Abstract
Abuse of methamphetamine (MA) increases the risk of infection of HIV-1, induces considerable neurotoxicity in several brain regions, and impairs the motor and cognitive function in individuals. HIV-1 transactivator of transcription (Tat) has also shown the potent capability to induce neuronal death and impaired brain function. The present study aims to study the synergistic effect of MA and Tat on cytokine synthesis in substantia nigra, striatal dopamine content, and behavioral performance in the rats. Although increased expression of cytokines (interleukin-1β and tumor necrosis factor-α) was observed in the substantia nigra in the rats receiving either MA or Tat alone, a combination of MA and Tat induced a larger and more sustained upregulation of cytokines. In the rats receiving either MA or Tat alone, significant loss in striatal dopamine content was found, which was further exacerbated in the rats receiving both MA and Tat. In the rats receiving either MA or Tat alone, significantly lower performance in the rotarod test and open-field test was observed, whereas the rats receiving both MA and Tat showed more sustained behavioral impairments. These results suggested that Tat protein synergized with MA to induce central neuroinflammation and impair the dopaminergic transmission, thus leading to sustained Parkinson’s-like behavior.
Keywords: methamphetamine, striatum, substantia nigra, transactivator of transcription
Introduction
Exposure to methamphetamine (MA), a widely abused psychostimulant, induces considerable neurotoxicity in several brain regions and impairs motor and cognitive function in individuals 1. A recent retrospective population-based large-scale cohort study reported a significantly increased risk for developing Parkinson’s disease (PD) in individuals with a history of MA or amphetamine use 2. Previous evidences have shown that exposure to MA induced significant oxidative stress resulting from the dysregulation of the dopaminergic system, hyperthermia, apoptosis, and neuroinflammation, thus mediating its neurotoxicity and impairments of brain function 1. MA is a potent inducer of dopamine release and is toxic to dopamine neurons. Although significant neurodegeneration of dopaminergic terminals and neuroinflammation have been reported extensively in the striatum 3, the effect of MA on the cell bodies of dopaminergic neurons in substantia nigra remains disputed.
It is well known that abuse of MA is particularly high in groups that are at a higher risk for HIV-1 infection. Although HIV-1 itself does not infect neurons, viral proteins, such as transactivator of transcription (Tat) and glycoprotein 120, show potent neurotoxicity in the central neurons 4. Exposure to Tat induced considerable synaptic degeneration, neuroinflammation, and neuronal death in the hippocampal CA1 area, striatum, and other brain regions 5. Emerging evidences suggest that Tat may impair the dopamine system in the brain and potentiate the motor and cognitive dysfunction induced by MA in the rodent model 6. The present study aims to investigate the synergistic effect of Tat and amphetamine on nigral neuroinflammation, striatal dopamine content, and motor function in a rodent model.
Materials and methods
Administration of methamphetamine
Adult male Wistar rats (weighing ∼180–210 g) were obtained from the Institutional Center of Experiment Animals and were housed under standard laboratory conditions (22±2°C and 12 : 12 h light cycle) with free access to food and water. All animal protocols were approved by the Institutional Animal Care and Use Committee and were carried out following the guidelines of National Institution of Health.
(+)-MA (Sigma-Aldrich, St. Louis, Mossouri, USA) hydrochloride was dissolved in 0.9% saline. (+)-MA (three doses of 10 mg/kg, at 3-h intervals) was injected intraperitoneally and saline in the same volume was injected into the rats in control group.
Cannula implantation and microinjection
The methods for site-specific cannula implantation and microinjection were similar to those reported previously 7. After anesthetization with sodium pentobarbital solution (50 mg/kg), rats were placed in a stereotaxic frame and implanted with bilateral 26-G stainless-steel guide cannulas (Plastics One, Roanoke, Virginia, USA) into the substantia nigra (anteroposterior, −5.3; mediolateral, ±2.2; dorsoventral, −7.0) 7. The guide cannula was then secured to the skull with dental cement and capped. After the implantation surgery, rats were allowed a recovery period of 1 week before subsequent experiments. The awake animals were administered an intranigral infusion of the recombinant HIV-1 protein Tat1–86 (10 µg/1 µl; Abcam, Cambridge, Massachusetts, USA) or scramble peptide (in same concentration) through a 33-G injector 30 min before the first injection of MA. All cannula placements for the substantia nigra were histologically verified afterward.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to detect the concentration of cytokine interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the substantia nigra. The substantia nigra tissues from the rats in all groups were collected and processed using commercial ELISA kits (R & D Systems, Minneapolis, Minnesota, USA) following the instructions provided by the manufacturer.
Measurement of dopamine contents in the striatum
The dopamine content in the striatum was measured following the protocols as described previously 8. Briefly, after decapitation of animals, the brain was removed and tissues from the dorsal striatum were dissected out, frozen in liquid nitrogen, and stored at −80°C until assayed. Each frozen tissue sample was weighed and then homogenized in 200 μl of 0.2 M perchloric acid containing 100 ng/ml isoproterenol as an internal standard. The homogenate was placed on ice for 30 min and then centrifuged at 20 000g for 15 min at 4°C. Dopamine contents in the supernatants were measured with HPLC (Eicom, San Diego, California, USA) and expressed as µg/g tissue weight.
Motor performance testing
Motor performance was evaluated using a rotarod apparatus as described 9. Before treatment with MA or Tat, the animals were placed in a rotarod with a 60-mm-diameter textured rod, 75 mm in length, rotating at a speed of 25 rpm for 5 days to ensure that they could maintain themselves on the rod for at least 180 s. Each animal was tested 5 times, with a 5-min interval between each trial, and the maximum duration of the test was 5 min. The time spent by the animal on the rotarod was considered as the latency to fall. All animals were tested at days 1, 3, 7, and 10 after the initial MA and/or Tat administration.
Locomotor activity
Locomotor experiments were conducted as described previously 10. The locomotor chambers were 40×40×40 cm (Coulbourn Instruments, Whitehall, Pennsylvania, USA) and had clear plexiglas walls with a stainless-steel floor covered with a thin layer of pine-chip bedding. Photobeams were arranged in a 16 (x-axis) photocell array, spaced 2.54 cm apart. During each locomotor test, session (60 min), a 70 dB white noise was generated to mask any possible background noise. Locomotor activity was measured by recording the moving distance in centimeters. All animals were tested at days 1, 3, 7, and 10 after the initial MA and/or Tat administration.
Drugs and data analysis
MA, Tat, and all other reagents were purchased from Sigma, Abcam, or other commercial source. All data were presented as mean±SEM and analyzed statistically using a t-test or analysis of variance, followed by post-hoc analysis. The criterion for statistical significance was P<0.05.
Results
Tat enhanced the MA-induced upregulation of cytokines
As shown in Fig. 1, administration of MA significantly increased the nigral cytokines (IL-1β and TNF-α) at day 1 and day 3, which was recovered at day 7 and day 10. It was observed that administration of Tat (10 µg) into the substantia nigra also significantly upregulated the striatal cytokines (IL-1β and TNF-α) at days 1, 3, and 7, which was also recovered at day 10. Moreover, a combination of both treatments significantly further enhanced the upregulation of nigral cytokines at day 1 through day 10. These results suggested that coadministration of Tat significantly enhanced and extended MA-induced upregulation of cytokines synthesis in the substantia nigra.
Fig. 1.
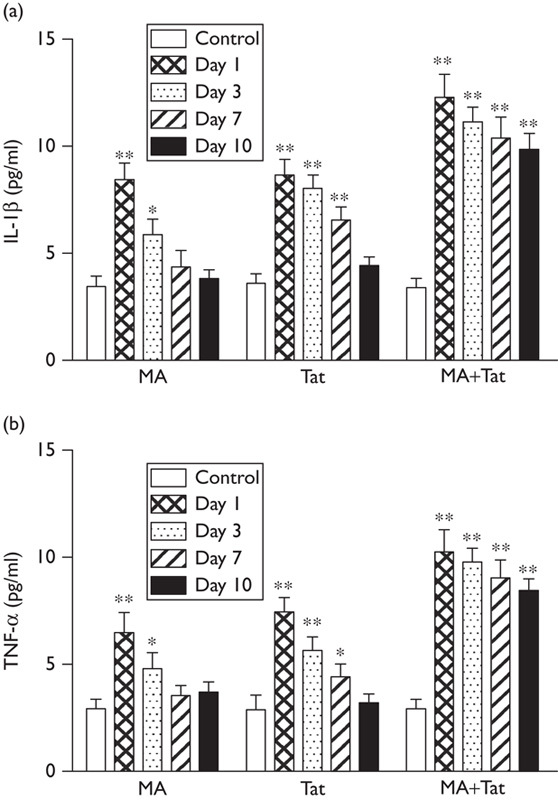
HIV Tat enhanced MA-induced upregulation of cytokines. (a) Administration of MA (3×10 mg/kg) or Tat (10 µg) alone significantly increased the IL-1β in the substantia nigra, whereas a combination of both treatments induced further upregulation and sustained expression of IL-1β (N=7–9 rats per group); (b) administration of either MA or Tat significantly increased the TNF-α in the substantia nigra, whereas a combination of both treatments further increased the expression of TNF-α (N=7–9 rats per group). *P<0.05; **P<0.01. MA, methamphetamine; Tat, transactivator of transcription.
Tat potentiated the MA-induced dopamine deficit
As shown in Fig. 2, administration of MA induced a significant reduction in dopamine content in the striatum at day 1 and day 3, which was eventually recovered at day 7 and day 10. It was also found that administration of Tat (10 µg) into the substantia nigra also significantly decreased the striatal dopamine at day 1 to day 10. Moreover, a combination of both treatments induced a further significant decrease in the striatal dopamine at day 1 through day 10. These results suggested that coadministration of Tat significantly exacerbated MA-induced reduction of striatal dopamine.
Fig. 2.
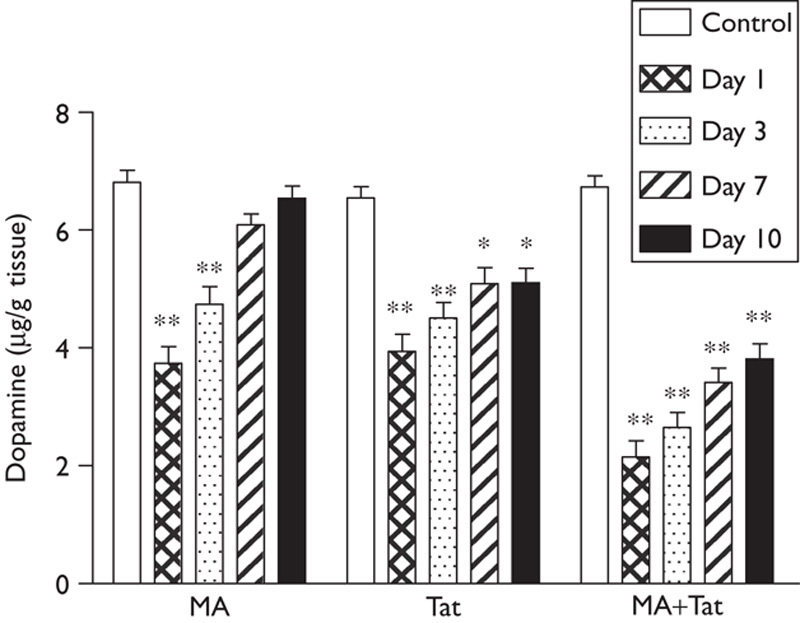
HIV Tat enhanced MA-induced striatal dopamine deficit. Administration of either MA or Tat reduced the dopamine content in the striatal, whereas a combination of both treatments induced further reduction in the dopamine content in the striatum (N=7–9 rats per group). *P<0.05; **P<0.01. MA, methamphetamine; Tat, transactivator of transcription.
Tat potentiated the MA-induced behavioral impairments
As shown in Fig. 3a, administration of MA significantly shortened the time that the rats spent on the rotarod at day 1 and day 3, which indicated an impaired motor balance. This impaired fall latency recovered gradually at day 7 and day 10. Meanwhile, delivery of Tat (10 µg) into the substantia nigra also significantly decreased the fall latency in the rats at day 1 to day 10. Notably, a combination of both treatments further decreased the fall latency at day 1 to day 10. These results suggested that coadministration of Tat significantly enhanced and extended MA-impaired motor balance.
Fig. 3.
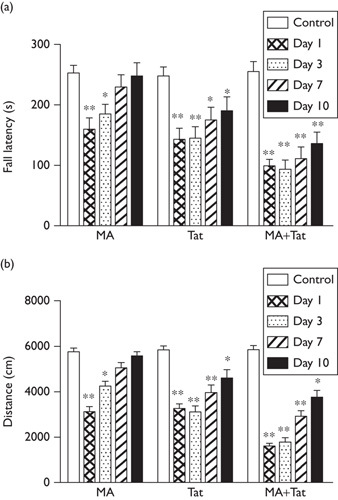
HIV Tat enhanced MA-impaired performance in the rotarod test and locomotor activity. (a) Administration of either MA or Tat decreased the fall latency, which was further decreased by the combination of both treatments. (b) Administration of either MA or Tat reduced the moving distance in the open-field test, which was further decreased by the combination of both treatments (N=9–11 rats per group). *P<0.05; **P<0.01. MA, methamphetamine; Tat, transactivator of transcription.
As shown in Fig. 3b, rats treated with MA showed considerable reduction in moving distance in the open-field test at day 1 and day 3 after treatment, which was gradually recovered to the control level at day 7 and day 10. Similarly, a microinjection of Tat (10 µg) into the substantia nigra also significantly decreased the moving distance in the rats at day 1 through day 10. It was further found that coadministration of MA and Tat protein further reduced in the moving distance at day 1 through day 10. These results indicated that Tat may synergize with MA to impair the locomotor activity in the rodents.
Discussion
It was well recognized that individuals with a history of MA use had a higher risk of developing PD later in life 2, whereas the underlying mechanism remained disputed. A study with transcranial sonography found an abnormally bright and enlarged substantia nigra, a strong risk factor for developing PD, in individuals with a history of MA use 11. Repeated administration of a high dose of MA led to a long-lasting reduction in dopamine uptake and dopamine content in the rat striatum 12. Significant neuronal loss and depletion of striatal dopamine content were observed in the mice treated with MA 13. A single high dose of MA induced considerable neuronal apoptotic death in the striatum 14 and substantia nigra 15, and a continuous injection of a low dose of MA induced long-term striatal dopamine depletion by destroying dopamine nerve fibers 16. MA induced oxyradical stress, autophagy, and neurite degeneration in the midbrain neuronal cultures 17. In the present study, we found that exposure to MA induced significant upregulation of cytokines (IL-1β and TNF-α) in the substantia nigra, decreased the dopamine content in the striatum, and impaired the behavioral performance in the rotarod test and open-field test. These results confirmed the detrimental effect of MA on the function of dopamine neurons.
Accumulating evidences suggested that Tat largely mediated the neurotoxicity and functional impairments of the brain in patients infected with HIV-1. A single local administration of Tat led to a significant reduction in evoked dopamine release in the nucleus accumbens 18. Induction of HIV-1 Tat in the transgenic mice reduced the number of apical dendritic spines, disrupted the distribution of synaptic proteins, and impaired synaptic plasticity in the hippocampal CA1 area, thus leading to cognitive dysfunction 5. Tat protein decreased the expression and function of dopamine transporter in cell surface and dopamine uptake, by a PKC-dependent mechanism, in rat striatal synaptosomes 19. Meanwhile, Tat also induced considerable microglia activation and synthesis of cytokines, impaired synaptic architecture, and neuronal death in the mice brain 20,21. In the present study, we found that administration of Tat significantly increased the synthesis of cytokines (IL-1β and TNF-α) in the substantia nigra, decreased the striatal dopamine content, and induced motor impairments in the rats, which indicated the detrimental effect of Tat on the function of dopamine neurons in the brain.
Previous studies have also implied that Tat protein may potentiate the neurotoxicity induced by illicit drugs. Tat and MA increased the release of matrix metalloproteinase-1 and urokinase plasminogen activator from cultured brain-derived cells 22. It was reported previously that Tat and opiates synergized to reduce dendritic spine number and induce neuronal apoptotic death in several brain regions including the striatum 23. It was also reported that Tat enhanced MA-induced reductions in striatal dopamine release and content 24 in a synergistic manner, and inhibition of TNF-α signaling largely attenuated the synergistic interaction between Tat and MA in the rodents 25. In the present study, Tat protein significantly enhanced the magnitude, as well as extended the duration, of MA-induced nigral cytokines (IL-1β and TNF-α) synthesis and loss of striatal dopamine content, which indicates their synergistic effect in inducing neurotoxicity. Further behavioral studies also showed that Tat protein exacerbated MA-impaired performance in rotarod and open-field tests, indicating their synergistic effect in impairing the brain function and inducing Parkinson’s-like behavior.
Although previous studies showed the distinguished, but somehow convergent, mechanisms underlying MA-induced or Tat alone-induced neurotoxicity in the rodent model, there were evidences to show that a combination of both reagents synergistically exacerbated the oxidative stress, microglia activation, and cytokine synthesis in the cortical, hippocampal, and striatal regions of the brain in rodent models 1. This might contribute toward the synergistic effect between MA and Tat in inducing more significant and sustained cellular and behavioral impairments in the rat in the present study.
Acknowledgements
Z.L. and Z.S. carried out the studies and contributed toward the manuscript. Z.L., J.L., and Y.W. designed the study and analyzed the data. J.L. and Y.W. supervised the study and finalized the manuscript. This work was supported by a grant from the Natural Science Foundation of Shandong Province (ZR2012HM026).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Zengxun Liu and Zhenchun Shi contributed equally to the writing of this article.
References
- 1.Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, Kumar A.Methamphetamine toxicity and its implications during HIV-1 infection.J Neurovirol 2011;17:401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan RC, Cunningham JK, Sykes J, Kish SJ.Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs.Drug Alcohol Depend 2012;120:35–40. [DOI] [PubMed] [Google Scholar]
- 3.Ares-Santos S, Granado N, Moratalla R.The role of dopamine receptors in the neurotoxicity of methamphetamine.J Intern Med 2013;273:437–453. [DOI] [PubMed] [Google Scholar]
- 4.Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE.Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia.Brain 2011;134:3616–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice.Biol Psychiatry 2013;73:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theodore S, Stolberg S, Cass WA, Maragos WF.Human immunodeficiency virus-1 protein Tat and methamphetamine interactions.Ann N Y Acad Sci 2006;1074:178–190. [DOI] [PubMed] [Google Scholar]
- 7.Lessard A, Couture R.Modulation of cardiac activity by tachykinins in the rat substantia nigra.Br J Pharmacol 2001;134:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabuki Y, Ohizumi Y, Yokosuka A, Mimaki Y, Fukunaga K.Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice.Neuroscience 2014;259:126–141. [DOI] [PubMed] [Google Scholar]
- 9.Marques MR, Stigger F, Segabinazi E, Augustin OA, Barbosa S, Piazza FV, et al. Beneficial effects of early environmental enrichment on motor development and spinal cord plasticity in a rat model of cerebral palsy.Behav Brain Res 2014;263:149–157. [DOI] [PubMed] [Google Scholar]
- 10.Arndt DL, Arnold JC, Cain ME.The effects of mGluR2/3 activation on acute and repeated amphetamine-induced locomotor activity in differentially reared male rats.Exp Clin Psychopharmacol 2014[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd G, Noyes C, Flavel SC, Della Vedova CB, Spyropoulos P, Chatterton B, et al. Illicit stimulant use is associated with abnormal substantia nigra morphology in humans.PloS One 2013;8:e56438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J.Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine.Brain Res 1980;181:151–160. [DOI] [PubMed] [Google Scholar]
- 13.Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED.Treatment of mice with methamphetamine produces cell loss in the substantia nigra.Brain Res 1996;738:172–175. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JP, Xu W, Angulo N, Angulo JA.Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration.Neurotoxicology 2006;27:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ares-Santos S, Granado N, Espadas I, Martinez-Murillo R, Moratalla R.Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining.Neuropsychopharmacology 2014;39:1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Callaghan JP, Miller DB.Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse.J Pharmacol Exp Ther 1994;270:741–751. [PubMed] [Google Scholar]
- 17.Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D.Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis.J Neurosci 2002;22:8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM.In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission.Synapse 2009;63:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Midde NM, Gomez AM, Zhu J.HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes.J Neuroimmune Pharmacol 2012;7:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin J, Lam L, Sadic E, Fernandez F, Tan J, Giunta B.HIV-1 Tat-induced microglial activation and neuronal damage is inhibited via CD45 modulation: a potential new treatment target for HAND.Am J Transl Res 2012;4:302–315. [PMC free article] [PubMed] [Google Scholar]
- 21.Lu SM, Tremblay ME, King IL, Qi J, Reynolds HM, Marker DF, et al. HIV-1 Tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells.PloS one 2011;6:e23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A.Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases.J Neurovirol 2004;10:21–28. [DOI] [PubMed] [Google Scholar]
- 23.Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons.Am J Pathol 2010;177:1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cass WA, Harned ME, Peters LE, Nath A, Maragos WF.HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat.Brain Res 2003;984:133–142. [DOI] [PubMed] [Google Scholar]
- 25.Theodore S, Cass WA, Nath A, Steiner J, Young K, Maragos WF.Inhibition of tumor necrosis factor-alpha signaling prevents human immunodeficiency virus-1 protein Tat and methamphetamine interaction.Neurobiol Dis 2006;23:663–668. [DOI] [PubMed] [Google Scholar]


