Abstract
Decreased function of human mitochondrial branched chain alpha-ketoacid dehydrogenase complex results in branched chain ketoacidemia or maple syrup urine disease. Activity of this multienzyme complex varies from 0 to approximately 15% of wild type branched chain alpha-ketoacid dehydrogenase complex activity within the population of homozygous affected individuals. We used the technique of Western Blotting with antibodies against purified bovine liver branched chain alpha-ketoacid dehydrogenase complex to screen mitochondrial proteins from cultured human fibroblasts for immunocrossreactive proteins. This method probes the physical structure of the proteins forming this multienzyme complex. One patient with branched chain ketoacidemia lacked an immunoreactive transacylase protein. This protein catalyzes the transfer of the branched chain acyl group from the decarboxylase to reduced coenzyme A. Kinetic analysis of the enzyme activity in cell lysates from this patient confirmed that the complex would not utilize coenzyme A. Thus, we have defined a structural basis for an impaired multienzyme complex of mitochondria in man.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuang D. T., Hu C. W., Patel M. S. Induction of the branched-chain 2-oxo acid dehydrogenase complex in 3T3-L1 adipocytes during differentiation. Biochem J. 1983 Jul 15;214(1):177–181. doi: 10.1042/bj2140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCIS J., LEVITZ M., MILLER S., WESTALL R. G. Maple syrup urine disease. Br Med J. 1959 Jan 10;1(5114):91–93. doi: 10.1136/bmj.1.5114.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Davidson E. D., Elsas L. J., 2nd Thiamine increases the specific activity of human liver branched chain alpha-ketoacid dehydrogenase. Nature. 1975 Apr 10;254(5500):529–530. doi: 10.1038/254529a0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Elsas L. J., 2nd Subcellular distribution and cofactor function of human branched chain alpha-ketoacid dehydrogenase in normal and mutant cultured skin fibroblasts. Biochem Med. 1975 May;13(1):7–22. doi: 10.1016/0006-2944(75)90135-0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Elsas L. J., 2nd Stabilization of mammalian liver branched-chain alpha-ketoacid dehydrogenase by thiamin pyrophosphate. Arch Biochem Biophys. 1980 Jun;202(1):23–28. doi: 10.1016/0003-9861(80)90401-4. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Priest J. H. Branched-chain ketoacid dehydrogenase activity and growth of normal and mutant human fibroblasts: the effect of branched-chain amino acid concentration in culture medium. Biochem Genet. 1983 Oct;21(9-10):895–905. doi: 10.1007/BF00483948. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Wheeler F. B., Lemmon S. K., Elsas L. J., 2nd In vivo and in vitro response of human branched chain alpha-ketoacid dehydrogenase to thiamine and thiamine pyrophosphate. Pediatr Res. 1978 Mar;12(3):235–238. doi: 10.1203/00006450-197803000-00016. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., 2nd, Danner D. J. The role of thiamin in maple syrup urine disease. Ann N Y Acad Sci. 1982;378:404–421. doi: 10.1111/j.1749-6632.1982.tb31214.x. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., Pask B. A., Wheeler F. B., Perl D. P., Truster S. Classical maple syrup urine disease: cofactor resistance. Metabolism. 1972 Oct;21(10):929–944. doi: 10.1016/0026-0495(72)90027-3. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Antibodies to bovine liver branched-chain 2-oxo acid dehydrogenase cross-react with this enzyme complex from other tissues and species. Biochem J. 1983 Aug 1;213(2):339–344. doi: 10.1042/bj2130339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Elsas L. J., Danner D. J. Direct physical evidence for stabilization of branched-chain alpha-ketoacid dehydrogenase by thiamin pyrophosphate. Am J Hum Genet. 1984 Jul;36(4):802–807. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MENKES J. H. Maple syrup disease; isolation and identification of organic acids in the urine. Pediatrics. 1959 Feb;23(2):348–353. [PubMed] [Google Scholar]
- Schatz G. How mitochondria import proteins from the cytoplasm. FEBS Lett. 1979 Jul 15;103(2):203–211. doi: 10.1016/0014-5793(79)81328-9. [DOI] [PubMed] [Google Scholar]
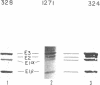
- Sheu K. F., Hu C. W., Utter M. F. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J Clin Invest. 1981 May;67(5):1463–1471. doi: 10.1172/JCI110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Abnormal mRNA for argininosuccinate synthetase in citrullinaemia. Nature. 1983 Feb 10;301(5900):533–534. doi: 10.1038/301533a0. [DOI] [PubMed] [Google Scholar]