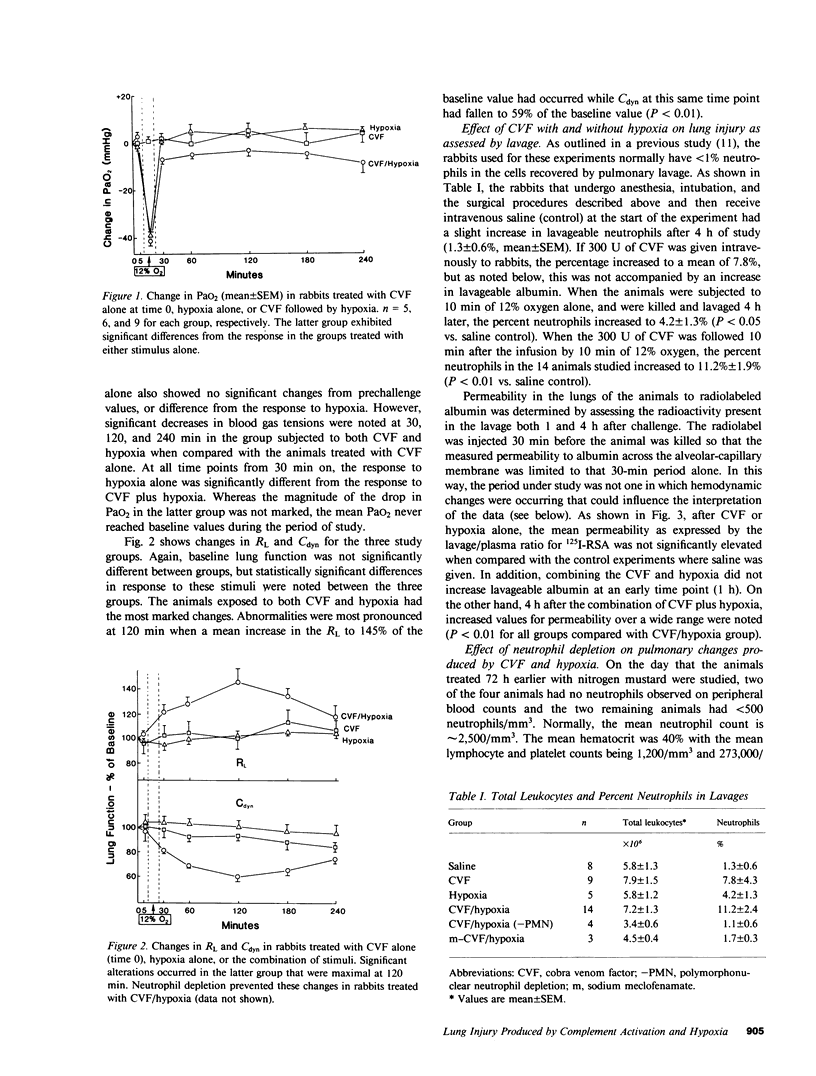
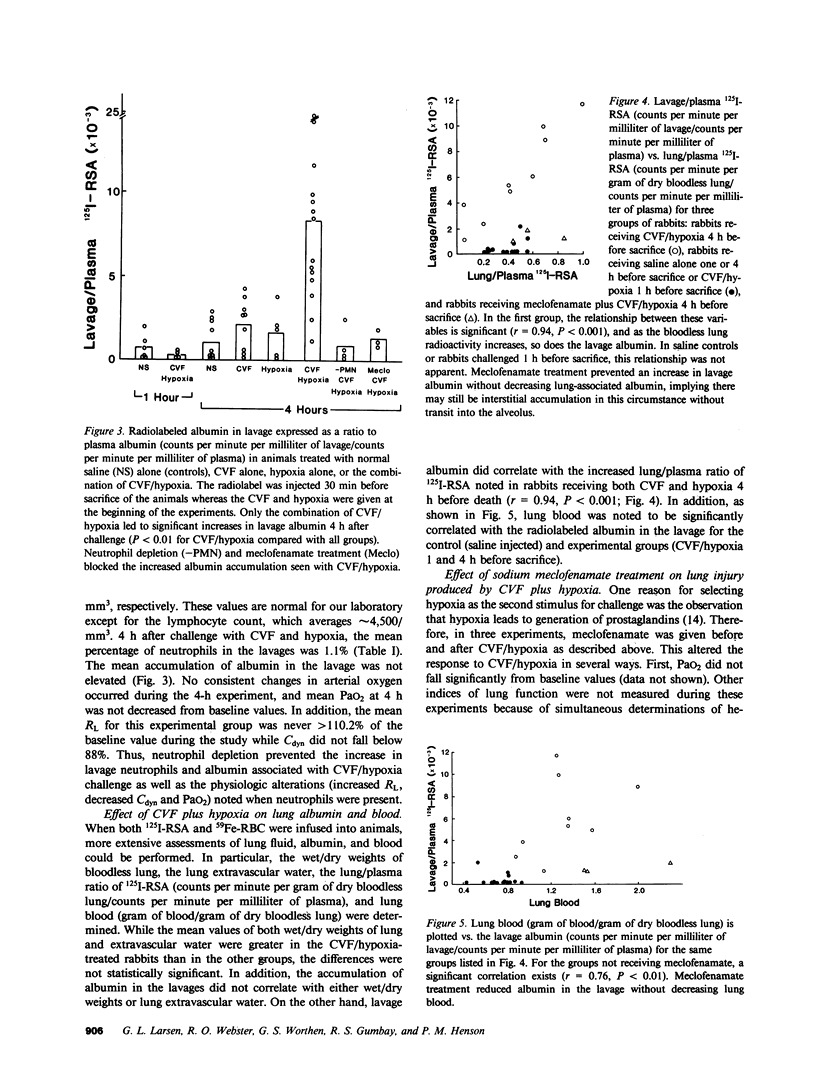
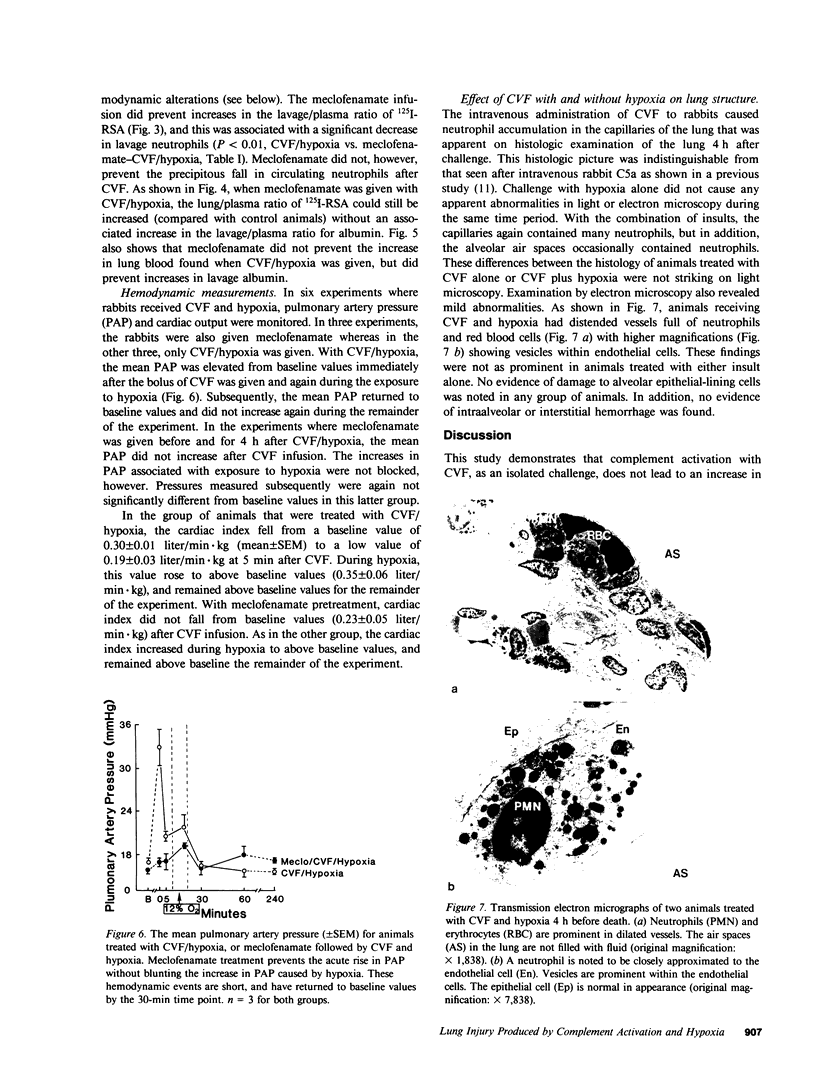
Abstract
Systemic complement activation with intravascularly administered cobra venom factor (CVF) or infusion of either zymosan-activated rabbit plasma or a fifth component of complement fragment with anaphylatoxin activity in the rabbit have not caused significant increases in bronchoalveolar lavage albumin in rabbits (Webster, R. O., G. L. Larsen, B. C. Mitchell, A. J. Goins, and P. M. Henson. 1982. Am. Rev. Respir. Dis. 125:335-340). To assess if another stimulus (hypoxia) acting in concert with complement activation can produce significant lung injury, rabbits were challenged with CVF alone, 10 min of 12% oxygen alone, or CVF followed by a 10-min exposure to 12% oxygen. Either stimulus alone caused no significant changes in arterial oxygen, pulmonary resistance, or dynamic compliance during the 240 min of observation after either stimulus, and neither stimulus alone caused increased albumin accumulation in bronchoalveolar lavage over a 30-min period at the end of the experiment. However, the combination of insults significantly altered arterial oxygen, pulmonary resistance, and dynamic compliance while also increasing albumin and neutrophils recovered by lavage. The increase in lavage albumin did not appear to be due to hemodynamic events in that the pulmonary artery pressure increased acutely after CVF infusion and again during the hypoxic exposure, but was normal when albumin accumulation in the lung was measured. Neutrophil depletion with nitrogen mustard abolished all of these changes induced by CVF plus hypoxia. In addition, meclofenamate pretreatment and infusion during the 4-h study abolished the increases in lavage albumin and neutrophils as well as the increase in pulmonary artery pressure after CVF. Meclofenamate pretreatment did not, however, block accumulation of albumin in the lung (interstitium). We conclude that complement activation, as an isolated event, will not cause a significant increase in lavage albumin in this model. However, combining complement activation with an episode of hypoxia will lead to an increase in lavage albumin that is dependent on the presence of neutrophils for its expression. Meclofenamate treatment will prevent increases in lavage albumin and neutrophils while not preventing albumin accumulation in the lung (interstitium), suggesting a product of the cyclooxygenase pathway of arachidonic acid metabolism is needed to produce movement of albumin and/or neutrophils across the alveolar epithelium in this model.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell E. J., Fry D. L. Pulmonary mechanics in the rabbit. J Appl Physiol. 1969 Aug;27(2):280–285. doi: 10.1152/jappl.1969.27.2.280. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Rowe J. G., Hugli T. E. A modified method for chemotaxis under agarose. J Immunol Methods. 1979;25(4):337–353. doi: 10.1016/0022-1759(79)90026-7. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Cooper J. D., McDonald J. W., Ali M., Menkes E., Masterson J., Klement P. Prostaglandin production associated with the pulmonary vascular response to complement activation. Surgery. 1980 Aug;88(2):215–221. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Desai U., Kreutzer D. L., Showell H., Arroyave C. V., Ward P. A. Acute inflammatory pulmonary reactions induced by chemotactic factors. Am J Pathol. 1979 Jul;96(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Drazen J. M., Loring S. H., Regan R. Validation of an automated determination of pulmonary resistance by electrical subtraction. J Appl Physiol. 1976 Jan;40(1):110–113. doi: 10.1152/jappl.1976.40.1.110. [DOI] [PubMed] [Google Scholar]
- Flick M. R., Perel A., Staub N. C. Leukocytes are required for increased lung microvascular permeability after microembolization in sheep. Circ Res. 1981 Mar;48(3):344–351. doi: 10.1161/01.res.48.3.344. [DOI] [PubMed] [Google Scholar]
- Giclas P. C., Keeling P. J., Henson P. M. Isolation and characterization of the third and fifth components of rabbit complement. Mol Immunol. 1981 Feb;18(2):113–123. doi: 10.1016/0161-5890(81)90077-8. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Weaver L. J., Hudson L. D., Craddock P. R., Jacob H. S. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980 May 3;1(8175):947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M., McCarthy K., Larsen G. L., Webster R. O., Giclas P. C., Dreisin R. B., King T. E., Shaw J. O. Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol. 1979 Oct;97(1):93–110. [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Meyers A. J., Gherini S. T., Beckmann A., Markison R. E., Churg A. M. Production of acute pulmonary injury by leukocytes and activated complement. Surgery. 1980 Jul;88(1):48–58. [PubMed] [Google Scholar]
- Issekutz A. C. Effect of vasoactive agents on polymorphonuclear leukocyte emigration in vivo. Lab Invest. 1981 Sep;45(3):234–240. [PubMed] [Google Scholar]
- Jacob H. S., Craddock P. R., Hammerschmidt D. E., Moldow C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. doi: 10.1056/NEJM198004033021407. [DOI] [PubMed] [Google Scholar]
- Johnson A., Malik A. B. Effect of granulocytopenia on extravascular lung water content after microembolization. Am Rev Respir Dis. 1980 Oct;122(4):561–566. doi: 10.1164/arrd.1980.122.4.561. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Burrowes C. E., Movat H. Z. The quantitation of hemorrhage in the skin. Measurement of hemorrhage in the microcirculation in inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Jan;163(1):126–131. doi: 10.3181/00379727-163-40733. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brodovich H. M., Stalcup S. A., Pang L. M., Lipset J. S., Mellins R. B. Bradykinin production and increased pulmonary endothelial permeability during acute respiratory failure in unanesthetized sheep. J Clin Invest. 1981 Feb;67(2):514–522. doi: 10.1172/JCI110061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Owen-Thomas J. B., Reeves J. T. Hypoxia and pulmonary arterial pressure in the rabbit. J Physiol. 1969 May;201(3):665–672. doi: 10.1113/jphysiol.1969.sp008779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARCE M. L., YAMASHITA J., BEAZELL J. MEASUREMENT OF PULMONARY EDEMA. Circ Res. 1965 May;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- Perkowski S. Z., Havill A. M., Flynn J. T., Gee M. H. Role of intrapulmonary release of eicosanoids and superoxide anion as mediators of pulmonary dysfunction and endothelial injury in sheep with intermittent complement activation. Circ Res. 1983 Nov;53(5):574–583. doi: 10.1161/01.res.53.5.574. [DOI] [PubMed] [Google Scholar]
- Reybrouck T., Amery A., Billiet L., Fagard R., Stijns H. Comparison of cardiac output determined by a carbon dioxide-rebreathing and direct Fick method at rest and during exercise. Clin Sci Mol Med. 1978 Nov;55(5):445–452. doi: 10.1042/cs0550445. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982 Apr 15;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S. I., Yoshida T., Kitamura S., Vreim C. Pulmonary alveolar hypoxia: release of prostaglandins and other humoral mediators. Science. 1974 Sep 27;185(4157):1181–1183. doi: 10.1126/science.185.4157.1181. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Henson P. M., Henson J., Webster R. O. Lung inflammation induced by complement-derived chemotactic fragments in the alveolus. Lab Invest. 1980 May;42(5):547–558. [PubMed] [Google Scholar]
- Shaw J. O., Roberts M. F., Ulevitch R. J., Henson P., Dennis E. A. Phospholipase A2 contamination of cobra venom factor preparations. Biologic role in complement-dependent in vivo reactions and inactivation with p-bromophenacyl bromide. Am J Pathol. 1978 Jun;91(3):517–530. [PMC free article] [PubMed] [Google Scholar]
- Snapper J. R., Bernard G. R., Hinson J. M., Jr, Hutchison A. A., Loyd J. E., Ogletree M. L., Brigham K. L. Endotoxemia-induced leukopenia in sheep. Correlation with lung vascular permeability and hypoxemia but not with pulmonary hypertension. Am Rev Respir Dis. 1983 Mar;127(3):306–309. doi: 10.1164/arrd.1983.127.3.306. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Swinscow T. D. Statistics at square one. XIX--Correlation (continued). Br Med J. 1976 Sep 25;2(6038):747–748. doi: 10.1136/bmj.2.6038.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Mitchell B. C., Goins A. J., Henson P. M. Absence of inflammatory lung injury in rabbits challenged intravascularly with complement-derived chemotactic factors. Am Rev Respir Dis. 1982 Mar;125(3):335–340. doi: 10.1164/arrd.1982.125.3.335. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]