missed and artifacts are excluded in the entire coding sequence of all clinically relevant genes [48].
Many academic institutions have implemented and developed clinical sequencing technologies that are currently used in the clinic. As well, broader commercial platforms, often involving hundreds of genes, are also gradually being incorporated into the clinic. Most recently, RNA-based platforms have been developed. These techniques allow for high confidence detection of translocations and other genomic rearrangements not easily detected with capture- based sequencing (reviewed by Mardis in this issue). Next-generation Sequencing Standardization Working Group Guidelines [49, 50] have been proposed to deal with the plethora of new sequencing platforms that are emerging, and these will have to be modified to deal with the rapidly evolving exigencies of clinical sequencing.
CONCLUSION
The advent of new techniques in molecular genetics and, specifically, of NGS is rapidly expanding our knowledge base of mechanisms of leukemogenesis and the nature of clonal evolution that occurs during the natural history of AML and in response to therapy. Patients with AML can be stratified into different risk subgroups according to genetic data which segregate patients into subgroups with biologic, prognostic, and therapeutic relevance. The available technologies provide a new window of opportunity to identify the patient population who will and will not benefit from standard treatments. In addition, the identification of novel mutations continues to inform the development of targeted therapies, many of which are currently in clinical trials.
Important challenges must be overcome to fully realize the utility of molecular genetics to improve clinical practice. The question of how to best utilize molecular data to guide patient therapy is a challenge which is not completely elucidated. Although several prognostic models have been developed to deal with such questions, important limitations on the applicability of these models remain. Further, these models continue to evolve as more pathogenic mutations are uncovered in AML.
The implementation of sequencing technology in the clinic remains fraught with challenges. These relate to the cost of these technologies, appropriate bioinformatics support, and the balance between the urgency to initiate AML therapy and the turnaround time required to generate a report. Indeed, the last problem has important clinical implications, as the identification of mutations at baseline has the potential to not only alter long term patient management decisions such as whether to use SCT or chemotherapy following remission but, more urgently, may be used to tailor induction therapy. Moreover, rapid, robust genomic profiling will allow accrual to appropriate clinical trials utilizing targeted therapy most appropriate to the genotype of each patient’s disease. As successful treatment of adults with AML represents a major medical challenge, the incorporation of molecular genetics in to risk stratification and selection of targeted therapies for these patients could have a significant impact on response to treatment and long-term outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yates JW, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485–8. [PubMed] [Google Scholar]
- 2.Fernandez HF, Yao X, et al. Anthracycline Dose Intensification in Acute Myeloid Leukemia. N Engl J Med. 2009;361:1249–59. doi: 10.1056/NEJMoa0904544. S.Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland DG. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1538–42. doi: 10.1182/blood-2002-02-0492. G.J.D., [DOI] [PubMed] [Google Scholar]
- 5.Ley TJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–33. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa ME, et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell. 2010;17(1):13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 8.Koreth J, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama. 2009;301(22):2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlenk RF, Krauter J, et al. Mutations and Treatment Outcome in Cytogenetically Normal Acute Myeloid Leukemia. N Engl J Med. 2008;358(18):1079–89. doi: 10.1056/NEJMoa074306. D.K., [DOI] [PubMed] [Google Scholar]
- 10.Ley TJ, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mardis ER, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–66. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan XJ, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43(4):309–15. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 13.Van Vlierberghe P, et al. PHF6 mutations in adult acute myeloid leukemia. Leukemia. 2011;25(1):130–134. doi: 10.1038/leu.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delhommeau F, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 15.Metzeler KH, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood. 2011;118(26):6920–9. doi: 10.1182/blood-2011-08-368225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Wahab O, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–7. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelsi-Boyer V, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145(6):788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- 18.Langemeijer SM, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41(7):838–42. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 19.Welch JS, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel JP, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlenk RF, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 22.Thol F, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29(21):2889–96. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- 23.Paschka P, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 24.Abbas S, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 2010;116(12):2122–6. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 25.Boissel N, et al. Prognostic impact of isocitrate dehydrogenase enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol. 2010;28(23):3717–23. doi: 10.1200/JCO.2010.28.2285. [DOI] [PubMed] [Google Scholar]
- 26.Green CL, et al. The prognostic significance of IDH2 mutations in AML depends on the location of the mutation. Blood. 2011;118(2):409–12. doi: 10.1182/blood-2010-12-322479. [DOI] [PubMed] [Google Scholar]
- 27.Grossmann V, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012;120(15):2963–72. doi: 10.1182/blood-2012-03-419622. [DOI] [PubMed] [Google Scholar]
- 28.Dohner H, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 29.Mrozek K, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–23. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastore F, et al. Combined Molecular and Clinical Prognostic Index for Relapse and Survival in Cytogenetically Normal Acute Myeloid Leukemia. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.52.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzeler KH, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26(5):1106–7. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itzykson R, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–52. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 33.Patel JP, Levine RL. How do novel molecular genetic markers influence treatment decisions in acute myeloid leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:28–34. doi: 10.1182/asheducation-2012.1.28. [DOI] [PubMed] [Google Scholar]
- 34.MacConaill LE. Existing and emerging technologies for tumor genomic profiling. J Clin Oncol. 2013;31(15):1815–24. doi: 10.1200/JCO.2012.46.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gargis AS, et al. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nat Biotechnol. 2012;30(11):1033–6. doi: 10.1038/nbt.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frampton GM, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotech. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]