Abstract
Recent epidemiological evidence suggests that effects of cardiovascular risk factors may vary depending on sex and age. In this study, we assessed the associations of metabolic syndrome (MetS) with sarcopenia and its components in older adults, and examined whether the associations vary by sex and age. We also tested if any one of the MetS components could explain the associations. We conducted a cross-sectional analysis of the baseline data from the cohort study conducted in Kashiwa city, Chiba, Japan in 2012 which included 1971 functionally-independent, community-dwelling Japanese adults aged 65 years or older (977 men, 994 women). Sarcopenia was defined based on appendicular skeletal muscle mass, grip strength and usual gait speed. MetS was defined based on the National Cholesterol Education Program’s Adult Treatment Panel-III criteria. The prevalence of sarcopenia was 14.2% in men and 22.1% in women, while the prevalence of MetS was 43.6% in men and 28.9% in women. After adjustment for potential confounders, MetS was positively associated with sarcopenia in men aged 65 to 74 years (odds ratio 5.5; 95% confidence interval 1.9–15.9) but not in older men or women. Among the sarcopenia components, MetS was associated with lower muscle mass and grip strength, particularly in men aged 65 to 74 years. The associations of MetS with sarcopenia and its components were mainly driven by abdominal obesity regardless of sex or age. In conclusion, MetS is positively associated with sarcopenia in older men. The association is modified by sex and age, but abdominal obesity is the main contributor to the association across sex and age.
Introduction
Metabolic syndrome (MetS) is a constellation of cardiovascular risk factors which include abdominal obesity, dyslipidemia, hypertension and elevated glucose [1]. Insulin resistance and chronic inflammation are considered central mechanisms responsible for MetS [2] and inextricably correlate with each other to exert detrimental metabolic effects and lead to cardiovascular morbidity and mortality [3]–[5]. Accumulating epidemiological evidence suggests that both insulin resistance and chronic inflammation cause adverse effects on skeletal muscle. Diabetes, or even insulin resistance without diabetes, is associated with greater declines in skeletal muscle mass and strength [6], [7]. A link between inflammation and muscle weakness has been reported in several studies [8], [9]. Therefore, we postulate that MetS can accelerate age-related loss of muscle mass and strength, leading to the development of sarcopenia, a syndrome characterized by loss of skeletal muscle mass and function with a risk of physical disability [10]. Indeed, recent studies showed that MetS is associated with physical capacity impairment and increased risk of developing physical and functional disabilities [11]–[13].
Several recent studies have suggested that the effects of MetS may vary depending on age and sex. Cardiovascular risk factors, whose adverse effects have been established in younger people, may have different impacts in the elderly or frail population. Obesity did not seem to be a risk factor for increased mortality in elderly hospitalized patients with or without diabetes [14], [15]. Elevated blood pressure was associated with lower mortality risk in physically frail elderly adults who could not walk 20 feet [16]. MetS was associated with lower probability of prevalent and incident functional disability in older adults [17]. The association between MetS and cardiovascular events was observed only in patients younger than 75, but not in patients aged 75 or over [18]. With regard to sex-related differences in the effects of MetS, MetS was associated with lower muscle strength in elderly men but not in elderly women [19]. However, data on sex- or age-related differences in the effect of MetS on sarcopenia are still scarce.
In the present study, we assessed the associations of MetS with sarcopenia and its components in functionally-independent community-dwelling Japanese older adults, and examined whether the associations were modified by sex or age. We hypothesized that MetS is positively associated with sarcopenia and its components, and that the associations are more pronounced in relatively young men. We also examined whether any of the individual MetS components could explain the associations and if the same MetS components contributed to the associations across sex and age.
Methods
Subjects
The Kashiwa study is a prospective cohort study designed to characterize the biological, psychosocial and functional changes associated with aging in a community-based cohort of 2044 older adults (1013 men, 1031 women). Those aged 75 and older accounted for 36.3% of men and 35.0% of women. The sampling and data collection process has been described in detail elsewhere [20]. Briefly, the inclusion criteria were age equal to or older than 65 years and functional independence (i.e., not requiring nursing care provided by long-term care insurance). The subjects were randomly selected from the resident register of Kashiwa city, Chiba, Japan, enrolled in 2012, and followed annually. The current study is a cross-sectional analysis of the Kashiwa study baseline data. Seventy three subjects who did not undergo bioimpedance analysis (BIA), usual gait speed or hand grip strength measurements were excluded, leaving an analytic sample of 1971 older adults (977 men, 994 women). Those excluded from the analysis were older compared to those included in the analysis (mean age 75.9 years vs. 72.9 years, p = 0.001), but did not significantly differ with respect to other characteristics including sex, height, weight, and prevalence of MetS.
The study was approved by the ethics committee of the Graduate School of Medicine, The University of Tokyo. All subjects provided written informed consent.
Definition of Sarcopenia
We followed the recommendations of the European Working Group on Sarcopenia in Older People (EWGSOP) for the diagnostic definition of sarcopenia [10]. The proposed diagnostic criteria required the presence of low muscle mass plus the presence of either low muscle strength or low physical performance. Muscle mass was measured by BIA using an Inbody 430 machine (Biospace, Seoul, Korea). Appendicular skeletal muscle mass (ASM) was derived as the sum of the muscle mass of the four limbs [10]. ASM was then normalized by height in meters squared to yield skeletal muscle mass index (SMI) (kg/m2). SMI values lower than two standard deviations below the mean values of young male and female reference groups were classified as low muscle mass (SMI <7.0 kg/m2 in men, <5.8 kg/m2 in women) [21]. Muscle strength was assessed by hand grip strength, which was measured using a digital grip strength dynamometer (Takei Scientific Instruments, Niigata, Japan). Hand grip strength values in the lowest quintile were classified as low muscle strength in this study (cutoff values: 30 kg for men, 20 kg for women). Physical performance was assessed by usual gait speed. Subjects were instructed to walk over an 11-meter straight course at their usual speed. Usual gait speed was derived from 5 meters divided by the time in seconds spent in the middle 5 meters (from the 3-meter line to the 8-meter line) [22]. Usual gait speed values in the lowest quintile were classified as low physical performance in the current study (cutoff values: 1.26 m/s for each sex).
Definition of metabolic syndrome
MetS was defined based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria [1]. The presence of any three of the following five abnormalities constitutes a diagnosis of MetS: (i) abdominal obesity; (ii) elevated triglycerides (TG) with fasting plasma triglycerides ≥150 mg/dL; (iii) low high density lipoprotein cholesterol (HDL-C) with fasting HDL-C <40 mg/dL in men and <50 mg/dL in women; (iv) elevated blood pressure with systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg; (v) elevated fasting plasma glucose with fasting plasma glucose ≥100 mg/dL. Abdominal obesity was defined by waist circumference using the thresholds recommended by the Japanese Obesity Society (≥85 cm in men and ≥90 cm in women) [1].
Waist circumference was measured at the umbilical level using a measuring tape with the subject in an upright position. Blood pressure was measured using a standard technique with an HEM-7080IT automated measuring device (Omron Co., Tokyo, Japan). Blood samples were obtained after an overnight fast. Total cholesterol, HDL-C and TG were analyzed by enzymatic methods using a JCA-BM8060 automated analyzer (Japan Electron Optics Laboratory Ltd., Tokyo, Japan). Fasting plasma glucose level was measured using a JCA-BM9030 automated analyzer (Japan Electron Optics Laboratory Ltd.).
Other measurements
Demographic information, medical history of doctor-diagnosed chronic conditions, use of medication, and food intake were obtained using a standardized self-reported questionnaire. Physical activity was assessed using the Global Physical Activity Questionnaire, and metabolic equivalents (METs)-minute per week was computed [23]. Height and weight were measured with the subject wearing light clothing and no shoes using a fixed stadiometer and a digital scale, and used to compute body mass index (BMI).
Statistical Analysis
Differences in subject characteristics between those with and without sarcopenia were examined using Student’s t-test or Wilcoxon rank-sum test (for continuous variables) and chi-square test (for categorical variables).
First, we employed logistic regression analysis to evaluate the association of MetS with sarcopenia. Our preliminary analysis suggested that the association of metabolic syndrome with sarcopenia was modified by sex (p<0.01), and therefore the following analyses were stratified by sex.
The model was initially adjusted for age only (model 1). We added height and weight to remove the confounding effect of body size (model 2). We then further adjusted for life-style risk factors for both sarcopenia and MetS, including physical activity and food intake (model 3). In the fully-adjusted model, the interaction between MetS and age was examined to test the hypothesis that the effect of MetS on sarcopenia varies by age.
To test if any MetS component could explain the MetS-sarcopenia association, we initially fitted a fully-adjusted logistic regression model to examine the association between each component of MetS and sarcopenia, followed by other logistic regression models between MetS and sarcopenia adjusted for MetS components.
Second, to examine the association of MetS with each component of sarcopenia (i.e., muscle mass, grip strength and usual gait speed), we employed multiple linear regression models. If the association between MetS and any one of the sarcopenia components was statistically significant, another multiple linear regression model with MetS components as independent variables instead of MetS was conducted to evaluate the association between MetS components and the sarcopenia component. Finally, each component of MetS was introduced as a covariate to the multiple linear regression model between MetS and the sarcopenia component to test if the MetS component could explain the association between MetS and the sarcopenia component. Considering that the number of combinations between MetS components and sarcopenia components is quite high, the analyses between MetS components and sarcopenia components were considered supplemental and carried out only when the association between MetS and any of the sarcopenia components was statistically significant, in order to decrease the possibility of finding associations that were significant just by chance alone.
There were no missing values of any variable in the entire analytic sample.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R statistical software version 2.15.2 (R Foundation, Vienna, Austria). Two-sided p<0.05 was considered statistically significant.
Results
Subject characteristics
The prevalence of sarcopenia was 14.2% in men and 22.1% in women, and 43.6% of men and 28.9% of women were classified as having MetS. The characteristics of the study subjects by the sarcopenia status in each sex are shown in Table 1. Those with sarcopenia were older and had smaller body size compared with those without sarcopenia in each sex. Those with sarcopenia were physically less active and had smaller food intake in each sex. The prevalence of MetS was higher in those without sarcopenia, but the difference was significant only in men (p = 0.048 in men, 0.052 in women). Among the five MetS components, abdominal obesity was significantly more prevalent in those without sarcopenia in each sex.
Table 1. Characteristics of all subjects and according to sarcopenia status in men and women.
| All | Sarcopenia | No sarcopenia | p | |
| Men | 977 | 139 (14.2%) | 838 (85.8%) | |
| Age (years) | 73.1±5.5 | 78.4±5.5 | 72.2±5.0 | <0.001 |
| Height (cm) | 164.2±5.8 | 160.0±5.6 | 164.9±5.5 | <0.001 |
| Weight (kg) | 62.8±8.6 | 54.1±7.2 | 64.3±8.0 | <0.001 |
| BMI (kg/m2) | 23.3±2.8 | 21.1±2.5 | 23.6±2.6 | <0.001 |
| SMI (kg/m2) | 7.28±0.68 | 6.34±0.48 | 7.44±0.58 | <0.001 |
| Hand grip strength (kg) | 34.8±6.0 | 27.5±4.3 | 36.0±5.3 | <0.001 |
| Usual gait speed (m/s) | 1.47±0.26 | 1.28±0.24 | 1.51±0.24 | <0.001 |
| MetS | 43.6% | 36.0% | 44.9% | 0.048 |
| MetS components | ||||
| Abdominal obesity | 55.5% | 36.0% | 58.7% | <0.001 |
| High TG | 22.7% | 21.6% | 22.9% | 0.73 |
| Low HDL-C | 21.4% | 20.9% | 21.5% | 0.87 |
| High BP | 90.4% | 88.5% | 90.7% | 0.41 |
| High FPG | 51.0% | 53.2% | 50.6% | 0.56 |
| Food intake | ||||
| Very large | 2.9% | 1.4% | 3.1% | <0.001 |
| Large | 15.3% | 5.8% | 16.8% | |
| Normal | 65.4% | 58.3% | 66.6% | |
| Small | 14.4% | 30.2% | 11.8% | |
| Very small | 2.1% | 4.3% | 1.7% | |
| Physical activity (Mets) | 3962.9±3981.0 | 3191.7±3612.2 | 4090.8±4026.7 | 0.01 |
| Medical history | ||||
| Hypertension | 47.2% | 51.1% | 46.5% | 0.32 |
| Diabetes | 15.4% | 18.0% | 14.9% | 0.36 |
| Dyslipidemia | 29.8% | 31.7% | 29.5% | 0.60 |
| Stroke | 7.2% | 12.2% | 6.4% | 0.01 |
| CAD | 8.0% | 11.5% | 7.4% | 0.10 |
| Cancer | 19.0% | 26.6% | 17.8% | 0.01 |
| Medication use | ||||
| Statin | 17.6% | 18.7% | 17.4% | 0.71 |
| Women | 994 | 220 (22.1%) | 774 (77.9%) | |
| Age (years) | 72.8±5.4 | 76.2±5.8 | 71.8±4.9 | <0.001 |
| Height (cm) | 151.4±5.5 | 148.2±5.6 | 152.3±5.1 | <0.001 |
| Weight (kg) | 51.5±7.7 | 46.4±5.7 | 52.9±7.6 | <0.001 |
| BMI (kg/m2) | 22.5±3.2 | 21.1±2.6 | 22.8±3.2 | <0.001 |
| SMI (kg/m2) | 5.84±0.65 | 5.25±0.41 | 6.02±0.60 | <0.001 |
| Hand grip strength (kg) | 22.4±3.9 | 18.4±3.2 | 23.6±3.3 | <0.001 |
| Usual gait speed (kg) | 1.46±0.26 | 1.26±0.26 | 1.51±0.23 | <0.001 |
| MetS | 28.9% | 23.6% | 30.4% | 0.052 |
| MetS components | ||||
| Abdominal obesity | 24.0% | 14.6% | 26.7% | <0.001 |
| High TG | 17.9% | 16.4% | 18.4% | 0.50 |
| Low HDL-C | 36.6% | 33.2% | 37.6% | 0.23 |
| High BP | 84.2% | 87.3% | 83.3% | 0.16 |
| High FPG | 33.7% | 34.1% | 33.6% | 0.89 |
| Food intake | ||||
| Very large | 2.0% | 1.4% | 2.2% | <0.001 |
| Large | 13.1% | 9.6% | 14.1% | |
| Normal | 72.4% | 64.1% | 74.8% | |
| Small | 11.2% | 20.9% | 8.4% | |
| Very small | 1.3% | 4.1% | 0.5% | |
| Physical activity (Mets) | 3722.7±3429.5 | 2748.0±2825.0 | 4000.0±3535.6 | <0.001 |
| Medical history | ||||
| Hypertension | 39.8% | 45.9% | 38.1% | 0.04 |
| Diabetes | 8.8% | 8.2% | 8.9% | 0.73 |
| Dyslipidemia | 46.9% | 45.5% | 47.3% | 0.63 |
| Stroke | 4.7% | 5.9% | 4.4% | 0.35 |
| CAD | 4.9% | 5.5% | 4.8% | 0.68 |
| Cancer | 11.2% | 11.8% | 11.0% | 0.73 |
| Medication use | ||||
| Statin | 30.3% | 29.1% | 30.6% | 0.66 |
Mean and standard deviation are shown for continuous variables, and proportions as percent for categorical variables. Percentages may not add up to 100 because of rounding.
Abbreviations: BMI, body mass index; SMI, skeletal muscle mass index; MetS, metabolic syndrome; TG, triglycerides; CAD, coronary artery disease; HDL-C, high density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose.
Association between MetS and sarcopenia
In multiple logistic regression adjusted for age, MetS was significantly associated with decreased risk of sarcopenia in each sex (Table 2, Model 1). However, after additional adjustment for body size (i.e., height and weight), MetS was significantly associated with increased risk of sarcopenia in men, while the association between MetS and sarcopenia became non-significant in women (Table 2, Model 2). Further adjustment for life-style risk factors had little effect on the association (Table 2, Model 3). Exclusion of subjects who did not meet the criteria for MetS but had one or two MetS components (i.e., comparing those with MetS and those with no MetS component) yielded stronger MetS-sarcopenia association in men (OR 8.25, 95% CI 2.17–31.37, p = 0.002), but the association remained non-significant in women (OR 1.10, 95% CI 0.48–2.94, p = 0.83). In the fully adjusted model, the interaction between MetS and age was statistically significant in men (p = 0.02), suggesting that the effect of MetS on sarcopenia may vary by age. We then divided the subjects into two groups according to age: “young old” (65–74 years) and “old old” (≥75 years). The characteristics of the subjects by the sarcopenia status in each subgroup (young-old and old-old) are shown in Table S1. In the age-stratified analysis, MetS was significantly associated with sarcopenia in “young old” men only (Table 2, Model 3b).
Table 2. Adjusted associations of metabolic syndrome with sarcopenia in men and women.
| Men | Women | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Model 1 | 0.58 (0.38, 0.87) | 0.008 | 0.55 (0.38, 0.79) | 0.001 |
| Model 2 | 2.05 (1.21, 3.47) | 0.007 | 1.06 (0.69, 1.65) | 0.79 |
| Model 3 | 2.08 (1.22, 3.54) | 0.007 | 1.03 (0.66, 1.61) | 0.89 |
| Model 3a | 1.49 (0.80, 2.76) | 0.21 | 1.02 (0.57, 1.85) | 0.94 |
| Model 3b | 4.99 (1.73, 14.40) | 0.003 | 1.03 (0.52, 2.04) | 0.93 |
Abbreviations: OR, odds ratio; CI, confidence interval.
Model 1: adjusted for age.
Model 2: adjusted for age, height and weight.
Model 3: adjusted for age, height, weight, physical activity and food intake.
Model 3a: Adjusted for the same covariates as in Model 3, restricted to those aged 75 or over.
Model 3b: Adjusted for the same covariates as in Model 3, restricted to those aged 65 to 74.
Associations of MetS components with sarcopenia
Multiple logistic regression models demonstrated that, of the five MetS components, only abdominal obesity was significantly associated with increased risk of sarcopenia in men (odds ratio [OR] 2.98, 95% confidence interval 1.55–5.63, p≤0.001) while none of the MetS components was significantly associated with sarcopenia in women (Figure 1). Abdominal obesity was significantly and independently associated with sarcopenia in men in the model including all five MetS components simultaneously (OR 2.89, 95% CI 1.51–5.53, p = 0.001). When abdominal obesity was added as a covariate to the logistic regression model between MetS and sarcopenia, the MetS-sarcopenia association became statistically non-significant (p = 0.12), suggesting that the MetS-sarcopenia association was mainly mediated by abdominal obesity. In the age-stratified analysis, abdominal obesity and elevated TG were significantly associated with sarcopenia (OR 6.22, 95% CI 1.82–21.22, p = 0.004 and OR 3.37, 95% CI 1.23–9.28, p = 0.02, respectively) in young-old men, but no significant associations were observed between MetS components and sarcopenia in old-old men or women. Abdominal obesity and elevated TG remained significantly associated with sarcopenia in young-old men in the model including all five MetS components simultaneously (OR 6.32, 95% CI 1.81–22.06, p = 0.004 and OR 3.30, 95% CI 1.19–9.13, p = 0.02, respectively). Addition of abdominal obesity and elevated TG to the model between MetS and sarcopenia in young-old men made the MetS-sarcopenia association statistically non-significant (p = 0.13).
Figure 1. Fully adjusted odds ratio and 95% confidence interval of sarcopenia by individual metabolic syndrome components in all subjects and according to age group.
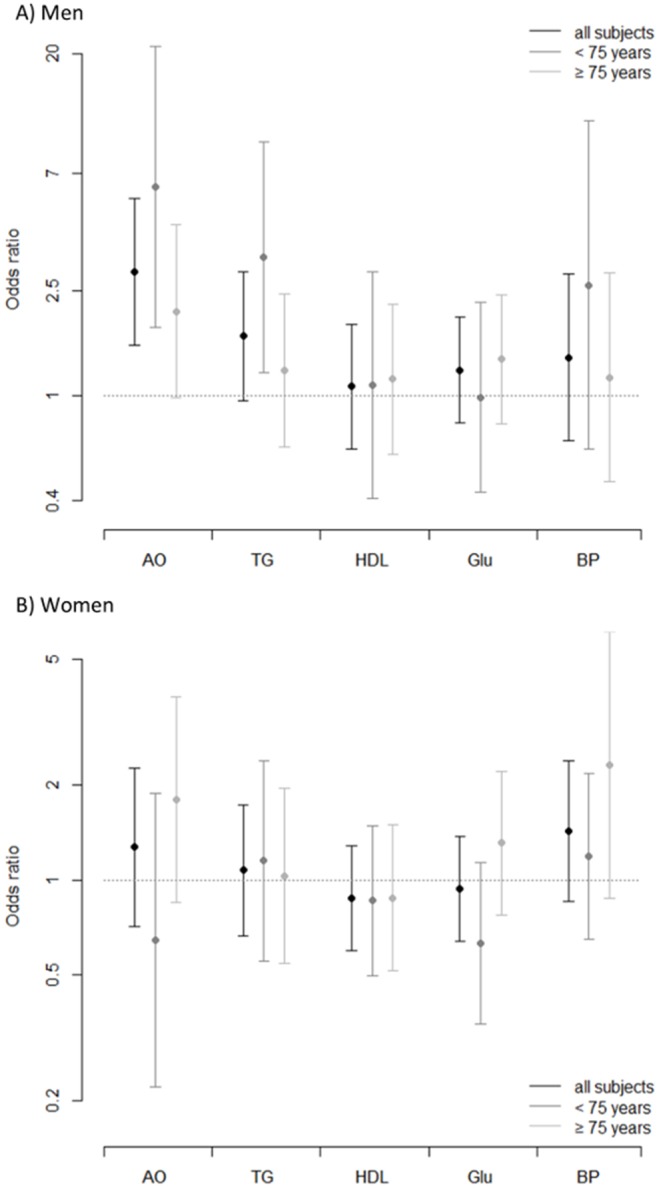
Black bars: all subjects, dark-gray bars: subjects aged 65 to 74 years, light-gray bars: subjects aged 75 years or over. All models are adjusted for age, height, weight, physical activity and food intake. AO, abdominal obesity; TG, elevated triglycerides; HDL, low high density lipoprotein; Glu, elevated fasting plasma glucose; BP, high blood pressure. A) Men. B) Women.
Associations of MetS with sarcopenia components
In fully-adjusted multiple linear regression models, MetS was associated with lower grip strength in each sex and lower muscle mass in men (Table 3). When analysis was stratified by age, the inverse associations of MetS with muscle mass and grip strength in men remained significant except for the association between MetS and muscle strength in the old-old group, which became statistically non-significant (Table 3). In women, the inverse association between MetS and grip strength was observed in the old-old group only. The association between MetS and muscle mass became significant in old-old women in the age-stratified analysis.
Table 3. Adjusted associations of metabolic syndrome with individual sarcopenia components in all subjects and according to age groups in men and women* †.
| Men | Women | |||
| beta (95% CI) | p | beta (95% CI) | p | |
| Skeletal muscle mass index | ||||
| All | −0.14 (−0.20, −0.09) | <0.001 | −0.05 (−0.10, 0.007) | 0.09 |
| Old-old | −0.13 (−0.24, −0.03) | 0.009 | −0.10 (−0.19, −0.005) | 0.04 |
| Young-old | −0.15 (−0.22, −0.08) | <0.001 | −0.02 (−0.09, 0.05) | 0.57 |
| Grip strength | ||||
| All | −0.98 (−1.68, −0.28) | 0.006 | −0.61 (−1.11, −0.10) | 0.02 |
| Old-old | −0.65 (−1.76, 0.45) | 0.25 | −0.84 (−1.64, −0.05) | 0.04 |
| Young-old | −1.26 (−2.17, −0.34) | 0.007 | −0.38 (−1.04, 0.27) | 0.25 |
| Usual gait speed | ||||
| All | −0.02 (−0.06, 0.01) | 0.22 | −0.01 (−0.05, 0.02) | 0.55 |
| Old-old | −0.006 (−0.06, 0.05) | 0.83 | −0.03 (−0.08, 0.03) | 0.36 |
| Young-old | −0.03 (−0.07, 0.009) | 0.13 | 0.004 (−0.04, 0.05) | 0.86 |
Abbreviations; CI, confidence interval.
*All the models were adjusted for age, height, weight, physical activity and food intake.
The young-old group refers to those aged 65 to 74 and the old-old group to those aged 75 or older.
In the subsequent supplementary analysis, abdominal obesity was significantly associated with lower grip strength in each sex and with lower muscle mass in men (Table S2). In addition, low HDL-C was associated with lower grip strength, and high TG was associated with lower muscle mass in men. These associations observed in men were significant in the young-old group only in the age-stratified analysis. For women, the only significant association observed was between high TG and lower muscle mass in the old-old group.
The association between MetS and grip strength became statistically non-significant after introduction of abdominal obesity into the model in each age group and sex. The introduction of abdominal obesity attenuated the association between MetS and muscle mass (i.e., decreased the magnitude of the regression coefficient) in each age group and sex by more than 10%, more markedly than did any other MetS component, consistent with abdominal obesity dominating the association of MetS with sarcopenia components (data not shown).
Discussion
In this cross-sectional analysis of 1971 functionally-independent, community-dwelling adults older than 65, MetS was associated with increased risk of sarcopenia, particularly in “young-old” men (aged 65 to 74), after adjustment for potential confounders including body size. Without adjustment for body size, MetS was associated with decreased risk of sarcopenia, suggesting that body size can confound the association between MetS and sarcopenia and should be taken into account when considering the impact of cardiovascular risk factors on muscle.
We demonstrated that MetS was associated with lower muscle mass and lower muscle strength, but the effects varied by sex and age. The adverse effects of MetS on muscle mass and strength were mainly observed in the young-old group for men. In stark contrast, women were mostly insusceptible to adverse effects of MetS on muscle, except for the marginally statistically significant associations of MetS with muscle mass and strength in the old-old group (age 75 or older). The mechanisms underlying the age- and sex-related differences in the associations between MetS and muscle mass/strength need to be explored in future research, but possible explanations may include the effects of sex hormones on skeletal muscle. MetS is associated with lower testosterone level [24]. Considering that testosterone is positively related to muscle strength [25], it is conceivable that one of the pathways through which MetS exerts its adverse effects on muscle is via testosterone. Since testosterone decreases with age [26] and is lower in women than in men, younger men, with relatively high levels of testosterone, may be especially vulnerable. Another possible explanation is cytokines secreted by adipose tissue, so-called adipokines. Adipose tissue produces and releases adipokines such as adiponectin and leptin as well as pro-inflammatory cytokines such as IL-6 [27]. Skeletal muscle is an important target tissue for these molecules, and circulating levels of such molecules are influenced by the amount of adipose tissue as well as age and sex [28], [29].
Several studies have reported an inverse association between MetS and muscle strength in younger men and women [30], [31]. One small cross-sectional study of older adults revealed an inverse association between MetS and muscle strength in men, but not in women [19]. This study also demonstrated that the association between MetS and muscle strength was more pronounced in men aged 65–74 compared to men aged 75 or older, consistent with our findings. Low muscle mass, with or without the presence of obesity, is associated with MetS in younger men and women [32]–[34]. Several studies in older adults showed an inverse association between MetS and muscle mass [35], [36], but these studies did not assess men and women separately.
We also demonstrated that the observed associations of MetS with the summary definition of sarcopenia or its individual components were mainly driven by abdominal obesity regardless of sex and age. Neither high BP nor elevated FPG showed a statistically significant association with sarcopenia or its components. Only a few studies have assessed which MetS components are main contributors to the association between MetS and the summary definition of sarcopenia or its components. An inverse association between MetS and physical performance was found in the cross-sectional analysis of a large-scale cohort study of older men, with obesity having the highest regression coefficient on physical performance among five MetS components [37]. Likewise, another large-scale cohort study of older adults found an association between MetS and poor physical performance, with abdominal obesity explaining the largest fraction of the variation in physical performance [38]. Our findings confirmed these previous studies and additionally demonstrated that abdominal obesity may be the main contributing factor for the associations of MetS with sarcopenia and its individual components regardless of sex and age, suggesting that there is a common mechanism underlying the adverse effects of MetS on muscle, for which abdominal obesity may partly be a marker, and that additional factors are at play causing sex- and age-related differences. Visceral fat accumulation, or abdominal obesity, is hypothesized to play an essential role in the development of MetS, given its propensity to cause insulin resistance, chronic inflammation and lower adiponectin levels [39]–[42]. All these factors may also be involved in the pathophysiological process of development of sarcopenia [6]–[9], [28], and we postulate that abdominal obesity may represent a clinical phenotype that is associated with increased risk of developing both MetS and sarcopenia. This study had several limitations. First, it could not be free of unmeasured or uncontrolled confounders due to its observational nature. In addition, since this study was cross-sectional, we could not infer a causal relationship between MetS and sarcopenia. Low muscle mass is associated with physical inactivity [10] and insulin resistance [43], and therefore could lead to the development of MetS. We speculate that, in reality, sarcopenia and MetS are deeply intertwined and cause adverse effects on each other, leading to frequent co-existence of these two syndromes. Second, medical history, use of medication and food intake were self-reported. Even though we used a standardized questionnaire, reporting bias was possible. Third, we did not collect information on or adjust for food composition such as total calories, which may confound the sarcopenia-MetS association. Finally, since the subjects were exclusively functionally-independent Japanese older adults, our findings may not be able to be generalized to older adults from other racial/ethnic groups.
In conclusion, this study comprehensively examined the associations of MetS with sarcopenia and its individual components in older adults, with particular attention to the modifying effects of sex and age. We demonstrated associations of MetS with sarcopenia, particularly muscle mass and strength. The associations were modified by sex and age, but were mainly driven by abdominal obesity regardless of sex and age. This study adds to the growing knowledge on the adverse effects of MetS on muscle. Further research is needed to elucidate the underlying mechanisms of the sex- and age-related differences in the association between MetS and sarcopenia.
Supporting Information
Characteristics of subjects according to sarcopenia status and age in men and women.
(DOCX)
Adjusted associations of metabolic syndrome components with individual sarcopenia components.
(DOCX)
Acknowledgments
All the following Kashiwa study investigators contributed by commenting on the manuscript: Takeshi Kikutani, The Nippon Dental University Graduate School of Life Dentistry; Takashi Higashiguchi, Fujita Health University School of Medicine; Kazuko Ishikawa-Takata, National Institute of Health and Nutrition; Shuichi P Obuchi, Tokyo Metropolitan Institute of Gerontology.
The authors wish to thank the staff members and participants of the Kashiwa study and the following individuals for helping with data acquisition: Dr. Koji Shibasaki and Masashi Suzuki, The University of Tokyo; Dr. Yoshiya Oishi, Oishi Dental Clinic; Dr. Hirohiko Hirano PhD DDS and Yuki Ohara, Tokyo Metropolitan Geriatric Institute of Gerontology; Dr. Noriaki Takahashi and Dr. Hiroyasu Furuya, The Nippon Dental University; Hisashi Kawai and Seigo Mitsutake, Tokyo Metropolitan Institute of Gerontology; staff members of The Institute of Healthcare Innovation Project, The University of Tokyo.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Health and Labor Sciences Research Grant (H24-Choju-Ippan-002 to KI) from the Ministry of Health, Labor, and Welfare of Japan (http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/hokabunya/kenkyujigyou/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 2. Romeo GR, Lee J, Shoelson SE (2012) Metabolic syndrome, insulin resistance, and roles of inflammation–mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol 32: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koh KK, Han SH, Quon MJ (2005) Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol 46: 1978–1985. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Ferrannini E (1991) Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14: 173–194. [DOI] [PubMed] [Google Scholar]
- 5. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, et al. (2007) Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 49: 403–414. [DOI] [PubMed] [Google Scholar]
- 6. Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, et al. (2011) Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc 59: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, et al. (2009) Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 32: 1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaap LA, Pluijm SM, Deeg DJ, Visser M (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119: 526 e529–517. [DOI] [PubMed]
- 9. Beenakker KG, Ling CH, Meskers CG, de Craen AJ, Stijnen T, et al. (2010) Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev 9: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenholm S, Koster A, Alley DE, Houston DK, Kanaya A, et al. (2010) Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women–results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 65: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penninx BW, Nicklas BJ, Newman AB, Harris TB, Goodpaster BH, et al. (2009) Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci 64: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carriere I, Peres K, Ancelin ML, Gourlet V, Berr C, et al. (2014) Metabolic syndrome and disability: findings from the prospective three-city study. J Gerontol A Biol Sci Med Sci 69: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss A, Boaz M, Beloosesky Y, Kornowski R, Grossman E (2009) Body mass index and risk of all-cause and cardiovascular mortality in hospitalized elderly patients with diabetes mellitus. Diabet Med 26: 253–259. [DOI] [PubMed] [Google Scholar]
- 15. Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, et al. (2000) Body mass index and mortality among hospitalized patients. Arch Intern Med 160: 2641–2644. [DOI] [PubMed] [Google Scholar]
- 16. Odden MC, Peralta CA, Haan MN, Covinsky KE (2012) Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 172: 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laudisio A, Bandinelli S, Gemma A, Ferrucci L, Incalzi RA (2013) Metabolic syndrome and functional ability in older age: The InCHIANTI study. Clin Nutr. pii S0261-5614: 00212–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawano Y, Ogihara T, Saruta T, Goto Y, Ishii M (2011) Association of blood pressure control and metabolic syndrome with cardiovascular risk in elderly Japanese: JATOS study. Am J Hypertens 24: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 19. Yang EJ, Lim S, Lim JY, Kim KW, Jang HC, et al. (2012) Association between muscle strength and metabolic syndrome in older Korean men and women: the Korean Longitudinal Study on Health and Aging. Metabolism 61: 317–324. [DOI] [PubMed] [Google Scholar]
- 20. Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, et al. (2014) Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int 14 Suppl 193–101. [DOI] [PubMed] [Google Scholar]
- 21. Tanimoto Y, Watanabe M, Sun W, Hirota C, Sugiura Y, et al. (2012) Association between muscle mass and disability in performing instrumental activities of daily living (IADL) in community-dwelling elderly in Japan. Arch Gerontol Geriatr 54: e230–233. [DOI] [PubMed] [Google Scholar]
- 22. Nagasaki H, Itoh H, Hashizume K, Furuna T, Maruyama H, et al. (1996) Walking patterns and finger rhythm of older adults. Percept Mot Skills 82: 435–447. [DOI] [PubMed] [Google Scholar]
- 23. Ainsworth BE, Bassett DR Jr, Strath SJ, Swartz AM, O’Brien WL, et al. (2000) Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc 32: S457–464. [DOI] [PubMed] [Google Scholar]
- 24. Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB (2008) Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab 93: 3403–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, et al. (2011) Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol 164: 811–817. [DOI] [PubMed] [Google Scholar]
- 26. Liu PY, Beilin J, Meier C, Nguyen TV (2007) Center JR, et al (2007) Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab 92: 3599–3603. [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115: 911–919; quiz 920. [DOI] [PubMed]
- 28. Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, et al. (2013) Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 14: 261–272. [DOI] [PubMed] [Google Scholar]
- 29. Zoico E, Di Francesco V, Mazzali G, Vettor R, Fantin F, et al. (2004) Adipocytokines, fat distribution, and insulin resistance in elderly men and women. J Gerontol A Biol Sci Med Sci 59: M935–939. [DOI] [PubMed] [Google Scholar]
- 30. Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, et al. (2004) Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc 36: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 31. Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, et al. (2007) Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc 39: 233–240. [DOI] [PubMed] [Google Scholar]
- 32. Moon SS (2014) Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J 61: 61–70. [DOI] [PubMed] [Google Scholar]
- 33. Park SH, Park JH, Park HY, Jang HJ, Kim HK, et al. (2013) Additional role of sarcopenia to waist circumference in predicting the odds of metabolic syndrome. Clin Nutr. pii S0261-5614: 00230–6. [DOI] [PubMed] [Google Scholar]
- 34. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, et al. (2013) Body size phenotypes and low muscle mass: the Korean sarcopenic obesity study (KSOS). J Clin Endocrinol Metab 98: 811–817. [DOI] [PubMed] [Google Scholar]
- 35. Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, et al. (2013) Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract 7: e301–307. [DOI] [PubMed] [Google Scholar]
- 36. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, et al. (2010) Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 33: 1652–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Everson-Rose SA, Paudel M, Taylor BC, Dam T, Cawthon PM, et al. (2011) Metabolic syndrome and physical performance in elderly men: the osteoporotic fractures in men study. J Am Geriatr Soc 59: 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beavers KM, Hsu FC, Houston DK, Beavers DP, Harris TB, et al. (2013) The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci 68: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsuzawa Y, Funahashi T, Nakamura T (2011) The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 18: 629–639. [DOI] [PubMed] [Google Scholar]
- 40. Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, et al. (2002) Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51: 2951–2958. [DOI] [PubMed] [Google Scholar]
- 41. Despres JP (2006) Is visceral obesity the cause of the metabolic syndrome? Ann Med 38: 52–63. [DOI] [PubMed] [Google Scholar]
- 42. Tchernof A, Despres JP (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404. [DOI] [PubMed] [Google Scholar]
- 43. Srikanthan P, Hevener AL, Karlamangla AS (2010) Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 5: e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of subjects according to sarcopenia status and age in men and women.
(DOCX)
Adjusted associations of metabolic syndrome components with individual sarcopenia components.
(DOCX)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All relevant data are within the paper and its Supporting Information files.


