Abstract
Excessive anthropogenic nitrogen (N) and phosphorus (P) inputs have caused an alarming increase in harmful cyanobacterial blooms, threatening sustainability of lakes and reservoirs worldwide. Hypertrophic Lake Taihu, China’s third largest freshwater lake, typifies this predicament, with toxic blooms of the non-N2 fixing cyanobacteria Microcystis spp. dominating from spring through fall. Previous studies indicate N and P reductions are needed to reduce bloom magnitude and duration. However, N reductions may encourage replacement of non-N2 fixing with N2 fixing cyanobacteria. This potentially counterproductive scenario was evaluated using replicate, large (1000 L), in-lake mesocosms during summer bloom periods. N+P additions led to maximum phytoplankton production. Phosphorus enrichment, which promoted N limitation, resulted in increases in N2 fixing taxa (Anabaena spp.), but it did not lead to significant replacement of non-N2 fixing with N2 fixing cyanobacteria, and N2 fixation rates remained ecologically insignificant. Furthermore, P enrichment failed to increase phytoplankton production relative to controls, indicating that N was the most limiting nutrient throughout this period. We propose that Microcystis spp. and other non-N2 fixing genera can maintain dominance in this shallow, highly turbid, nutrient-enriched lake by outcompeting N2 fixing taxa for existing sources of N and P stored and cycled in the lake. To bring Taihu and other hypertrophic systems below the bloom threshold, both N and P reductions will be needed until the legacy of high N and P loading and sediment nutrient storage in these systems is depleted. At that point, a more exclusive focus on P reductions may be feasible.
Introduction
Excessive phosphorus (P) and nitrogen (N) inputs promote hypertrophic conditions in lakes worldwide [1], [2], often manifested as harmful (toxic, hypoxia-generating, food-web disrupting) cyanobacterial blooms [3], [4]. Some cyanobacteria can convert atmospheric nitrogen (N2) to biologically-available ammonia via N2 fixation, providing diazotrophic cyanobacteria with an advantage in waters that are replete in other essential nutrients (e.g., P, Fe, trace metals) but deficient in N [5]. This observation forms a basis for the paradigm that low N:P ratios favor cyanobacterial dominance [6], [7]. However, N2 fixation is energetically unfavorable, and cyanobacteria relying on atmospheric N2 often do not achieve maximal growth rates [8].
Nutrient loading dynamics have changed, however, since this paradigm was first introduced [6], [7]. Agricultural, urban, and industrial expansion in developed and developing regions has dramatically increased reactive N discharges [9], while P loading has stabilized or decreased after recognition that P enrichment influenced eutrophication in freshwater ecosystems [c.f., 10]. Today, unprecedented amounts of biologically-available N are discharged over land, through subsurface aquifers, and via the atmosphere [9]. Freshwater and marine ecosystems are now N:P enriched and more eutrophic [11], [12]. Recent studies have shown that these ecosystems can become even more eutrophic when they receive persistent N loading (i.e., they remain sensitive to N inputs) [1], [2], [13].
The increasing dominance and geographic expansion of non-N2 fixing cyanobacterial genera, specifically blooms of the widespread, toxin-producing genus Microcystis, are a troubling indicator of excessive N loading [14]. This pattern has been observed in large lake systems (e.g., Lake Erie, North American Great Lakes; Lake Kasumigaura, Japan; Lake Okeechobee, Florida; Lake Taihu, China), and estuarine and coastal waters (e.g., Baltic Sea, Europe; Potomac River-Chesapeake Bay and the San Francisco Bay Delta, North America; Peel Harvey and Swan River Estuaries, Australia) [15]. Microcystis spp., and some benthic analogues (e.g., non-N2 fixing Lyngbya and Oscillatoria), are indicative of N-over-enriched conditions [16]. The continuing threat from N-driven eutrophication persists because: 1) some N can be ‘lost’ by denitrification, creating a demand for continued N inputs to sustain eutrophication [17]; 2) historically high P loads accumulate in sediments, providing a reservoir for recycled P to sustain eutrophication; and 3) in nutrient-affected eutrophic lakes, N2 fixation does not meet ecosystem-scale N requirements [12], [18], [19]. These studies indicate that eutrophication is accelerated by increasing external N inputs, and they point to the importance of controlling N inputs (in addition to P) for reducing and reversing eutrophication along the freshwater to marine continuum [2], [20].
Schindler et al. [21] and others [22], [23] suggest that N2 fixing cyanobacteria will replace non-N2 fixers once N inputs are reduced, especially if P remains available. This possibility is troubling in lakes and reservoirs used for drinking water, fishing, and recreational purposes, because some N2 fixers (e.g., Anabaena spp., Aphanizomenon spp., Nodularia spp.) also produce toxins. We evaluated this potential scenario in highly eutrophic (hypertrophic) Lake Taihu, China’s third largest freshwater lake, which has experienced massive cyanobacterial blooms dominated by Microcystis spp. [24]–[26]. Previous in situ bioassay work [26], [27] in Lake Taihu has shown that P inputs control algal production in the winter-spring, but N-limitation and N and P co-limitation are common during the summer-fall bloom period. Blooms in Lake Taihu remain dominated by non-N2 fixing Microcystis, with potentially N2 fixing Anabaena and Aphanizomenon present as sub-dominant genera. The blooms are initiated in late spring (April–May) and persist well into fall (November).
Using in situ 1000 L mesocosms deployed for several weeks during the peak bloom period (June-August), we examined the response of the native phytoplankton community to a range of nutrient enrichments, including one in which only P was administered to enhance N-limitation. The aim of these experiments was to assess the potential for N2 fixing cyanobacteria to replace non-N2 fixers under N limited conditions in Lake Taihu.
Methods
Location and field sites
Lake Taihu (Taihu means ‘large lake’ in Mandarin) is located approximately 150 km west of Shanghai, China (Lake coordinates 31° 10′ N, 120° 9′ E). Taihu is China’s third largest freshwater lake at ∼2400 km2 (Fig. 1). It is shallow (mean depth ∼2 m), polymictic, and hypertrophic, and it was formed as a former oxbow of the nearby Yangtze River [28]. Taihu’s drainage basin is 36,500 km2 and contains more than 30 input sources, ranging from rivers to small streams and man-made drainage canals. Water exits the southeastern corner of Taihu via the Taipu River, which drains through Shanghai into the East China Sea (Fig. 1). About 40 million people live within Taihu’s watershed, which supports approximately 11% (and expanding) of the Chinese economy [28]. The lake is the key drinking water source for ∼10 million people, but also serves as a repository for waste from the urban, agricultural, and industrial segments of the local area.
Figure 1. Map of Lake Taihu showing major tributaries and nearby cities.
The location of Taihu in China is shown on the inserted map.
No specific permissions were required for field sampling and studies. These studies did not involve endangered or protected species.
Mesocosm bioassays were located at the interface of one of the northern bays, Meiliang Bay, and the lake proper (Fig. 1). This area was chosen because it is the site of recurring, intensive annual Microcystis spp. blooms [24], [28], previous nutrient limitation studies [27], and is located near the Nanjing Institute of Geography and Limnology’s Taihu Laboratory for Lake Ecosystem Research (TLLER) at Wuxi. Meiliang Bay receives freshwater inputs from the Liangxi and Zhihu Gang rivers, which drain partially-treated wastewater from industrial, residential, and agricultural areas. The named rivers in Fig. 1 contribute more than 85% of the lake’s freshwater inflow.
Mesocosms
Twelve cylindrical, 1 m diameter, 1.8 m tall, 1000 L volume, semi-translucent fiberglass mesocosms, which are open at the top and closed at the bottom, were constructed by the shipyard in Wuxi. The mesocosms were installed at the lakeshore near TLLER (Fig. 2), providing ambient natural light and temperature conditions. The mesocosms were outfitted with a flotation flange to ensure that the mesocosms would remain upright and above the lake surface water line. We also monitored daily ambient physical and chemical parameters (temperature, transparency, dissolved oxygen, pH, conductivity), nutrient concentrations (N, P, and carbon species), and phytoplankton community biomass and composition in the mesocosms and nearby lake water. The mesocosm experiment was started on 15 July and ended on 31 July 2013.
Figure 2. Photographs of mesocosm array, located on the shore of Taihu near the Taihu Laboratory for Lake Ecosystem Research (TLLER), at Wuxi, China.
We measured phytoplankton community biomass and compositional response to N limitation (i.e., exclusive P enrichment) vs. P limitation (N enrichment), as well as co-limitation, over the two-week period, which allowed the phytoplankton community to adjust to selective nutrient enrichment over numerous generations. The potential for a shift from non-N2 fixing to actively N2 fixing cyanobacteria is supported by previous (and ongoing) microscopic observations showing that both groups co-existed during N replete and N-limited periods [24], [27].
Prior to deployment, the mesocosms were cleaned and ‘conditioned’ by filling several times with water from Meiliang Bay (Fig. 1). Once filled for the experiments, the mesocosms were allowed to stabilize for 24 h prior to initiating the following triplicated treatments: 1) Control (no nutrients); 2) nitrogen only (N-only); 3) phosphorus only (P-only); and 4) nitrogen and phosphorus addition (N+P). Nitrogen was added as a solution of 0.3 mg L−1 NO3-N (as KNO3) and 0.2 mg L−1 NH4-N (as NH4Cl; final concentration 0.5 mg L−1 N). Phosphorus was added as 0.02 mg L−1 P (as K2HPO4). These nutrient enrichments were based on recent (since 2007) nutrient measurements during spring in Meiliang Bay prior to the bloom initiation [26], [27]. Nitrogen and P were supplied every two days throughout the experiment.
While we recognize the importance of sediments as a source or sink for nutrients, we did not add sediments to the mesocosm experiment because: 1) the objective of this study was to evaluate the potential for planktonic N2 fixers to dominate under well-defined water column N and P regimes; 2) preliminary experiments (in 2012) indicated that the presence or absence of sediments had little effect on phytoplankton community structure and N2 fixation rates over the course of the mesocosm deployments; and 3) we were concerned that sediments would affect DIN availability via denitrification and excessive microbial (non-phytoplankton) uptake, which could introduce variability, lead to poor replication, and defeat the purpose of the experiment (examining water column utilization of nutrients).
Mesocosms were thoroughly stirred with a plastic paddle following nutrient additions and daily prior to sampling to maintain homogeneous nutrient and seston distributions. Growth of periphyton on the mesocosm walls was controlled by scrubbing the walls of mesocosms every two days with a pre-cleaned brush. This procedure proved effective in minimizing periphyton growth during the experimental period. Subsamples were collected every two days for nutrient analyses (dissolved inorganic N and P forms, Total N and P), carbon and N content of the seston and chlorophyll a (Chl a)). Samples were filtered within 2 h of collection on 25 mm Whatman GF/F filters (pre-combusted at 450°C for elemental C and N analyses). Retained material was used for particulate analyses and filtrate was analyzed for dissolved nutrients. Subsamples for photopigments (fucoxanthin, 9′cis-neoxanthin, violaxanthin, diadinoxanthin, antheraxanthin, myxoxanthophyll, alloxanthin, lutein, zeaxanthin, chlorophyll b, ß-carotene, echinenone), which are diagnostic of major phytoplankton taxonomic groups, N2 fixation potential, and microscopic identification and enumeration of phytoplankton were collected on GF/F filters every four days. All subsamples were collected prior to nutrient additions. Water temperature, dissolved oxygen, and pH were measured with a YSI Model 6600 multiprobe.
Nutrient and other chemical analyses
Soluble reactive P (SRP) was determined using the molybdenum blue method [29]. NH4 +-N was measured by the indophenol blue method, and NOx (NO3 –-N + NO2 − N) was analyzed with the cadmium reduction method [29]. Total phosphorus (TP), total dissolved phosphorus (TDP), total nitrogen (TN), and total dissolved nitrogen (TDN) were analyzed using a combined persulphate digestion [30], followed by spectrophotometric analysis, as for SRP and NO3 –-N.
Photopigment determinations
Chlorophyll a served as an indicator of total phytoplankton biomass, while diagnostic chlorophyll and carotenoid photopigments were used as indicators of major phytoplankton classes [31]. For Chl a, 50–100 mL subsamples were collected from mesocosms and filtered onto 25 mm GF/F filters, which were blotted dry, folded in foil wrappers and frozen until analysis. Chl a concentrations were determined spectrophotometrically after extraction in 90% hot ethanol [32].
Diagnostic photopigments were measured using high performance liquid chromatography (HPLC). Approximately 100–150 mL of sample water was vacuum filtered using 25 mm GF/F filters (nominal pore size 0.7 µm). Filters were blotted dry, frozen and transported (frozen) to The University of North Carolina at Chapel Hill, Institute of Marine Sciences for analysis. Filters were extracted in 100% acetone, sonicated, and stored at 20°C for approximately 24 h. Extracts (200 µL) were then injected (using an autosampler) into a Shimadzu Model SIL-20AC HT HPLC equipped with a Model SPD-M10Avp photo diode array detector, following procedures described by Van Heukelem et al. [33] and Pinckney et al. [31]. Pigments were identified according to their absorption spectra, which were determined using commercially obtained pigment standards (DHI-Danish Hydraulic Institute, Denmark).
Contributions of the dominant four algal classes (diatoms, chlorophytes, cryptophytes, and cyanobacteria) to Chl a were calculated using Chemtax [34]. The input pigment ratio matrix for the four classes was adapted from Schlüter et al.’s [35] study of lacustrine phytoplankton species. Average ratios for each class were calculated based on species averages within their light availability experiments. With only four dominant classes identified microscopically, the number of pigments used for Chemtax was reduced to twelve. Dominant photopigments, algal classes represented, and input ratios used for the Chemtax analysis are shown in Table 1. Phytoplankton samples were fixed with Lugol’s iodine solution (2% final concentration) and sedimented for 48 h. Cell density was measured with a Sedgwick–Rafter counting chamber under microscopic magnification of X200–400. Phytoplankton species were identified according to Hu et al. [36].
Table 1. Dominant algal classes and input accessory pigment ratios used for Chemtax determination of class contribution to total chlorophyll a.
| Fuco | Neo | Viol | Diad | Anth | Myx | Allo | Lut | Zea | Chl b | β Car | Ech | |
| Diatom | 0.51 | 0 | 0 | 0.074 | 0 | 0 | 0 | 0 | 0 | 0 | 0.003 | 0 |
| Cryptophyte | 0 | 0 | 0 | 0 | 0 | 0 | 0.37 | 0 | 0 | 0 | 0.004 | 0 |
| Chlorophyte | 0 | 0.038 | 0.026 | 0 | 0.016 | 0 | 0 | 0.15 | 0 | 0.36 | 0.003 | 0 |
| Cyanobacteria | 0 | 0 | 0 | 0 | 0 | 0.14 | 0 | 0 | 0.28 | 0 | 0.097 | 0.076 |
Fuco = fucoxanthin; Neo = 9′cis-neoxanthin, Viol = violaxanthin; Diad = diadinoxanthin; Anth = antheraxanthin; Myx = myxoxanthophyll; Allo = alloxanthin; Lut = lutein; Zea = zeaxanthin; Chl b = chlorophyll b; β Car = β-carotene; Ech = echinenone.
Nitrogen fixation
Nitrogen fixation (nitrogenase activity) rates were estimated using the acetylene reduction (AR) assay, as described by Paerl [37], by dispensing 90 mL of sample into 125 mL stoppered serum vials, and adding 7 mL of acetylene generated from calcium carbide (Aldrich). Triplicate light and dark bottles, as well as 0.2 µm filtered (sterile PALL Inc. nitrocellulose filters) lake water blanks were incubated in situ in Taihu for 4 h, after which 4 mL headspace samples were collected in pre-evacuated Vacutainer (Becton Dickinson Inc.) tubes. The tubes were then transported to the laboratory, and 0.2 mL was withdrawn for measurements of ethylene production (from acetylene) by flame ionization gas chromatography using a Shimadzu GC9 gas chromatograph.
Statistical analyses
Phytoplankton responses to the factorial nutrient addition design were assessed using an unsupervised, objective, nutrient-limitation classification model [38]. The model is based on a generalized linear model of treatment contrasts and time effects accounted for by orthogonal polynomials. Aikake information criteria (AIC) was used to determine the model that achieves the greatest degree of parsimony with biomass responses to treatments and time. Separating the time effects from the treatment effects provides a classification of which nutrient, if any, was limiting, or whether both nutrients were limiting. Seven classifications of nutrient limitation status are possible: 1-null, meaning no nutrient effects; 2 and 3- exclusive N or P limitation, where only N or P stimulates biomass above the control, and addition of the non-limiting nutrient in the N+P treatment provides no additional stimulation; 4 and 5-primary N or P limitation, where addition of one nutrient stimulates growth above the control, but addition of the non-limiting nutrient provides additional stimulation; 6-combined limitation, where both N, P, and N+P additions are different from the control; and 7-exclusive combined limitation, when neither N nor P additions alone stimulate biomass above the control, but the combined N+P treatment stimulates growth. This classification model was used to analyze responses of total phytoplankton biomass, as Chl a, and the biomass responses of specific taxa based on diagnostic photopigments or microscopic enumeration. To determine confidence in each nutrient limitation classification, bootstrapping with replacement was used to produce 1000 resampled data sets from the original triplicated mesocosm treatments. The frequency of bootstrapped nutrient limitation determinations provides a measure of confidence in the determination made from the original experimental dataset [38].
Results
Nutrient addition effects on phytoplankton biomass
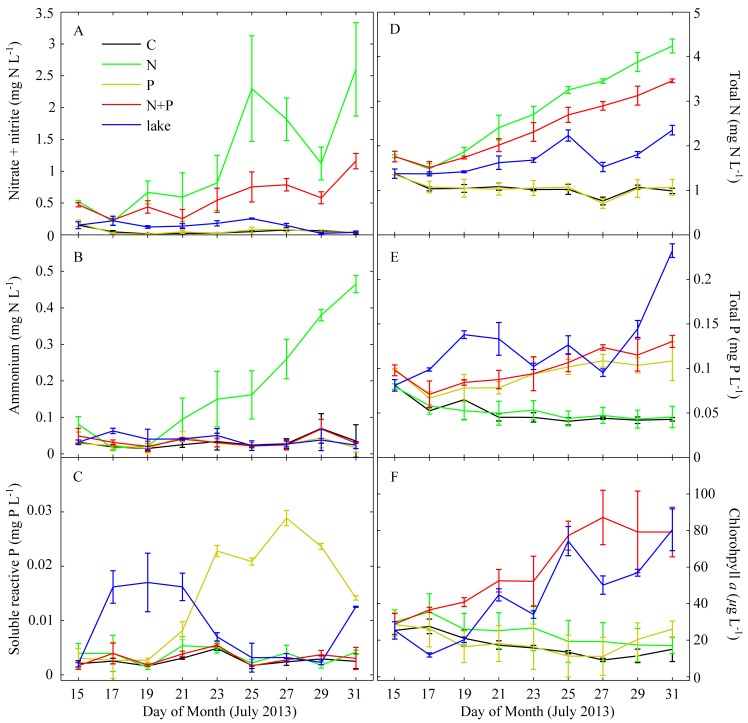
For the first two days, N-only additions led to a small but significant stimulation of phytoplankton biomass relative to controls and P additions (Fig. 3). Similarity between N-only and N + P additions showed that the initial community was N limited rather than co-limited. After two days, only the N+P treatment showed continued growth, with Chl a concentrations reaching 60 µg L−1 or higher, reflecting typical bloom conditions [24]–[26]. All other treatments showed similar declines in Chl a through 27 July 2013.
Figure 3. Time series of dissolved and total nitrogen and phosphorous forms and phytoplankton biomass as chlorophyll a from the summer 2013 mesocosm experiment.
Solid lines connect means of triplicate mesocosms. Error bars are one standard deviation.
Overnight on 27 July into early morning 28 July 2013, the TLLER meteorological station recorded 48.6 mm rainfall during a strong thunderstorm. Nutrient concentrations (in mg L−1 N or P) in the rainwater were 3.41 TN, 3.39 TDN, 0.46 NH4 +, 0.69 NO3 −, 0.032 TP, 0.20 TDP, and 0.025 PO4 3−. If only inorganic nutrients are assumed bioavailable, the N and P added to the 1000 L mesocosms would have increased bioavailable N and P concentrations by ∼0.04 and 0.0009 mg L−1 (102∶1 molar ratio), respectively. If all N and P added from the rain were assumed bioavailable, N and P bioavailability would have increased by 0.12 and 0.0012 mg L−1 (221∶1 molar ratio), respectively. This N enriched and P poor rain event served as a ‘natural experiment’ to evaluate the effects of N addition to the experimentally-manipulated mesocosm phytoplankton communities. The atmospheric N addition led to a rapid stimulation of biomass in the P addition treatments and, to a lesser extent, in the controls. N-only and N + P treatments showed no response, indicating that growth in those treatments was not N limited during the time of the storm. Assessments of nutrient limitation status by the unsupervised nutrient limitation classification model were affected by the storm and corroborate the overall N limitation status of the phytoplankton community. For the entire experimental period, the nutrient limitation model best supported by the data was a primary combined N and P limitation (Table 2), but 23% of the resampled datasets supported a primary N limitation determination. When only data prior to the storm were analyzed, confidence in a primary N limitation status for Chl a response was high (93% of resampled datasets), with a small portion (6.3%) of primary combined limitation determinations (Table 2).
Table 2. Nutrient limitation classifications (N.L.C.) and ambiguity of classification for total phytoplankton biomass and specific algal taxa (Andersen et al. 2005).
| Group | N.L.C. | Null | XN | N1 | XP | P1 | XC | C1 |
| Total | C1 | 0 | 0 | 22.5 | 0 | 3.3 | 1.1 | 73.1 |
| phytoplankton | (N1) | (0) | (0) | (93.3) | (0) | (0.2) | (0.2) | (6.3) |
| Diatoms | P1 | 0 | 0 | 1.3 | 0 | 51.4 | 1.6 | 45.7 |
| (XC) | (0) | (0) | (20.0) | (0) | (9.9) | (45.5) | (24.6) | |
| Cryptophytes | N1 | 0 | 0 | 54.2 | 0 | 18.7 | 11.5 | 15.6 |
| (N1) | (0) | (0) | (64.2) | (0) | (5.2) | (25.3) | (5.3) | |
| Chlorophytes | C1 | 0 | 0 | 31.9 | 0 | 0 | 8.5 | 59.6 |
| (N1) | (0) | (0) | (9.7) | (0) | (3.2) | (7.9) | (79.2) | |
| Cyanobacteria | N1 | 0 | 0 | 38.4 | 0 | 30.6 | 12.1 | 18.9 |
| (N1) | (0) | (0) | (40.5) | (0) | (29.5) | (9.5) | (20.5) | |
| Microcystis sp. | P1 | 0 | 0 | 1.4 | 0 | 87.7 | 0.3 | 10.6 |
| (P1) | (0) | (12.0) | (2.8) | (0) | (69.1) | (0.5) | (15.6) | |
| Anabaena spp. | – | 7.8 | 35.7 | 18.1 | 11.5 | 28.0 | 1.5 | 13.7 |
| (–) | (11.3) | (64.0) | (7.7) | (0) | (5.7) | (9.2) | (2.1) |
Total phytoplankton biomass as spectrophotometric Chl a. Ambiguity of the classification of the nutrient limitation status is shown by the frequency (%) of different classifications from 1000 bootstrapped data sets. Values in parentheses are results excluding data from the period following the 27 July 2013 rain event. A – indicates that the null model was chosen in greater than 5% of boot-strapped cases.
Phytoplankton community comparisons between mesocosms and ambient lake water
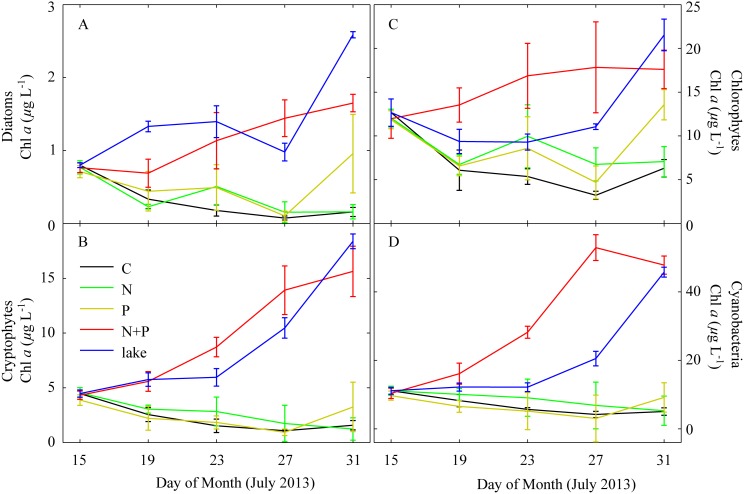
When examined over time for phytoplankton biomass and composition, ambient lake water tended to show elevated levels of total community biomass and major algal groups relative to the control (no nutrient addition) mesocosms (Fig. 4). These differences were observed shortly after the initiation and remained throughout the duration of the experiment (16 d), indicating that nutrient-limited conditions were maintained in the controls throughout the experiment. While total biomass (as Chl a) was consistently lower in controls, the phytoplankton composition remained similar between controls and ambient lake water, indicating that potentially confounding issues, such as selective grazing, death, or major shifts in phytoplankton composition due to ‘container effects’ were minimal. While phytoplankton biomass remained low in control mesocosms, Chl a and other diagnostic photopigment concentrations remained detectable throughout the experiment (Fig. 4).
Figure 4. Time series of biomass of the dominant algal classes from the summer 2013 mesocosm experiment.
Solid lines connect means of triplicate mesocosm tanks. Error bars are one standard deviation.
Nutrient addition effects on phytoplankton community composition
Overall, the addition of either N or P alone showed little stimulation of the dominant phytoplankton classes over the control; however, combined N+P additions stimulated all four major classes (Fig. 4). For diatoms, P additions led to small increases in biomass above the control. The nutrient classification status was assessed as primary P limitation, but confidence in this determination was weak with primary combined limitation being nearly as likely (Table 2). Exclusion of the post-storm periods revealed that, prior to this point, the diatom community had likely been co-limited (Table 2). Cryptophytes showed weak stimulation by N-only additions (Fig. 4) and were classified as having a primary N limitation status. However, confidence in this assessment was not strong with primary P limitation, exclusive N + P co-limitation, and primary combined limitation being collectively as likely (Table 2). Chlorophytes were assessed as primary combined limitation, and exclusion of the post-storm period increased confidence in this assessment. Cyanobacteria were assessed as primarily being N limited but, as with the other phytoplankton classes, confidence in this determination was weak. It is instructive that for none of the four phytoplankton classes, in any of the 1000 bootstrapped data sets, was there a single instance where nutrient limitation status was assessed as exclusive N or exclusive P limitation. This underscores the finding that the dominant phytoplankton classes were largely co-limited by N and P during the experiment. N and P availability nearly balanced demand such that, when N or P was added alone, it drove growth limitation to limitation by the other nutrient. In this case, only combined additions of N and P led to continued accumulation of biomass.
The lack of cyanobacterial biomass stimulation by P additions was surprising, since ambient phosphate concentrations were quite low at the start of the experiment (∼2–4 µg P-PO4 L−1). Total dissolved inorganic N (DIN) (NH4 + + NOx) concentrations in the P treatments were also quite low (∼20–40 µg DIN L−1), providing presumably favorable conditions (DIN:DIP of approximately 10) for selective stimulation of cyanobacterial growth, especially among N2 fixing genera in response to P additions [7].
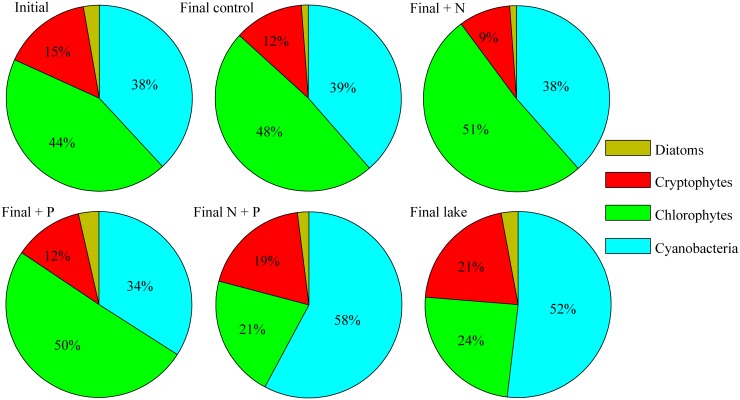
N+P additions promoted the growth of all groups relative to controls, with cyanobacteria showing particularly strong stimulation (Fig. 3). This appeared to mirror conditions in ambient lake water, which showed a steady stimulation of cyanobacterial biomass and bloom development in parallel with the experimental period. By the end of the experiment, community composition of the N+P treatments were very similar to ambient lake water (Fig. 5). In comparison, community composition of the controls, P-only, and N-only treatments was similar to the initial community composition (Fig. 5).
Figure 5. Pie graphs depicting proportion of total chlorophyll a comprised by each of the dominant four algal classes at the initial sampling of the mesocosm experiments (average of all samples collected) and final sampling (average of triplicate mesocosms) for each treatment and the ambient lake water.
Nutrient conditions during the mesocosm experiment
Dissolved inorganic and total N and P concentration showed strong patterns related to nutrient additions and biological utilization (Fig. 3). Control and P only addition mesocosms showed almost no detectable DIN, indicating that N was likely the most limiting nutrient throughout the experimental period. Even with N additions, for the first two and four days, NOx and NH4 + concentrations decreased in both the N-only and N+P addition treatments. This indicates that N availability did not meet N demand at the beginning of the experiment and suggests that NH4 + was preferentially utilized over NOx. After this initial period, both NOx and NH4 + increased in the N-only additions. However, the N+P additions treatments showed half the NOx buildup and no significant NH4 accumulation, indicating that while N limitation was more evident than P limitation, N+P co-limitation most likely characterized conditions during the experimental period. Total N predictably increased in response to N-only and N+P enrichment, but notably it did not increase in response to P-only enrichment. This served as independent (of acetylene reduction measurements) evidence that N2 fixation did not respond in a significant manner to the P-only treatments and that microbial production in response to the P-only treatments was likely supported on regenerated sources of N. SRP remained at low levels similar to the control in the N-only and N+P treatments. In the P-only treatment, SRP accumulated after four days and reached a peak on 27 July. After the 27 July storm, SRP dropped by 50%, indicating significant uptake in response to increased N availability from rainwater. Total P increased in response to both P-only and N+P additions, but there were no significant differences in response to these treatments. Overall, nutrient concentration results support previous findings that during summer months this region of Taihu is N+P co-limited [26], [27]. Exclusive P limitation was not detected throughout the course of these experiments.
Microscopic observations of phytoplankton community dynamics
In general, microscopic observations tended to confirm HPLC diagnostic pigment analyses with respect to the responses of major phytoplankton groups to various nutrient additions. Specific attention was paid to the dominant N2 fixing (Anabaena sp.) vs. non-N2 fixing (Microcystis sp.) cyanobacterial genera responses to various nutrient additions (Figs. 6, 7).
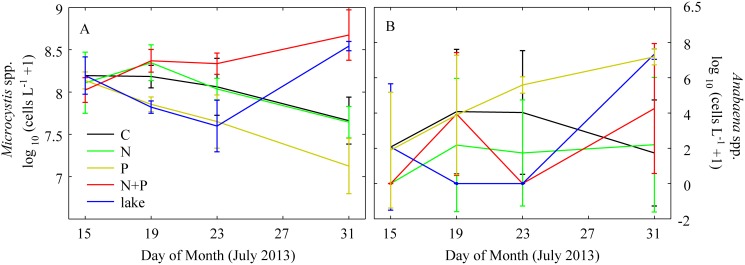
Figure 6. Time series of cell abundances for Microcystis spp. and Anabaena spp., the dominant non-nitrogen fixing and nitrogen fixing genera during the summer 2013 mesocosm experiment.
Solid lines connect means of log10 values of triplicate mesocosm tanks. Error bars are one standard deviation.
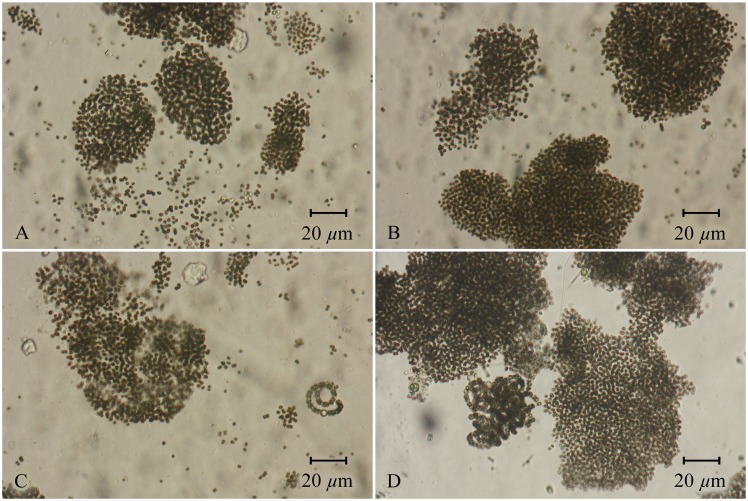
Figure 7. Representative microscopic observations (X200, brightfield) of dominant cyanobacterial genera in the mesocosm experiment conducted during 2013.
Samples were collected towards at the end of the experiment, 31 July 2013. (A) Control (no nutrient addition). (B) N-only addition. (C) P-only addition. (D) N+P addition. Note the overall dominance by Microcystis spp. colonies. However, an increase in number of filaments of the N2 fixing genus Anabaena was observed in the phosphorus and phosphorus and nitrogen treatments. No significant N2 fixation was observed in either of these treatments however. Biomass-wise, Microcystis spp. clearly dominated the cyanobacterial community in all treatments.
The diatom community was dominated by a mix of solitary centric (e.g., Cyclotella sp.), pennate (e.g., Navicula sp.), and chain-forming genera (e.g., Melosira sp.). Chlorophytes proved to be highly diverse throughout all treatments, but the largest fraction of chlorophyte biomass was comprised of colonial (e.g., Scenedesmus spp., Crucigenia sp., Pediastrum spp., Eudorina sp.) and filamentous (Ulothrix sp.) forms. Cryptophytes were largely comprised of nanoplanktonic Cryptomonas spp. and Chroomonas spp. In general, dominant members of these eukaryotic groups were similar to those reported during a long-term monitoring study [24]. Dominant cyanobacterial genera in order of prominence were Microcystis, Oscillatoria, Anabaena, Aphanizomenon, and Merismopedia.
Among these, Anabaena and Aphanizomenon are capable of N2 fixation. Microcystis responded in two ways to the nutrient addition treatments: stimulation by N+P additions, and a negative response to P-only additions (Fig. 6). The nutrient limitation classification for Microcystis was assessed as primary P limitation because the nutrient classification does not differentiate between positive or negative effects [38]. For more than 8% of the resampled datasets, a null model fit the data equally as parsimoniously as a model that included treatment contrasts for cell abundances of Anabaena. This is conceptually equivalent to an insignificant F-value in an ANOVA and indicates that high variability within replicates and a high frequency of zero counts precluded an objective assessment of its nutrient limitation status. However, by the end of the experiment, Anabaena had highest cell densities in the P-only treatments (Fig. 6). Aphanizomenon abundance was also highly variable but showed declines in abundance for all treatments.
Nitrogen fixation responses
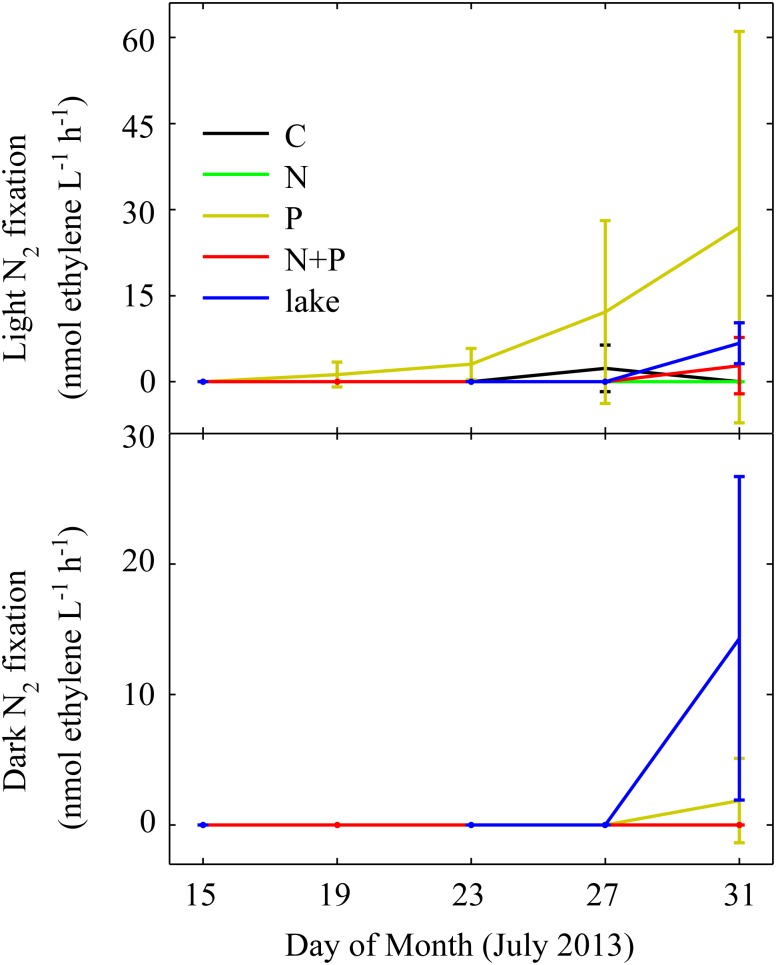
Nitrogen fixation (as acetylene reduction) responses were monitored throughout the experimental period. Extremely low (and statistically insignificant) rates of N2 fixation were observed relative to filtered lake water and reagent blanks in both the lake and among mesocosm treatments (Fig. 8). These results support the above-mentioned microscopic observations indicating that, while filamentous N2 fixing genera Anabaena and Aphanizomenon were present, and Anabaena increased in the P-only treatment (Fig. 6), heterocyst frequency was very low, and only traces of N2 fixation were observed at the end of the experiment (Figs. 7, 8). Therefore, while the potential for this process exists, there was no significant activity. These results were supported by direct measurements of total N (TN) in the mesocosms, which showed no evidence that N2 fixation led to a significant increase in TN in response to P additions. This proved true for all treatments, including the P-only treatment, which was expected to yield the highest potential for N2 fixation.
Figure 8. Time series of light and dark acetylene reduction rates from the summer 2013 mesocosm experiment.
Solid lines connect means of triplicate mesocosms. Error bars are one standard deviation.
We calculated the percentage of Anabaena’s N requirement (based on growth rates and cell N quotas) that may have been satisfied by N2 fixation, based on our AR measurements and conversions to N2 fixed using a 4∶1 ethylene production:N2 fixation ratio. At most, only 20% of Anabaena’s N needs were met this way. If we extrapolate these results to the entire phytoplankton community, less than 5% of N needs are met by N2 fixation under P-enriched conditions. There were no significant rates of N2 fixation in any of the other treatments.
These results indicate that despite the presence of N limited and N+P co-limited conditions, diazotrophic genera must be competing with non-N2 fixing genera (i.e., Microcystis and eukaryotes) for combined N sources to sustain growth. This situation persisted throughout the experimental period.
Interestingly, the P only treatments did not stimulate total cyanobacterial community production. Instead, only diatoms showed selective stimulation by this treatment, and even then, this stimulation only occurred early in the experiment. Overall, enhanced N limitation (through selective P enrichment) did not lead to a significant increase in cyanobacterial biomass or result in N2 fixation, but the N+P treatment did enhance cyanobacterial biomass (Fig. 4).
Discussion
Results from the mesocosm nutrient addition experiment confirm prior work based on short-term (<1 week) microcosms, indicating that, during summer bloom periods, growth of Taihu’s cyanobacteria-dominated phytoplankton community is primarily controlled by N supply and further stimulated by N and P together [26], [27]. Despite summer N limitation, N2 fixing cyanobacteria did not significantly increase in abundance, heterocyst frequency, or N2 fixing activity, either in the mesocosm experiments or in ambient lake water. These results do not support Schindler et al.’s [21] conclusion that diazotrophic cyanobacteria should dominate and quantitatively fix N2 to compensate for N requirements needed to sustain phytoplankton community production rates. The length of mesocosm experiments of up to a month should have provided plenty of time for numerous generations of N2 fixers to proliferate and for vegetative cells to differentiate into heterocysts [5], [8]. Hence, the low N fixation rates were not attributable to short incubation time. The question therefore arises: Why were non-N2 fixing genera (Microcystis) not replaced by active diazotrophs (Anabaena, Aphanizomenon) during P enrichment, despite the fact that these genera were observed in the lake throughout the experimental period?
In addressing this question, we note that phytoplankton growth also may be constrained by other factors, including temperature, availability of micronutrients (Fe, trace metals), and adequate light for supporting energy-demanding processes, such as N2 fixation. Other ecological factors could play additional roles, including the ability to regulate buoyancy (important for accessing light and nutrients), grazing, and beneficial as well as antagonistic associations with other microbes. These factors are known to play a role in the competitive interactions between diazotrophic and non-diazotrophic cyanobacterial species [3].
In shallow, well mixed, highly turbid Taihu, the ability of non-diazotrophs to access and store nutrients, while maintaining high photosynthetic rates and competing for N sources with diazotrophs, is likely important in the initiation and maintenance of blooms. The non-diazotroph Microcystis dominated prior to the experiment and remained dominant while N was severely limiting (i.e., P alone treatment). N limitation constrained the ability of a bloom, equal in biomass to the ambient lake, to form. Microcystis maintained its dominance over N2 fixing genera under N limitation and during a decrease in overall biomass. Its ability to effectively compete for dwindling N sources, especially NH4 + regenerated from sediments and organic N, provides an advantage [39], [40]. Microcystis dominance may also be related to: 1) effective cellular N storage; 2) access to sediment and water column regenerated N (as NH4 +) via buoyancy regulation; 3) close associations with heterotrophic bacteria present around colonies, which enhanced ‘phycosphere’-scale nutrient (including N and P) recycling [41]; and 4) more efficient use of light for supporting photosynthetic growth and nutrient sequestration than diazotrophic genera. The latter selective mechanism may be quite important in Taihu’s turbid, shallow waters, in which Microcystis may be particularly effective in vertically orienting itself [42].
Since N2 fixation is a very energy-demanding process [5], light availability in highly turbid Taihu may be insufficient for diazotrophs to compete with the effective N sequestration mechanisms that Microcystis possesses [39], [43]. Secchi depths in Taihu are commonly in the range of 8–85 cm (mean = 38 cm; [44], [45], which indicates that a substantial amount of the 2 m deep water column experiences very low light levels (<5% of incident light). Suspended solids, and not chlorophyll or chromophoric organic matter, accounts for the vast majority of light attenuation in Taihu [45]. Therefore, we suggest that light limitation may interact with nutrient limitation in this hypertrophic lake to favor non-N2 fixing cyanobacteria, especially during maximum bloom periods, when surface scums effectively shade the underlying water column. Other shallow, highly turbid lakes (e.g., Lake Okeechobee, FL., Dutch lakes) show similar patterns, with Microcystis maintaining dominance over N2-fixing genera in N and light limited conditions [46], [47].
Iron (Fe) limitation could have played a role in the inability of N2 fixers to assume dominance under the extreme N-limited conditions imposed in these experiments [5]. However, recent examinations indicated Fe-replete conditions during the summer blooms in Taihu [48]. Therefore, this is not a likely explanation for the absence of this process.
From a nutrient management perspective, results from our mesocosm experiments indicate that more N limited conditions induced by selectively enrichment with P did not lead to replacement of non-diazotrophs with diazotrophs in Taihu. This scenario may become more probable as overall nutrient reductions, leaching of stored nutrient supplies in sediments, and increases in transparency take place over time. However, this scenario will likely take several years to decades to emerge in this large, relatively long residence time (∼1 year) lake. Therefore, we conclude that N input reductions, in concert with P reduction (i.e., a dual nutrient strategy), is the most effective near-term strategy for reducing phytoplankton biomass, bloom potentials, and improving the overall trophic state of Taihu, as well as downstream N limited Yangtze estuary and South China Sea coastal waters. This approach requires a specific set of nutrient reduction targets, which are being formulated through the combined use of nutrient addition and dilution bioassays [26], [27]. At present, it appears that N input reductions would not lead to replacement of Microcystis with N2 fixers, even though this outcome could be considered a positive outcome, given the tendency for lower toxicity, growth rates, and ultimate biomasses of N2 fixers [8].
The use of microcosm and mesocosm experiments for determining phytoplankton responses to nutrient inputs has been criticized by Carpenter [49] as not reflective of ecosystem-level conditions. These approaches employ small-scale containers over short (days to weeks) incubation times and may not adequately mimic temporal and spatial scales for nutrient cycling and fluxes. However, prior work has shown remarkable agreement among nutrient enrichment and limitation experiments carried out over these time intervals with a wide size range of incubation vessels [50], [51]. Furthermore, incubations of at least two weeks should be adequate to elicit taxa-specific (including diazotrophic) responses to nutrient enrichment and other ecophysiological factors (e.g., changes in irradiance, temperature, pO2) [52].
We previously examined this question in Lake Taihu using 1 L to 4 L polyethylene Cubitainers over a time span of 1 week or less [26], [27]. These experiments indicated that N and P co-limitation was prevalent, especially during the critical summer bloom period. Others [51], [53] have shown similar results in eutrophic to hypereutrophic lakes. The question persists, however; can these results be extrapolated over longer time scales, and do they reflect natural conditions, such as sediment-water column nutrient exchange and seasonally-variable physical (temperature. light), chemical, and biological (grazing, microbial interactions) conditions? Can species and group succession patterns be replicated over the relatively short time scales that these experiments are conducted? This question is particularly relevant to the issue of replacement of non-N2 fixing with N2 fixing cyanobacterial species.
In shallow, hypertrophic Lake Taihu, there was remarkable agreement between ambient lake water and mesocosms deployed in the lake. As N limitation was intensified during the summer period, the non-N2 fixing cyanobacterial community was not replaced by a N2 fixing community. Most likely, Microcystis remained dominant during P enriched conditions in the lake because it effectively competes for regenerated N (as NH4 +) from sediments and the water column [27], [54], as noted in previous mesocosm experiments in a shallow, eutrophic, Swedish lake [39]. Energetically, Microcystis must be able to access regenerated N more efficiently than Anabaena and Aphanizomenon can fix N2 to meet their N requirements. The tradeoffs of regenerated N vs. fixed N need further study, especially relative to selective nutrient management strategies aimed at bringing hypertrophic lakes, like Taihu, below bloom thresholds. Our results, combined with the fact that sediment N:P is low in Taihu [55] and most eutrophic lakes, show that both N and P reductions are needed in the short-term, in large part due to the legacy of high N and P loading and sediment nutrient storage common in this and other eutrophic lakes [7], [47], [56]. For this reason, reducing only P loading to Lake Taihu, without parallel N reductions, is unlikely to improve water quality (i.e., reductions in bloom frequency and intensity) on a time scale that will be acceptable to the public and managers.
Recent evidence suggests that N loading may indirectly cause P release from sediments via disruption of the sulfur and Fe cycles [57], providing additional justification for a dual nutrient management approach. Failure to control N loading also has consequences for aquatic systems located downstream [20]. Excessive N loads to coastal and estuarine systems is linked to widespread development of algal blooms, such as red tide, and bottom-water hypoxia (e.g., the northern Gulf of Mexico). A P-only approach to watershed nutrient management for Taihu and other freshwater systems simply shunts N-driven eutrophication issues further downstream to the N sensitive coastal zone [20], an approach that we believe is irresponsible.
Acknowledgments
We appreciate the in depth review of this manuscript by 2 anonymous reviewers. Technical support was provided by Betsy Abare, Randy Sloup, Jianrong Ma, and Jianming Deng. The Taihu Laboratory for Lake Ecosystem Research (TLLER), Chinese Academy of Sciences, provided the environmental monitoring data.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by the US National Science Foundation (0826819, 1230543, and 1240851), the National Science Foundation of China (41003043, 41230744), Natural Science Foundation of Jiangsu Province (BK2012895), and the External Cooperation Program of Chinese Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, et al. (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10: 1124–1134. [DOI] [PubMed] [Google Scholar]
- 2. Lewis WM Jr, Wurtsbaugh WA, Paerl HW (2011) Rationale for control of anthropogenic nitrogen and phosphorus in inland waters. Environ. Sci. & Technol. 45: 10030–10035. [DOI] [PubMed] [Google Scholar]
- 3. Paerl HW, Fulton RS III, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. The Scientific World 1: 76–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huisman JM, Matthijs HCP, Visser PM (2005) Harmful cyanobacteria. Springer Aquatic Ecology Series 3. Springer. [Google Scholar]
- 5.Fogg GE (1974) Nitrogen fixation. In: Stewart WDP, editor. Algal physiology and biochemistry. Blackwell, Oxford. 560–583. [Google Scholar]
- 6. Schindler DW (1975) Whole-lake eutrophication experiments with phosphorus, nitrogen and carbon. Verh. Int. Ver. Theor. Angew. Limnol. 19: 3221–3231. [Google Scholar]
- 7. Smith VH (1983) Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221: 669–671. [DOI] [PubMed] [Google Scholar]
- 8. Attridge EM, Rowell P (1997) Growth, heterocyst differentiation and nitrogenase activity in the cyanobacteria Anabaena variabilis and Anabaena cylindrica in response to molybdenum and vanadium. New Phytol. 135: 517–526. [Google Scholar]
- 9. Howarth RW, Swaney DP, Billen G, Garnier J, Hong B, et al. (2012) Nitrogen fluxes from large watersheds to coastal ecosystems controlled by net anthropogenic nitrogen inputs and climate. Front. Ecol. & the Environ. 10: 37–43. [Google Scholar]
- 10.Likens GE editor (1972) Nutrients and eutrophication, American Society of Limnology Oceanography special symposium 1. Am. Soc. Limnol. Oceanography.
- 11. Nixon SW (1995) Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219. [Google Scholar]
- 12. Lewis WM Jr, Wurtsbaugh WA (2008) Control of lacustrine phytoplankton by nutrients: Erosion of the phosphorus paradigm. Inter. Rev. Ges. Hydrobiol. 93: 446–465. [Google Scholar]
- 13. Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, et al. (2009) Controlling eutrophication: Nitrogen and phosphorus. Science 323: 1014–1015. [DOI] [PubMed] [Google Scholar]
- 14. Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: Causes, consequences and controls. Microb. Ecol. 65: 995–1010. [DOI] [PubMed] [Google Scholar]
- 15. Paerl HW, Hall NS, Calandrino ES (2011a) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 409: 1739–1745. [DOI] [PubMed] [Google Scholar]
- 16.Paerl HW, Fulton RS III (2006) Ecology of harmful cyanobacteria. In: Graneli E, Turner J, editors. Ecology of harmful marine algae. Springer-Verlag. 95–107. [Google Scholar]
- 17. Seitzinger SP, Harrison JA, Boehlke JK, Bouwman AF, Lowrance R, et al. (2006) Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 16: 2064–2090. [DOI] [PubMed] [Google Scholar]
- 18. Paerl HW, Scott JT (2010) Throwing fuel on the fire: Synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. & Technol. 44: 7756–7758. [DOI] [PubMed] [Google Scholar]
- 19. Scott JT, McCarthy MJ (2010) Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 55: 1265–70. [Google Scholar]
- 20. Paerl HW (2009) Controlling eutrophication along the freshwater–marine continuum: Dual nutrient (N and P) reductions are essential. Estuar. Coasts 32: 593–601. [Google Scholar]
- 21. Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, et al. (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37 year whole ecosystem experiment. Proc. Nat. Acad. Sci. USA 105: 11254–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryhn AC, Håkanson L (2009) Coastal eutrophication: Whether N and/or P should be abated depends on the dynamic mass balance. Proc. Natl. Acad. Sci. USA 106: E3 doi:10.1073/pnas.0810905106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paterson MJ, Schindler DW, Hecky RE, Findlay DL, Rondeau KJ (2011) Comment: Lake 227 shows clearly that controlling inputs of nitrogen will not reduce or prevent eutrophication of lakes. Limnol. Oceanogr. 56: 1545–1547. [Google Scholar]
- 24. Chen Y, Qin B, Teubner K, Dokulil MT (2003) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankt. Res. 25: 445–453. [Google Scholar]
- 25. Qin B, Zhu G, Gao G, Zhang Y, Li W, et al. (2010) A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environ. Man. 45: 105–112. [DOI] [PubMed] [Google Scholar]
- 26. Xu H, Paerl HW, Qin B, Zhu G, Gao G (2010) Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 55: 420–432. [Google Scholar]
- 27. Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, et al. (2011b) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 45: 1973–1983. [DOI] [PubMed] [Google Scholar]
- 28. Qin B, Liu B, Havens K (2007) Eutrophication of shallow lakes with special reference to Lake Taihu, China. Hydrobiologia 581: 1–327. [Google Scholar]
- 29.American Public Health Association [APHA] (1995) Standard methods for the examination of water and wastewater, 19th ed. APHA, American Water Works Association, Water Environment Federation, Washington, DC.
- 30. Ebina J, Tsutsui T, Shirai T (1983) Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Wat. Res. 17: 1721–1726. [Google Scholar]
- 31. Pinckney JL, Richardson TL, Millie DF, Paerl HW (2001) Application of photopigment biomarkers for quantifying microalgal community composition and in situ growth rates. Org. Geochem. 32: 585–595. [Google Scholar]
- 32. Papista E, Acs E, Boeddi B (2002) Chlorophyll a determination with ethanol–a critical test. Hydrobiologia 485: 191–198. [Google Scholar]
- 33. Van Heukelem L, Lewitus A, Kana T, Craft N (1994) Improved separations of phytoplankton pigments using temperature-controlled high performance liquid chromatography. Mar. Ecol. Progr. Ser. 114: 303–313. [Google Scholar]
- 34. Mackey MD, Mackey DJ, Higgins HW, Wright SW (1996) CHEMTAX - a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 144: 265–283. [Google Scholar]
- 35. Schlüter L, Lauridsen TL, Krogh G, Jørgensen T (2006) Identification and quantification of phytoplankton groups in lakes using new pigment ratios – a comparison between pigment analysis by HPLC and microscopy. Freshw. Biol. 51: 1474–1485. [Google Scholar]
- 36.Hu H, Li Y, Wei Y, Zhu H, Shi Z (1980) Freshwater Algae in China. Shanghai Science and Technology Press. [in Chinese]. [Google Scholar]
- 37.Paerl HW (1998) Microbially-mediated nitrogen cycling. In: Burlage R, editor. Techniques in microbial ecology. Oxford University Press. 3–30. [Google Scholar]
- 38. Andersen T, Saloranta TM, Tamminen T (2005) A statistical procedure for unsupervised classification of nutrient limitation bioassay experiments with natural phytoplankton communities. Limnol. Oceanogr. : Methods 5: 111–118. [Google Scholar]
- 39. Blomqvist P, Pettersson A, Hyenstrand P (1994) Ammonium-nitrogen: A key regulatory factor causing dominance of non-nitrogen-fixing cyanobacteria in aquatic systems. Arch. Hydrobiol. 132: 141–164. [Google Scholar]
- 40. Flores E, Herrero A (2005) Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Transact. 33: 164–167. [DOI] [PubMed] [Google Scholar]
- 41. Paerl HW, Millie DF (1996) Physiological ecology of toxic cyanobacteria. Phycologia 35: 160–167. [Google Scholar]
- 42. Reynolds CS, Jaworski GMH, Cmiech HA, Leedale GF (1981) On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz. emend. Elenkin. Phil. Trans. Roy. Soc. Lond. Ser. B 293: 419–477. [Google Scholar]
- 43.Kappers FI (1980) The cyanobacterium Microcystis aeruginosa and the nitrogen cycle of the hypertrophic Lake Brielle (The Netherlands), In: Barica J, Mur L, editors. Hypertrophic ecosystems. Dr. W. Junk. 37–43. [Google Scholar]
- 44. Zhang Y, Qin B, Zhu G, Gao G, Luo L, et al. (2006) Effect of sediment resuspension on underwater light field in shallow lakes in the middle and lower reaches of the Yangtze River: A case study in Longgan Lake and Taihu Lake. Science in China: Series D Earth Sciences 49: 114–125. [Google Scholar]
- 45. Shi K, Zhang Y, Liu X, Wang M, Qin B (2014) Remote sensing of diffuse attenuation coefficient of photosynthetically active radiation in Lake Taihu using MERIS data. Remote Sensing of Environment 140: 365–377. [Google Scholar]
- 46. Scheffer M, Rinaldi S, Gragnani A, Mur LR, van Nes EH (1997) On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78: 277–282. [Google Scholar]
- 47. Havens KE, James RT, East TL, Smith VH (2003) N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ. Pollut. 122: 379–390. [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Zhu G, Qin B, Paerl HW (2012) Growth response of the cyanobacterium Microcystis spp. to nitrogen, phosphorus and iron enrichment in different regions of Lake Taihu, China. Hydrobiologia 700: 187–202. [Google Scholar]
- 49. Carpenter SR (1996) Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 77: 677–680. [Google Scholar]
- 50. Elser JJ, Marzolf E, Goldman CR (1990) The roles of phosphorus and nitrogen in limiting phytoplankton growth in freshwaters: A review of experimental enrichments. Can. J. Fish. Aquat. Sci. 47: 1468–1477. [Google Scholar]
- 51. Spivak AC, Vanni MJ, Mette EM (2010) Moving on up: can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshw. Biol. 56: 279–291. [Google Scholar]
- 52. Paerl HW (1990) Physiological ecology and regulation of N2 fixation in natural waters. Adv. Microb. Ecol. 11: 305–344. [Google Scholar]
- 53. Dodds WK, Johnson KR, Priscu JC (1989) Simultaneous nitrogen and phosphorus deficiency in natural phytoplankton assemblages: Theory, empirical evidence and implications for lake management. Lake and Res. Man. 5: 21–26. [Google Scholar]
- 54. McCarthy MJ, Lavrentyev PJ, Yang L, Zhang L, Chen Y, et al. (2007) Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China). Hydrobiologia 581: 195–207. [Google Scholar]
- 55. Trolle D, Zhu G, Hamilton D, Luo L, McBride C, et al. (2009) The influence of water quality and sediment geochemistry on the horizontal and vertical distribution of phosphorus and nitrogen in sediments of a large, shallow lake. Hydrobiologia 627: 31–44. [Google Scholar]
- 56. Jeppesen E, Søndergaard M, Meerhoff M, Lauridsen TL, Jensen JP (2007) Shallow lake restoration by nutrient loading reduction–some recent findings and challenges ahead. Hydrobiologia 584: 239–252. [Google Scholar]
- 57. Smolders AJP, Lucassen ECHET, Bobbink R, Roelofs JGM, Lamers LPM (2010) How nitrate leaching from agricultural lands provokes phosphate eutrophication in groundwater fed wetlands: The sulphur bridge. Biogeochemistry 98: 1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.