Abstract
A number of soil-borne microorganisms, such as mycorrhizal fungi and rhizobacteria, establish mutualistic interactions with plants, which can indirectly affect other organisms. Knowledge of the plant-mediated effects of mutualistic microorganisms is limited to aboveground insects, whereas there is little understanding of what role beneficial soil bacteria may play in plant defense against root herbivory. Here, we establish that colonization by the beneficial rhizobacterium Azospirillum brasilense affects the host selection and performance of the insect Diabrotica speciosa. Root larvae preferentially orient toward the roots of non-inoculated plants versus inoculated roots and gain less weight when feeding on inoculated plants. As inoculation by A. brasilense induces higher emissions of (E)-β-caryophyllene compared with non-inoculated plants, it is plausible that the non-preference of D. speciosa for inoculated plants is related to this sesquiterpene, which is well known to mediate belowground insect-plant interactions. To the best of our knowledge, this is the first study showing that a beneficial rhizobacterium inoculant indirectly alters belowground plant-insect interactions. The role of A. brasilense as part of an integrative pest management (IPM) program for the protection of corn against the South American corn rootworm, D. speciosa, is considered.
Introduction
Soil-borne microorganisms, including mycorrhizal fungi and rhizobacteria, can colonize roots and establish mutualistic interactions, inducing a range of plant responses, from growth promotion to pathogen defense [1]. From a plant’s perspective, up to 25% of photosynthesis is allocated toward root exudation, providing an organic-rich nutrient base for the rhizosphere microbiome [2]. Given these changes in plant nutritional quality and defense status, it is expected that mutualistic associations with beneficial microorganisms can impact above- and belowground plant-insect interactions [3].
Knowledge on the plant-mediated effects of mutualistic microorganisms is currently limited to aboveground insects (review by Pineda et al. [4]). Herbivores attacking plants in association with beneficial bacteria can either develop poorly or better depending on both their degree of specialization [5] and the specific plant-beneficial microorganism interaction [3], [6]. For some systems, the association of plants with beneficial microorganisms results in resistance to generalist herbivores, but not always to specialists [7], [8]. However, in other cases, beneficial microorganism-plant mutualism may benefit from herbivore attack [9].
Members of the bacterial genus Azospirillum are found in association with plant cereals worldwide [10] and have been used as inoculants for improving crop yields [11], [12]. In Brazil, Azospirillum brasilense Tarrand, Krieg and Döbereiner (Rhodospirillaceae) is the predominantly applied plant growth-promoting rhizobacterium (PGPR) in corn and wheat crops [13]. Despite the well-established beneficial effect of Azospirillum on crop plant growth and resistance to pathogens, the impact of inoculant application on herbivore performance and behavior is not well understood.
The South American corn rootworm, Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae), is a polyphagous herbivore, particularly during the adult stage, when this species causes severe damage to corn (Zea mays L.), beans (Phaseolus spp.) and soybeans (Glycine max (L.) Merrill) [14]. The larval stage lives in the soil and is usually found in corn roots, where its development is optimal compared with in other crop plants [15].
Considering the substantial damage inflicted by D. speciosa larvae to corn roots in Brazil and the wide use of A. brasilense as an inoculant, the present study investigated the plant-mediated effects of A. brasilense in corn on the host selection and performance of D. speciosa larvae. As host choice by rhizophagous insects is guided by root volatiles in particular [16], we also assessed whether root volatile emissions are altered in response to colonization by A. brasilense. To the best of our knowledge, this is the first study showing that a beneficial rhizobacterium inoculant indirectly alters belowground plant-insect interactions.
Materials and Methods
Plant Growth Conditions and Inoculation
Corn seeds (Z. mays, variety Delprim; Delley Semences et Plants SA, Delley, Switzerland) were inoculated with Azospirillum brasilense (strains AbV5 and AbV6) via mixture with Nitro 1000 (Cascavel, PR, Brazil) at a concentration of 1×107 colony formation units (CFU)/mL. Although A. brasilense is commercially referred to as a plant-growth-promoting rhizobacterium (PGPR), we did not detect any plant growth promotion, at least in terms of root and shoot biomass, within 10 days after inoculation (Figures S1 and S2). Therefore, we have adopted the term plant-beneficial rhizobacteria throughout this work. Control corn seeds were prepared in the same way as inoculated seeds but using distilled water in place of the inoculum. Seeds were sown in Basiplant potting soil (250 cm3) with no additional fertilization and were grown in an insect-free greenhouse from July to October 2013 (winter-spring) under natural light (Piracicaba, SP, Brazil).
Insect Rearing
Adults of the South American corn rootworm, D. speciosa, were collected in the field (from cucumber crops in Piracicaba, SP, Brazil, 22°43′14″ to 22°42′ 01″ S and 47°38′46″ to 47°36′49″ W, where the insect is an agricultural pest and no specific permission is required) and multiplied under laboratory conditions (25°C, 60% RH, 12 L:12 D) for three to five generations. Briefly, adults were fed on common beans (Phaseolus vulgaris L.) and larvae on corn seedlings. Eggs were collected every other day from black-dyed gauze that served as a substrate for oviposition and then transferred to Petri dishes with wet filter paper until hatching. Newly hatching larvae were immediately transferred to corn seedlings germinated in vermiculite. After approximately 30 days, adults emerged. For details on the method used for D. speciosa laboratory rearing, see reference [17]. The field collections involved only D. speciosa adults and therefore did not involve any endangered or protected species.
Olfactometer Assays
Host selection by D. speciosa larvae were evaluated in a glass six-arm olfactometer consisting of a central chamber (8 cm length, 10 cm diameter) with six arms connected to side chambers (5 cm length, 3 cm diameter) [18]. Three days before the bioassay, 7- to 8-day-old corn seedlings were transferred to the olfactometry side chambers, and the remaining space was filled with a mixture of sterile sand and rocks, in a 2∶1 ratio, moistened with 10% water (dry sand:water; g/g). Each side chamber containing wet soil and seedlings was weighed before being transferred to the greenhouse. To maintain the moisture content at approximately 10% during the three days in the greenhouse, we weighed the chamber set daily and added the appropriate volume of water to return it to its initial weight. The treatment chambers were alternated with controls, and a total of 30 second-third instar D. speciosa larvae were released in the central chamber, where they could freely choose among the 6 chambers over 24 hours. Thereafter, the olfactometer was disassembled, and the number of larvae in each side chamber was registered. When a larva did not leave the central chamber, it was recorded as a non-choice. Each assay replicate employed fresh plants and insect sets.
Larval Performance
Newly hatched D. speciosa larvae were allowed to feed on 5-day-old inoculated or non-inoculated corn seedlings grown in pots containing sterile vermiculite (25±1°C, 60% RH, 12 L:12 D). Each pot contained 30 corn seedlings, with one larva/seedling. The larvae were recovered ten days after feeding and weighed individually.
Volatile Collection and Analysis
The shoots and roots of inoculated and non-inoculated plants were harvested and weighed, and the roots were flash frozen in N2(l) and stored for volatile analysis. To collect volatiles, frozen roots were ground into a powder in liquid nitrogen, and a 30 mg aliquot was transferred to a 4 mL screw cap septum vial. According to Rasmann et al. [18], an SPME syringe carrying 100 µm polydimethylsiloxane (PDMS) (Supelco, Bellefonte, PA, USA) was used to puncture the septum, and the tissue was exposed for 60 minutes at 25°C. The SPME fiber was then immediately subjected to GC injection into an HP5-MS capillary column (JeW Scientific, Folsom, CA; 30 m×0.25 mm×0.25 µm) using Helium as the carrier gas. The SPME fiber was held in the injector (250°C) for 5 min to completely elute volatiles from the fiber. The column temperature was maintained at 40°C for five min, then increased to 150°C (5°C/min) for one min, then raised again (5°C/min) until reaching the final temperature of 250°C. Compounds were MS identified (Varian 4000) based on a comparison of the obtained mass spectra and retention times with those of authentic standards and the Kovats Index (KI) using n-alkane (C7–C30) standards [19] (Table 1).
Table 1. Identification of compounds in the root volatile profile.
| Identified compound | Rt (min) | KI | % | |
| Inoculated | Non-inoculated | |||
| decanal | 20.351 | 1206 | 6.68 | 22.66 |
| unknown hydrocarbon I | 23.968 | 1336 | 10.54 | 11.38 |
| unknown hydrocarbon II | 24.240 | 1346 | 6.84 | 6.56 |
| α-copaene | 25.136 | 1380 | 4.69 | 4.92 |
| 1-tetradecene | 25.450 | 1393 | 10.96 | 17.74 |
| (E)-β-caryophyllene | 26.289 | 1424 | 50.85 | 22.15 |
| geranyl acetone | 27.026 | 1454 | 9.44 | 14.60 |
Retention time (Rt), Kovats Index (KI), peak area (%) and identification of compounds emitted by inoculated and non-inoculated corn roots through combined GC-MS analysis.
Scanning Electron Microscopy
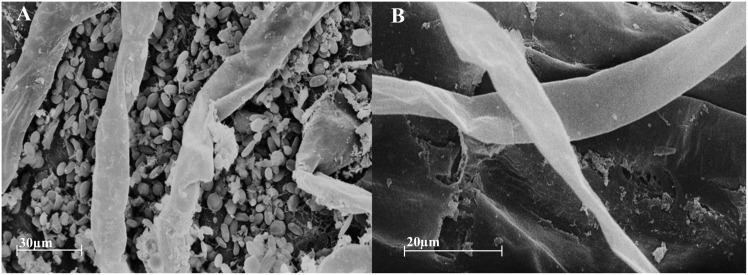
The roots of inoculated and non-inoculated corn were cut, rinsed in tap water and fixed in 3% glutaraldehyde in 50 mM cacodylate buffer (pH 7.0). Tissue dehydration was performed in a graded series ending in absolute acetone, followed by critical point drying. Then, the samples were mounted on stubs and subjected to gold sputtering (SDC-050 Sputter Coater, BAL-TEC). Finally, the samples were analyzed on a Zeiss LEO 435 VP scanning electron microscope.
Statistical Analysis
Kolmogorov–Smirnov and Levene’s tests were carried out to determine the normality and homogeneity of the data. Data from the olfactometer choice assays and the larval weights obtained in the performance experiments were analyzed using either general log-linear model (glm) or general log-linear mixed model (glmm) (P<0.05). The relative amounts of volatile compounds released by inoculated and control corn roots were Log10(x+1) transformed and analyzed through One-Way ANOVA followed by Tukey’s HSD (P<0.05). The statistical tests were performed using the software package R (www.R-project.org) version 2.8.1 and Minitab Release 14.
Results
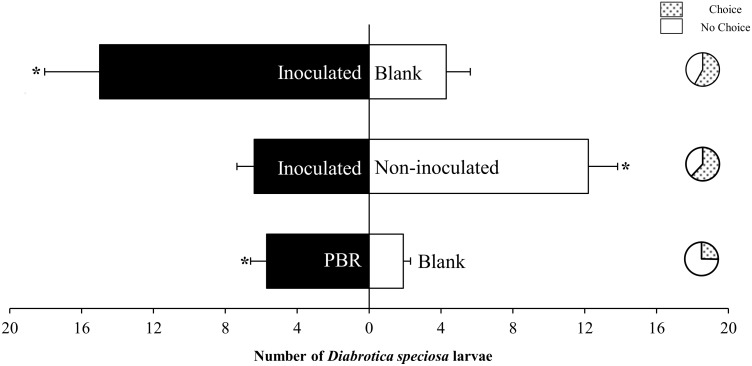
Scanning electron microscopy revealed that the roots of inoculated plants were successfully colonized by A. brasilense (Figure 1). To determine whether corn roots inoculated with A. brasilense influence insect behavior, a six-arm olfactometer was employed, in which South American corn rootworm larvae were allowed to move through soil to one of two stimulus choices. Regarding the choice of plants that were either inoculated or not inoculated with A. brasilense, the beetle larvae preferred non-inoculated plants (Figure 2, n = 10; glm, F1,18 = 10.13, P = 0.005), which emitted 72% less (E)-β-caryophyllene (Figure 3). To confirm that the outcome of the interaction was not a result of odors derived from the inoculant itself, larvae were presented with soil either inoculated with A. brasilense or not, but few larvae left the central chamber (75% of larvae were non-responsive), and the responsive larvae preferentially chose arms with the inoculant (Figure 2, n = 10; glm, F1,18 = 17.46, P<0.001). When larvae were allowed to choose between non-inoculated soil (blank) and inoculated plants, the larvae preferentially oriented toward the inoculated plants (Figure 2, n = 3; glm, F1,4 = 12.06, P = 0.025). Therefore, in the absence of a suitable host, the larvae oriented toward non-preferred hosts to survive. No chamber bias was observed, with an equal larval distribution between soil without plants and soil with non-inoculated plants (Figure S3).
Figure 1. Scanning electron microscopy images of inoculated and non-inoculated corn roots.
Scanning electron microscopy images showing the colonization of corn roots by the plant-beneficial rhizobacterium Azospirillum brasilense. (A) Inoculated corn roots and (B) non-inoculated corn roots.
Figure 2. Effect of Azospirillum brasilense on Diabrotica speciosa larval host choice.
Diabrotica speciosa larval choice between inoculated plants and the blank treatment (non-inoculated soil), inoculated and non-inoculated corn, and the plant-beneficial rhizobacterium (PBR) inoculant and the blank. Bars represent the mean number of larvae ± SE. Pie charts on the right represent non-responsive (no choice) and responsive (choice) larvae. Asterisks indicate a significant difference between treatments according to a quasi-Poisson glm (n = 10, P<0.05).
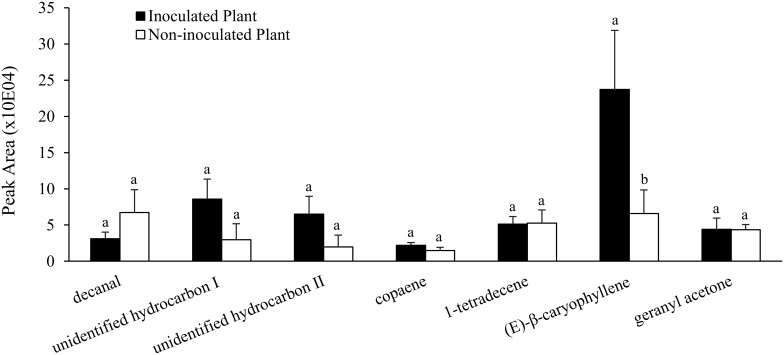
Figure 3. Root volatile profile induced by Azospirillum brasilense colonization.
Emissions of volatile compounds from inoculated and non-inoculated corn roots. Bars represent the mean ± SE. Different letters indicate a significant difference between treatments according to One-Way ANOVA followed by Tukey’s HSD test (n = 4, P<0.05).
To determine the role of the plant-beneficial rhizobacterium interaction in belowground plant defenses against insect pests, the root volatile profile was chemically characterized and quantified in A. brasilense inoculated roots (Table 1). The sesquiterpene (E)-β-caryophyllene was detected at a 3.6-fold higher level in inoculated plants than in the non-inoculated control via in vitro SPME analysis (Figure 3 and Table 1, Tukey’s HSD, One-Way ANOVA, F1,6 = 7.96, P = 0.030). Other volatile metabolites detected in the corn roots included decanal, copaene, 1-tetradecene and geranyl acetone as well as two unidentified hydrocarbons that were not induced by the plant-beneficial rhizobacteria (Figure 3 and Table 1).
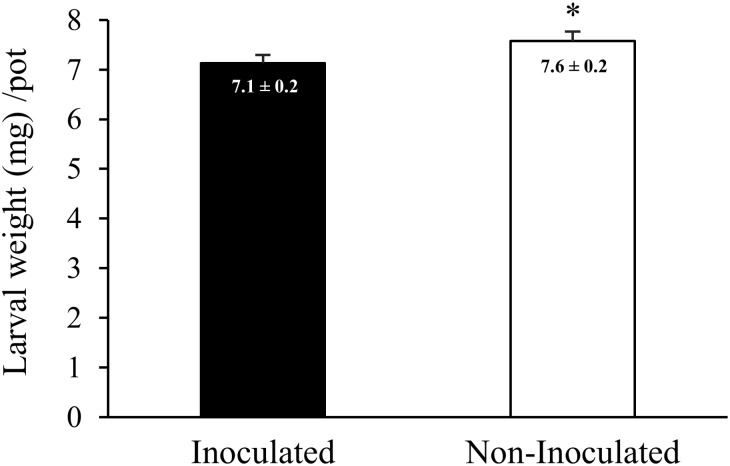
In addition to testing larval host selection for inoculated and non-inoculated plants, larval performance was assayed by monitoring larval weight in relation to diet. The D. speciosa larvae were heavier when they fed on non-inoculated plants than on inoculated plants (Figure 4, n = 10; glmm F1,432 = 5.05 P = 0.025).
Figure 4. Effect of Azospirillum brasilense on Diabrotica speciosa performance.
Diabrotica speciosa larval performance when fed on inoculated and non-inoculated corn plants. Bars represent the mean larval weight ± SE. Asterisks indicate a significant difference between treatments according to according to a glmm (n = 10, P<0.05).
Discussion
Root colonization by rhizobacteria induces plant resistance against pathogens in numerous agricultural systems [20] as well as plant protection against herbivory in a number of examined crops [3], [21], [22], [23]. Previous studies on rhizobacterium- and mycorrhizal-inducible plant volatiles have primarily focused on aboveground plant-herbivore interactions [24], [25].
The present study shows that colonization by the plant-beneficial rhizobacterium A. brasilense alters host choice by D. speciosa larvae, which preferentially oriented toward the roots of non-inoculated versus inoculated plants (Figure 2). As odors from the inoculant itself were attractive to the larvae (Figure 2), it is clear that the effect of plant inoculation on D. speciosa behavior was associated with the beneficial rhizobacterium-plant interaction. Odors produced by plant-beneficial soil bacteria are known to induce plant defenses [26] and may play a role in interactions with higher trophic levels [27]. However, as the inoculant used here contains components responsible for maintaining bacterial colony survival, it was not possible to infer that bacterial odors act as attractants for D. speciosa larvae.
In the present study, we established that root colonization by the plant-beneficial rhizobacterium A. brasilense specifically augments (E)-β-caryophyllene emissions in corn roots. The larvae of D. v. virgifera can exploit (E)-β-caryophyllene concentrations as a signal to select corn plants infested by a suitable number of conspecifics [28]. Therefore, larval behavior ranges from attraction to repellence by (E)-β-caryophyllene emissions depending on host nutritional quality and defense status. Given that the performance of D. speciosa is poorer when feeding on plants inoculated with plant-beneficial rhizobacteria versus non-inoculated plants (Figure 4), elevated (E)-β-caryophyllene root emissions may provide a chemical signal for larvae to avoid unsuitable hosts.
Indeed, entomopathogenic nematodes have been shown to use elevated levels of (E)-β-caryophyllene released from D. virgifera-damaged roots as a chemical cue in host location [18]. In addition to (E)-β-caryophyllene mediating subterranean tritrophic interactions between plants, herbivores and natural enemies of herbivores, this biologically active signal also functions in plant pathogen defense to inhibit microbial and fungal growth and may be rhizobacterium induced during the activation of induced systemic induction [29], [30].
Although A. brasilense inoculated corn has been reported to exhibit an enhanced biomass [31], we did not observe growth promotion in inoculated ten-day-old seedlings (Figures S1 and S2), as this effect may occur in late developmental stages of corn [8]. Therefore, plant biomass alone did not directly impact larval performance or preference. It is more likely that the low larval weight associated with feeding on inoculated plants is due to induced plant defenses, though metabolic profiling has yet to be performed. Nonetheless, we cannot discard the possibility that the root-colonizing rhizobacterium A. brasilense displays entomopathogenicity [32].
In general, our data show that the beneficial rhizobacterium A. brasilense confers protection to corn plants against attack by D. speciosa larvae. It has been established that treatment of cucumber with plant-beneficial rhizobacteria results in greater control of populations of Diabrotica sp. compared with non-treated plots and even chemical control [21]. In addition, elevated emissions of (E)-β-caryophyllene from undamaged inoculated corn may recruit entomopathogenic nematodes, contributing to Diabrotica control. The potential for employing plant-beneficial rhizobacteria such as A. brasilense as a component of an integrative pest management (IPM) program for protection of corn against D. speciosa clearly warrants further investigation.
Supporting Information
Shoot biomass of inoculated and non-inoculated corn plants. Dry weight of the shoots of inoculated and non-inoculated corn plants (mean ± SE). No significant difference was detected according to Student’s t-test (n = 6).
(TIF)
Root biomass of inoculated and non-inoculated corn plants. Fresh weight of the roots of inoculated and non-inoculated corn plants (mean ± SE). No significant difference was detected according to Student’s t-test (n = 6).
(TIF)
Diabrotica speciosa larval choice in control assays in a six-arm olfactometer. Diabrotica speciosa larval choice in control assays using non-inoculated plants vs. non inoculated plants (control) and blank vs. blank (no stimulus). Bars represent the mean number of larvae ± SE. The pie charts on the right represent non-responsive (no choice) and responsive (choice) larvae. No significant difference was detected according to a quasi-Poisson glm (n = 5).
(TIF)
Density of the distribution of larval weight data. Density of the distribution of raw data on the weight of Diabrotica speciosa when fed on non-inoculated (pink area - control) and inoculated (light green area - inoc) corn. A total of 444 data points: 227 for larval weight on non-inoculated corn and 217 on inoculated corn.
(JPG)
Data set of Diabrotica speciosa host choice, larval performance, corn root volatile emissions, and root and shoot biomass.
(XLSX)
Acknowledgments
The authors are grateful to Carla F. Favaro and Prof. Antônio Augusto Franco Garcia for assisting with the identification of compounds and providing statistical advice. The authors thank NAP/MEPA (ESALQ/USP) for technical support in microscopy and “Nitro 1000 inoculantes biológicos” for proving the inoculant.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by the Instituto Nacional de Ciência e Tecnologia (INCT) Semioquimicos na Agricultura, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo Pesquisa do Estado de São Paulo (FAPESP 2008/57701-2). MFGVP and PAS are funded by the Fundação de Amparo Pesquisa do Estado de São Paulo (FAPESP 2012/12252-1 and 2013/11993-0). PWP was funded by the Fulbright Scholar Program (U.S. Scholar 2013-2014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Badri DV, Weis TL, van Der Lelie D, Vivanco JM (2009) Rhizosphere Chemical dialogues: plant-microbe interactions. Curr Opin Biotech 20: 642–650. [DOI] [PubMed] [Google Scholar]
- 2. Huang B, North GB, Nobel PS (1993) Soil sheath, photosynthate distribution to roots, and rhizosphere water relations for Opuntia ficus-indica. . Int J Plant Sci 154: 425–431. [Google Scholar]
- 3. Dean JM, Mescher MC, De Moraes CM (2009) Plant-rhizobia mutualism influences aphid abundance on soybean. Plant Soil 323: 187–196. [Google Scholar]
- 4. Pineda A, Zheng SJ, van Loon JJA, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-born microrganisms. Trends Plant Sci 15: 507–514. [DOI] [PubMed] [Google Scholar]
- 5. van Oosten VR, Bodenhausen N, Reymond P, van Pelt JA, van Loon LC, et al. (2008) Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis . Mol Plant-Microbe Interact 21: 919–930. [DOI] [PubMed] [Google Scholar]
- 6. Gange AC, Brown VK, Aplin DM (2003) Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol Lett 6: 1051–1055. [Google Scholar]
- 7. Pineda A, Zheng SJ, van Loon JJA, Dicke M (2011) Rhizobacteria modify plant-aphid interactions: a case of induced systemic susceptibility. Plant Biol 14: 83–90. [DOI] [PubMed] [Google Scholar]
- 8. Walker V, Bertrand C, Bellvert F, Moenne-Loccoz Y, Bally R, et al. (2011) Host plant secondary metabolite profiling shows a complex, strain-dependent response of maize to plant growth-promoting rhizobacteria of the genus Azospirillum . New Phytol 186: 494–506. [DOI] [PubMed] [Google Scholar]
- 9. Heath KD, Lau JA (2011) Herbivores alter the fitness benefits of a plant–rhizobium mutualism. Acta Oecol 37: 87–92. [Google Scholar]
- 10.Hungria M (2011) Inoculação com Azospirillum brasilense: inovação em rendimento e baixo custo. Embrapa Soja. http://www.cnpso.embrapa.br/download/doc325.pdf. Accessed 29 January 2014.
- 11. Salomone IG, Dobereiner J (1996) Maize genotype effects on the response to Azospirillum inoculation. Biol Fert Soils 21: 193–196. [Google Scholar]
- 12. Herschkovitz Y, Lerner A, Davidov Y, Okon Y, Jurkevitch E (2005) Azospirillum brasilense does not affect population structure of specific rhizobacterial communities of inoculated maize (Zea mays). Environ Microbiol 7: 1847–1852. [DOI] [PubMed] [Google Scholar]
- 13. Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010) Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331: 413–425. [Google Scholar]
- 14. Ventura MU, Mello EP, Oliveira ARM, Siminelli F, Marques FA, et al. (2001) Males Are Attracted by Female Traps: A New Perspective for Management of Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) Using Sexual Pheromone. Neotrop Entomol 30: 361–364. [Google Scholar]
- 15. Walsh GC (2003) Host Range and Reproductive Traits of Diabrotica speciosa (Germar) and Diabrotica viridula (F.) (Coleoptera: Chrysomelidae), Two Species of South American Pest Rootworms, with Notes on Other Species of Diabroticina. Environ Entomol 32: 276–285. [Google Scholar]
- 16. Robert CAM, Erb M, Hibbard BE, French BW, Zwahlen C, et al. (2012a) A specialist root herbivore reduces plant resistance and uses an induced plant volatile to aggregate in a density-dependent manner. Funct Ecol 26: 1429–1440. [Google Scholar]
- 17. Milanez JM, Parra JRP (2000) Biologia e exigências térmicas de Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) em laboratório. An Soc Entomol Bras 29: 23–29. [Google Scholar]
- 18. Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, et al. (2005) Recruitment of entomopathogenic nematodes by insect damaged maize roots. Nature 434: 732–737. [DOI] [PubMed] [Google Scholar]
- 19. Kovats E (1965) Gas chromatographic characterization of organic substances in the retention index system. Adv Chromatogr 1: 229–247. [Google Scholar]
- 20. Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Smaiyappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot 20: 1–11. [Google Scholar]
- 21. Zehnder G, Kloepper J, Yao C, Wei G (1997a) Induction of systemic resistance against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth-promoting rhizobacteria. J Econ Entomol 90: 391–396. [Google Scholar]
- 22. Zehnder G, Kloepper J, Yao C, Wei G (1997b) Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol Exp Appl 83: 81–85. [Google Scholar]
- 23. Valenzuela-Soto JH, Estrada-Hernandez MG, Ibarra-Laclette E, Delano-Frier JP (2010) Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 231: 397–410. [DOI] [PubMed] [Google Scholar]
- 24. Fontana A, Reichelt M, Hempel S, Gershenzon J, Unsickers SB (2009) The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J Chem Ecol. 35: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pineda A, Soler R, Weldegergis BT, Shimwela MM, van Loon JJA, et al. (2013) Non-Pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ 36: 393–404. [DOI] [PubMed] [Google Scholar]
- 26. Ryu CM, Murphy JF, Mysore KS, Kloepper JW (2004) Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber Mosaic Virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant Journal 39: 381–392. [DOI] [PubMed] [Google Scholar]
- 27. D’Alessandro M, Erb M, Ton J, Brandenburg A, Karlen D, et al. (2014) Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ 37: 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robert CAM, Erb M, Hibbard BE, French BW, Zwahlen C, et al. (2012b) A specialist root herbivore reduces plant resistance and uses an induced plant volatile to aggregate in a density-dependent manner. Funct Ecol 26: 1429–1440. [Google Scholar]
- 29. Ulubelen A, Topcu G, Eris C (1994) Terpenoids from Salvia sclarea . Phytochem 36: 971–974. [DOI] [PubMed] [Google Scholar]
- 30. Sabulal B, Dan M, Anil JJ, Kurup R, Pradeep NS, et al. (2006) Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochem 67: 2469–2473. [DOI] [PubMed] [Google Scholar]
- 31. Arruda L, Beneduzi A, Martins A, Lisboa B, Lopes C, et al. (2012) Screening of rhizobacteria isolated from maize (Zea mays L.) in Rio Grande do Sul State (South Brazil) and analysis of their potential to improve plant growth. Appl Soil Ecol 63: 15–22. [Google Scholar]
- 32. Kupferschmied P, Maurhofer M, Keel C (2013) Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front Plant Sci 4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shoot biomass of inoculated and non-inoculated corn plants. Dry weight of the shoots of inoculated and non-inoculated corn plants (mean ± SE). No significant difference was detected according to Student’s t-test (n = 6).
(TIF)
Root biomass of inoculated and non-inoculated corn plants. Fresh weight of the roots of inoculated and non-inoculated corn plants (mean ± SE). No significant difference was detected according to Student’s t-test (n = 6).
(TIF)
Diabrotica speciosa larval choice in control assays in a six-arm olfactometer. Diabrotica speciosa larval choice in control assays using non-inoculated plants vs. non inoculated plants (control) and blank vs. blank (no stimulus). Bars represent the mean number of larvae ± SE. The pie charts on the right represent non-responsive (no choice) and responsive (choice) larvae. No significant difference was detected according to a quasi-Poisson glm (n = 5).
(TIF)
Density of the distribution of larval weight data. Density of the distribution of raw data on the weight of Diabrotica speciosa when fed on non-inoculated (pink area - control) and inoculated (light green area - inoc) corn. A total of 444 data points: 227 for larval weight on non-inoculated corn and 217 on inoculated corn.
(JPG)
Data set of Diabrotica speciosa host choice, larval performance, corn root volatile emissions, and root and shoot biomass.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.