Abstract
Objective
To examine associations of chronic insufficient sleep with mid-childhood cardio-metabolic health.
Design and methods
At 6 months and yearly from 1–7 years, mothers participating in the Project Viva cohort reported children’s 24-hour sleep duration. The main exposure was a sleep curtailment score, ranging from 0 (maximal curtailment) to 13 (never having curtailed sleep). The main outcome was a mid-childhood metabolic risk score, derived as the mean of 5 sex- and cohort-specific z-scores for waist circumference, systolic blood pressure, HDL cholesterol (scaled inversely), and log-transformed triglycerides and HOMA-IR; higher scores indicate higher risk.
Results
The mean (standard deviation [SD]) sleep score was 10.0 (2.8); 5.1% scored 0–4, 13.9% scored 5–7, 14.1% scored 8–9, 28.7% scored 10–11, and 38.3% scored 12–13. Mean (SD, range) metabolic risk score was −0.03 (0.6, −1.8 to 2.6). In multivariable models, the metabolic risk score difference for children with most versus least curtailed sleep was 0.29 units (95% Confidence Interval [CI]: 0.02, 0.57). Further adjustment for mid-childhood BMI-z score attenuated this difference to 0.08 units (95% CI: −0.14, 0.30).
Conclusions
Chronic insufficient sleep from infancy to school-age was associated with higher mid-childhood metabolic risk. This association was explained by sleep duration’s influence on mid-childhood adiposity.
Keywords: sleep curtailment, cardio-metabolic health, early childhood
Introduction
Cross-sectional and longitudinal studies have established associations between shorter sleep duration and increased risk of obesity in children.1, 2 Patterns of shorter sleep duration (<10 hours/day) during early childhood are preserved over long periods and increase risk of excess weight in later childhood.3 Independent of obesity, inadequate sleep may contribute to metabolic dysfunction: in adults, experimental evidence supports a role for shorter sleep in decreased insulin sensitivity,4 disrupted glucose homeostasis and appetite regulation,5 as well as cardiac risk factors such as hypertension6 and incident diabetes.7 Among adults, suggested mechanisms include the impact of insufficient sleep on glucose regulation as well as adipokines, e.g. increasing interleukin-6 (IL-6) and decreasing adiponectin.
Limited research has examined sleep duration and metabolic dysfunction in children. Recently, Spruyt et al found longer actigraph-recorded sleep duration among school-age children was cross-sectionally associated with a lower probability of metabolic dysfunction, as measured through glucose, insulin, cholesterol, triglycerides and high sensitivity C-reactive protein (hsCRP), independent of body mass index (BMI).8 Most, but not all,9 cross-sectional studies have linked shorter sleep duration to adverse metabolic outcomes in children (e.g. higher Homeostatic Model Assessment for assessing Insulin Resistance (HOMA-IR), lower adiponectin,10 increased non-high-density lipoprotein (HDL) cholesterol,11 and higher blood pressure).12 To our knowledge, no previous study has assessed prospective relationships of chronic insufficient sleep with an array of biomarkers of glucose and lipid metabolism, blood pressure, adiponectin, and inflammation in a cohort of young children without diagnosed sleep disorders.
The goal of this study was to examine whether chronic insufficient sleep from infancy to mid-childhood was associated with cardio-metabolic risk in mid-childhood, and the extent to which associations were explained by attained adiposity. This biomarker study builds on prior research showing associations of chronic sleep curtailment from infancy to mid-childhood with adiposity in early and mid-childhood.13 We hypothesized that chronic insufficient sleep would be associated with an adverse cardio-metabolic state in mid-childhood, and that adjustment for mid-childhood BMI z-scores would attenuate these associations. A better understanding of the contributions of chronic insufficient sleep to later metabolic health may inform early life interventions to prevent obesity and cardiovascular disease.
Methods
Subjects/Study Design
Study subjects were participants in Project Viva, a prospective cohort study that recruited women during early pregnancy from Harvard Vanguard Medical Associates, a multi-specialty group practice in eastern Massachusetts. Details of recruitment and retention procedures are available elsewhere.14 Of the 2128 women who delivered a live infant, 1116 families attended a 7 year (“mid-childhood”) in-person visit and 702 children provided blood samples. Since our main exposure was chronic sleep curtailment from 6 months to 7 years, we further excluded participants who did not have sleep data for these time points. Our sample for analysis was 652 children. Participants that were not included had similar characteristics to those who were: 56% not included versus 62% included had household incomes >$70,000/year, 63% versus 67% had a college degree, and maternal age at enrollment was 32 years for both groups.
After obtaining informed consent, we performed in-person study visits with the mother at the end of the first and second trimesters of pregnancy, and with mother and child in the first days after delivery and in infancy (median 6.2 months), early childhood (median 3.3 years) and mid-childhood (median 7.7 years). Mothers completed mailed questionnaires at 1, 2, 4, 5, and 6 years after birth. The Institutional Review Board of Harvard Pilgrim Health Care approved the study.
Measurements
Main Exposures
At 6 months and yearly from 1 to 7 years, mothers reported children’s sleep duration in a usual 24-hour period. At 6 months, we asked mother to report separately in hours/minutes their baby’s average length of morning nap, afternoon nap and nighttime sleep in the past month. At 1-year, mothers reported in hours/minutes the child’s usual 24-hour sleep duration in the past month including morning naps, afternoon naps, and nighttime sleep. Between 2–7 years, mothers reported number of hours the child slept in a usual 24-hour period in the past month, separating weekends and weekdays. Response categories included, “< 9, 9, 10, 11, 12, 13, and ≥14 hours/day”; at age 7 the response option was in hours/minutes.
The main exposure was a sleep curtailment score from infancy to mid-childhood. The sleep score was derived from mean sleep duration at each of the 8 measurement times: 6 months and yearly from 1–7 years. Using established thresholds from the published literature and durations associated with an increased risk for elevated BMI (≥ 95th percentile) in childhood15 and the National Sleep Foundation age-specific recommendations,16 we scored sleep duration as follows: from 6 months to 2 years, the score was 0 for <12 hours/day and 1 for ≥12 hours/day; from 3 to 4 years, <10 hours/day = 0, 10-<11 hours/day = 1, and ≥ 11 hours/day = 2; at 5 to 7 years, <9 hours/day = 0, 9-<10 hours/day = 1, and ≥ 10 hours/day = 2. The range of the total score was 0–13, where 0 indicated maximal sleep curtailment and 13 indicated never having curtailed sleep. To examine potential non-linear associations we categorized this score and collapsed scores of 0–4, 5–7 and 8–9 due to small frequencies and also of 10–11 and 12–13 due to comparable results shown in prior analyses for a total of five categories.13
Outcome Measures
At the early and mid-childhood visits, we measured height and weight using a calibrated stadiometer (Shorr Productions, Olney, MD) and scale (Seca model 881, Seca Corporation, Hanover, MD). We calculated age- and sex-specific BMI z-scores using US national reference data.17 At the mid-childhood visit, we measured waist circumference (cm) using a Lefkin tape and systolic (SBP) and diastolic (DBP) blood pressures with a Dinamap (Critikon, Inc., Tampa, Florida) Pro 100 automated oscillometric recorder. We obtained 5 blood pressure measurements taken 1 minute apart and calculated SBP as the average of 5 measurements since the intra-class coefficient (ICC) was high (ICC = 0.74). Research assistants performing measurements followed standardized techniques18 and participated in training to ensure validity.19
Phlebotomists collected blood from the antecubital vein in vacutainer tubes protected from sunlight and transported on ice to the laboratory for processing and storage within 24-hours. We centrifuged samples and stored plasma aliquots in liquid nitrogen at −80°C until analysis. We measured plasma fasting insulin using an electrochemiluminescence immunoassay on the Roche E Modular system. Fasting glucose was measured enzymatically using Roche Diagnostics reagents (Roche Diagnostics, Indianapolis, IN). We calculated insulin resistance using the HOMA-IR (fasting insulin [μU/mL] x fasting glucose [mg/dL]/405). Triglycerides were measured enzymatically with correction for endogenous glycerol. We measured plasma concentrations of adiponectin with a radioimmunoassay (Linco Research Inc., St Charles, MO).20, 21 We used an immunoturbidimetric high-sensitivity assay on a Hitachi 911 analyzer to determine hsCRP concentrations (Roche Diagnostics – Indianapolis, IN). Plasma IL-6 was measured by enzyme-linked immunosorbent assay (ELISA).
The main outcome was a mid-childhood metabolic risk score, derived as the mean of 5 sex- and cohort-specific z-scores for waist circumference, SBP, HDL cholesterol (scaled inversely), and log-transformed triglycerides and log-transformed HOMA-IR. While there is no consistent definition of the metabolic syndrome in young children, prior research has utilized similar metabolic risks scores.22–24 Higher scores indicate higher metabolic risk. We also examined the individual components of the metabolic risk score as outcomes, as well as adiponectin, IL-6, hsCRP and Tumor Necrosis Factor Alpha (TNF-α) because of previously observed associations of these biomarkers with sleep duration and with cardio-metabolic health.25, 26
Other measures
At enrollment, we collected information about maternal age, education, parity, and household income. At the early childhood visit parents reported the child’s race and ethnicity. In mid-childhood, we asked mothers to report their children’s average hours of TV/video time in the past month, separating weekdays and weekends. Similarly, mothers reported children’s weekly active play and physical activity on an average week in the past month through separate questions about walking and light, moderate or vigorous activities such as sports. Mothers reported the child’s consumption of different types of sugary drinks, including fruit drinks and soda, which we combined into a single sugary beverages variable in servings/day. Mothers reported their pre-pregnancy weight and height, from which we calculated their BMIs.
Statistical Analysis
We first examined bivariate relationships of the sleep curtailment score with each covariate and with anthropometric (waist circumference) and biomarker outcomes. We then used multivariable linear regression models to examine associations of the sleep score as a continuous and categorical variable. Since prior research has suggested sex differences in the metabolic response to chronic sleep curtailment,27, 28 we repeated analyses separately in boys and girls.
Our first model, Model 1, was adjusted for child age and sex only. We additionally adjusted for potential confounding by household income, maternal education, age, and pre-pregnancy BMI and child race/ethnicity (Model 2). Finally, we assessed the extent to which associations were attenuated after adjustment for attained adiposity as represented by BMI z-score in mid-childhood (Model 3). We also considered adjustment for BMI z-score in early childhood, servings/day of sugary drinks and hours/day of TV/video-viewing and active play in mid-childhood, but these did not change estimated associations or conclusions and were not included in final models.
In sensitivity analyses, we considered alternative derivations of the sleep score in which sleep duration in infancy and toddlerhood was treated in three categories, allowing each period of childhood to contribute equally to the total score. We also adjusted for the time of day of blood draw to assess whether circadian rhythms in the biomarkers might influence the results.
We examined the residuals of each model, which appeared normal with the exception of IL-6 and hsCRP; these we log-transformed. Point estimates and confidence intervals were exponentiated for presentation in tables. Thus, model coefficients can be interpreted as the mean difference in the metabolic outcomes in their native scale with the exception of IL-6 and hsCRP, which should interpreted as relative changes in these outcomes.
The confounding variables in our analyses were not available for all subjects. We used multiple imputation to generate several plausible values for each missing value.29, 30 We used a chained equations approach with predictive mean matching based on linear regressions for approximately continuous variables and logistic or generalized logistic regression for dichotomous or more generally categorical variables. The “completed” data set comprises the observed data and one imputed value for each missing value. We replicated this analysis across completed data sets and combined them in a structured fashion that reflects the true amount of information in the observed data, i.e., without presuming that the imputed values are known true values, but recovering the information in partially observed subjects. We generated 50 complete data sets31 and combined multivariable modeling results (Proc MI ANALYZE) in SAS version 9.3 (SAS Institute, Cary NC). From these multiple imputation results, we report adjusted effect estimates from regressions and 95% confidence intervals (CI) for each sleep category with the lowest risk sleep category as the reference.
Results
Characteristics of study participants overall, and by sleep curtailment score, are shown in Table 1. Children from families with lower incomes and lower education had lower sleep scores, indicating greater exposure to insufficient sleep from infancy to mid-childhood (Table 1). In addition, black and Hispanic children, as well as children of “other” races/ethnicities, had more curtailed sleep than non-Hispanic white children (Table 1). In mid-childhood, curtailed sleep was associated with greater hours of television and greater servings of sugary drinks but not with physical activity (Table 1). In bivariate analyses (Table 1), children with the lowest sleep scores (most curtailed sleep) had higher indices of BMI, waist circumference and HOMA-IR.
Table 1.
Characteristics of 652 Children from Project Viva, Overall, and by Sleep Curtailment Score †
| Characteristics | All N=652 |
Sleep Curtailment Score | ||||
|---|---|---|---|---|---|---|
| 0 to 4 33 (5.1%) |
5 to 7 91 (13.9%) |
8 to 9 92 (14.1%) |
10 to 11 187 (28.7%) |
12 to 13 250 (38.3%) |
||
| Maternal and Household |
Mean (SD) or %
|
|||||
| Maternal age at enrollment, years | 32.1 (5.4) | 30.0 (6.7) | 31.0 (6.9) | 31.4 (7.4) | 32.6 (5.7) | 32.6 (4.9) |
| Maternal BMI, kg/m2 | 24.9 (5.3) | 27.2 (7.8) | 25.1 (6.6) | 26.2 (7) | 24.9 (5.8) | 24.1 (4.6) |
| Nulliparous, % | 43.1 | 23.0 | 41.0 | 47.6 | 40.9 | 46.5 |
| Education, ≥ College grad, % | 67.3 | 23.2 | 45.3 | 60.2 | 75.7 | 77.4 |
| Household income >$70k/yr, % | 61.6 | 21.6 | 40.6 | 49.0 | 66.3 | 75.6 |
| Child | ||||||
| Age at blood draw, years | 7.9 (0.8) | 8.1 (1.2) | 8.1 (1.1) | 8.1 (1) | 7.9 (0.8) | 7.7 (0.7) |
| Girl, % | 47.6 | 51.8 | 40.4 | 49.2 | 43.8 | 51.8 |
| Race/ethnicity, % | ||||||
| Black | 19.4 | 42.5 | 39.8 | 34.3 | 15.9 | 5.9 |
| Hispanic | 4.1 | 10.8 | 7.7 | 4.3 | 2.3 | 3.3 |
| Other | 14.6 | 28.1 | 22.2 | 17.9 | 13.8 | 9.4 |
| White | 61.9 | 18.7 | 30.3 | 43.5 | 67.9 | 81.4 |
| Mid-childhood behaviors | ||||||
| Television viewing, hours/day | 1.6 (1.0) | 2.6 (1.5) | 2.0 (1.3) | 1.7 (1.2) | 1.6 (0.9) | 1.4 (0.8) |
| Physical activity, hours/day | 1.8 (1.3) | 1.8 (1.8) | 1.8 (1.5) | 1.7 (1.6) | 1.8 (1.4) | 1.9 (1.3) |
| Sugary drinks, servings/day | 0.4 (0.8) | 0.7 (1.4) | 0.7 (1.2) | 0.5 (1.2) | 0.4 (0.7) | 0.3 (0.5) |
| Cardio-metabolic outcomes | ||||||
| Body mass index, z-score | 0.40 (1.01) | 1.00 (5.58) | 0.60 (3.16) | 0.58 (3.07) | 0.35 (2.12) | 0.21 (1.61) |
| Metabolic Risk Score* | −0.03 (0.6) | 0.22 (0.9) | 0.05 (0.7) | 0.05 (0.7) | −0.04 (0.7) | −0.11 (0.6) |
| Waist circumference, cm | 59.9 (8.5) | 65.0 (13.1) | 61.9 (11.2) | 61.2 (9.8) | 59.8 (8.9) | 58.1 (7.1) |
| Diastolic blood pressure, mm/Hg | 54.3 (5.7) | 54.6 (6.9) | 54.6 (6.8) | 54.2 (5.9) | 53.9 (5.7) | 54.5 (6.2) |
| Systolic blood pressure, mm/Hg | 94.4 (8.8) | 95.6 (11.1) | 95.1 (11.5) | 95.5 (9.6) | 94.4 (9.7) | 93.7 (8.7) |
| Triglycerides, mg/dL | 57.9 (26.3) | 60.6 (34.6) | 56.2 (24.9) | 56.4 (26.3) | 58.6 (29.1) | 58.1 (26.2) |
| Total cholesterol, mg/dL | 160.1 (29.0) | 160.9 (34.3) | 160.8 (32.6) | 161.3 (33.1) | 159.4 (30.1) | 160.0 (29.5) |
| HDL cholesterol, mg/dL | 57.4 (14.2) | 55.3 (16.3) | 58.1 (13.9) | 57.1 (14.2) | 58.0 (15.4) | 57.0 (14.5) |
| HOMA-IR | 1.8 (1.6) | 2.4 (2.1) | 2.0 (2.1) | 2.0 (1.8) | 1.8 (1.9) | 1.5 (1.2) |
| Fasting glucose, mg/dL | 94.6 (16.1) | 95.6 (19.9) | 95.2 (17.7) | 95.8 (17.8) | 95.4 (16.6) | 93.2 (16.1) |
| Fasting insulin, uU/mL | 7.5 (5.9) | 10.2 (8.4) | 8.4 (7.4) | 8.2 (6.8) | 7.4 (6.9) | 6.7 (4.8) |
| Adiponectin, ug/mL | 15.6 (8.9) | 15.0 (11.9) | 14.8 (8.9) | 16.1 (11) | 15.3 (9.7) | 15.9 (9.4) |
| TNF-α, pg/mL | 2555 (605) | 2568 (771) | 2577 (646) | 2481 (604) | 2539 (622) | 2586 (647) |
|
Medan (IQR)
|
||||||
| IL-6, pg/mL | 0.6 (0.7) | 0.9 (0.8) | 0.7 (0.7) | 0.6 (0.6) | 0.6 (0.6) | 0.6 (0.7) |
| hsCRP, mg/dL | 0.2 (0.5) | 0.3 (1.6) | 0.2 (0.6) | 0.2 (0.5) | 0.2 (0.5) | 0.1 (0.3) |
The range of the total sleep score is 0–13, where 0 indicates the maximal sleep curtailment and 13 indicates never having curtailed sleep.
The metabolic risk score is composed of the mean of 5 sex-specific internal z-scores for waist circumference, systolic blood pressure, inverted HDL cholesterol, and log-transformed triglycerides and HOMA-IR.
The mean (SD, range) of the sleep curtailment score from infancy to mid-childhood was 10.0 (2.8, 0 – 13) units. Score frequencies are shown in Table 1. Metabolic risk scores for children with the most versus least curtailed sleep (sleep score 0–4 versus 12–13) were 0.29 units higher (95% CI: 0.02, 0.57; Table 2, Model 2 and Figure 1) in multivariable models adjusted for child’s age, sex, and race/ethnicity and maternal age, education, pre-pregnancy BMI, parity and household income. Of the metabolic risk score components, waist circumference contributed most to this difference; waist circumference for children with the most versus least curtailed sleep was 4.76 cm higher (95% CI: 1.18, 8.34). Further adjustment for mid-childhood BMI-z score attenuated the mean differences in metabolic risk score to 0.08 units (95% CI: −0.14, 0.30; Table 2, Model 3 and Figure 1) and in waist circumference to 0.86 cm (95% CI: −1.25, 2.98; Table 2, Model 3). Though results did not achieve statistical significance for other markers, there was a suggestion of a trend towards increased risk from high to low sleep score categories for insulin, systolic blood pressure, triglycerides, HDL cholesterol, IL-6, and hsCRP (Supplemental Table 1).
Table 2.
Association of Sleep Curtailment Score with the Metabolic Risk Score and Components (n = 652)†
| Sleep Curtailment Score | 0 to 4 5.1% |
5 to 7 13.9% |
8 to 9 14.1% |
10 to 11 28.7% |
12 to 13 38.30% |
Continuous Score |
|---|---|---|---|---|---|---|
|
|
||||||
| Metabolic Risk Score |
Mean Difference (95% Confidence Interval)
|
|||||
| Model 1. Child age & sex | 0.28 (0.01, 0.54) | 0.12 (−0.04, 0.27) | 0.11 (−0.05, 0.27) | 0.05 (−0.07, 0.17) | 0.0 (ref) | −0.03 (−0.04, −0.01) |
| Model 2. Model 1 + SES | 0.29 (0.02, 0.57) | 0.16 (0.00, 0.33) | 0.12 (−0.05, 0.28) | 0.05 (−0.07, 0.17) | 0.0 (ref) | −0.03 (−0.05, −0.01) |
| Model 3. Model 2 + BMI | 0.08 (−0.14, 0.30) | 0.05 (−0.09, 0.18) | 0.03 (−0.10, 0.16) | 0.01 (−0.08, 0.11) | 0.0 (ref) | −0.01 (−0.03, 0.01) |
| Components | ||||||
| Waist circumference, cm | ||||||
| Model 1. Child age & sex | 5.56 (2.04, 9.08) | 2.74 (0.55, 4.93) | 1.98 (−0.26, 4.23) | 1.34 (−0.31, 2.98) | 0.0 (ref) | −0.51 (−0.74, −0.27) |
| Model 2. Model 1 + SES | 4.76 (1.18, 8.34) | 2.7 (0.46, 4.94) | 1.34 (−0.84, 3.52) | 1.07 (−0.51, 2.64) | 0.0 (ref) | −0.46 (−0.72, −0.20) |
| Model 3. Model 2 + BMI | 0.86 (−1.25, 2.98) | 0.6 (−0.74, 1.94) | −0.17 (−1.47, 1.13) | 0.42 (−0.52, 1.35) | 0.0 (ref) | −0.06 (−0.22, 0.09) |
| Systolic blood pressure, mm/Hg | ||||||
| Model 1. Child age & sex | 1.07 (−2.81, 4.94) | 0.74 (−1.65, 3.12) | 1.15 (−1.17, 3.47) | −0.75 (−1.93, 0.43) | 0.0 (ref) | −0.14 (−0.40, 0.12) |
| Model 2. Model 1 + SES | 1.22 (−2.75, 5.19) | 0.98 (−1.53, 3.48) | 0.70 (−1.67, 3.08) | 0.29 (−1.48, 2.06) | 0.0 (ref) | −0.16 (−0.45, 0.12) |
| Model 3. Model 2 + BMI | −0.85 (−4.51, 2.81) | −0.14 (−2.51, 2.23) | −0.10 (−2.28, 2.08) | −0.06 (−1.68, 1.56) | 0.0 (ref) | 0.05 (−0.22, 0.32) |
| Triglycerides, mg/dL | ||||||
| Model 1. Child age & sex | 2.36 (−8.10, 12.82) | −1.91 (−8.83, 5.02) | −1.77 (−8.36, 4.82) | 0.62 (−4.66, 5.89) | 0.0 (ref) | −0.01 (−0.78, 0.75) |
| Model 2. Model 1 + SES | 6.78 (−4.40, 17.95) | 2.25 (−5.08, 9.59) | 1.50 (−5.39, 8.39) | 1.72 (−3.59, 7.02) | 0.0 (ref) | −0.64 (−1.51, 0.23) |
| Model 3. Model 2 + BMI | 3.54 (−7.42, 14.49) | 0.50 (−6.64, 7.63) | 0.24 (−6.58, 7.06) | 1.18 (−4.07, 6.42) | 0.0 (ref) | −0.31 (−1.17, 0.55) |
| HDL cholesterol, mg/dL | ||||||
| Model 1. Child age & sex | −2.16 (−7.95, 3.64) | 0.41 (−3.23, 4.06) | −0.32 (−3.97, 3.34) | 0.58 (−2.18, 3.35) | 0.0 (ref) | 0.12 (−0.31, 0.55) |
| Model 2. Model 1 + SES | −3.38 (−9.64, 2.87) | −0.81 (−4.74, 3.11) | −1.21 (−5.02, 2.61) | 0.16 (−2.65, 2.96) | 0.0 (ref) | 0.30 (−0.19, 0.78) |
| Model 3. Model 2 + BMI | −1.84 (−8.16, 4.48) | 0.02 (−3.85, 3.88) | −0.61 (−4.37, 3.15) | 0.41 (−2.35, 3.17) | 0.0 (ref) | 0.14 (−0.35, 0.63) |
| HOMA-IR | ||||||
| Model 1. Child age & sex | 0.83 (0.17, 1.50) | 0.49 (0.07, 0.91) | 0.40 (−0.02, 0.81) | 0.26 (−0.06, 0.58) | 0.0 (ref) | −0.09 (−0.13, −0.04) |
| Model 2. Model 1 + SES | 0.47 (−0.23, 1.18) | 0.31 (−0.15, 0.77) | 0.22 (−0.20, 0.65) | 0.19 (−0.12, 0.51) | 0.0 (ref) | −0.06 (−0.11, −0.002) |
| Model 3. Model 2 + BMI | 0.17 (−0.49, 0.83) | 0.15 (−0.29, 0.59) | 0.11 (−0.30, 0.51) | 0.14 (−0.15, 0.44) | 0.0 (ref) | −0.02 (−0.08, 0.03) |
All analyses use linear regression. Model 1 is adjusted only for child age and sex. Model 2 is additionally adjusted for maternal education, pre-pregnancy BMI, age at enrollment and nullparity; household income; and child race/ethnicity. Model 3 is further adjusted for child BMI z-score at mid-childhood.
The range of the total sleep score is 0–13, where 0 indicates the maximal sleep curtailment and 13 indicates never having curtailed sleep.
Figure 1.
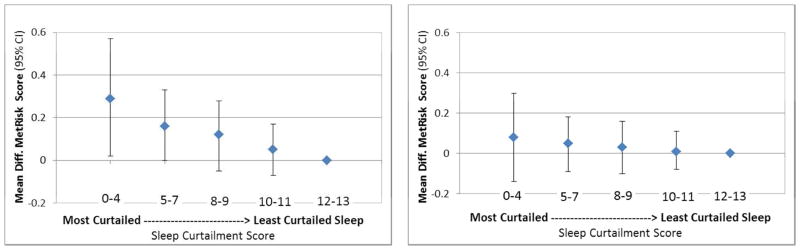
Figure 1a: Metabolic Risk Score by Sleep Curtailment without adjustment for BMI z-score in Mid-Childhood
Figure 1b: Metabolic Risk Score by Sleep Curtailment with adjustment for BMI z-score in Mid-Childhood
Adjusted for maternal age, education, pre-pregnancy BMI, parity; household income; child race/ethnicity & age at outcome assessment, then additionally for BMI z-score in mid-childhood
Results using the continuous sleep score mirrored those with the categorical score: before adjustment for mid-childhood BMI z-score, for each incremental increase in the sleep score, the metabolic risk score was 0.03 units (95% CI: −0.05, −0.01) lower and waist circumference was 0.46 cm (95% CI: −0.72, −0.20) lower (Table 2, Model 2). Associations of higher sleep scores with lower HOMA-IR (−0.06 units; 95% CI: −0.11, −0.002) and insulin (−0.21 mg/dL; 95% CI: −0.41, −0.003) were of borderline significance (Table 2, Model 2 and Supplemental Table 1, Model 2). The sleep score was not associated with other cardio-metabolic markers in mid-childhood, and all associations were attenuated after adjustment for BMI z-score in mid-childhood.
When we repeated analyses separately by sex, we found stronger associations among girls than boys; however, confidence intervals overlapped and tests for interactions of sex with the sleep score were non-significant. Sensitivity analyses considering alternative scoring methods for deriving the sleep curtailment score and adjusting for time of day of blood draw yielded nearly identical results (data not shown).
Discussion
In this prospective cohort, chronic sleep curtailment from infancy to mid-childhood was associated with higher metabolic risk score in mid-childhood, as well as higher levels of certain metabolic components including higher waist circumference and insulin. All associations attenuated after adjustment for mid-childhood BMI z-score, suggesting that increased metabolic risk was related to mid-childhood adiposity.
Prior work in Project Viva has demonstrated cumulative effects of sleep curtailment on mid-childhood adiposity; in this study, we show that while chronic insufficient sleep is an obesity risk factor, young children may not yet experience metabolic derangements beyond the direct consequences of excess adiposity. Early childhood is a time when weight trajectories are being established that may carry forward into adolescence and adulthood, underscoring the importance of establishing healthy sleep routines at a young age before more severe metabolic consequences are apparent.32 Proposed mechanisms linking insufficient sleep to metabolic risk include direct effects as well as indirect effects, such as shorter sleep’s influence on weight gain and obesogenic behavior. For example, sleep deprivation appears to impact hormonal signaling, leading to elevated evening cortisol levels and disrupted growth hormone, which could lead eventually to disrupted glucose homeostasis. Experimentally, sleep deprivation has been shown to increase ghrelin and decrease leptin levels in adults, stimulating hunger. This may combine with decreased impulse control and extended exposure to an obesogenic environment to decrease diet quality and increase energy intake.7, 33 In children, these behaviors are unfolding in a home environment under some degree of parental control, suggesting that changes to household routines focused on improving not only sleep habits but also screen time, dietary intake and other obesogenic behaviors might have mutually reinforcing beneficial effects.34
While chronic insufficient sleep among the young children in this study did not exert metabolic effects beyond those attributable to adiposity, this may not hold true in older children or for quality rather than quantity of sleep. In cross-sectional studies shorter and poorer quality sleep have adverse metabolic consequences independent of BMI. For example, shorter sleep measured by actigraphy predicts elevated blood pressure independent of obesity among adolescents, with stronger associations for sleep efficiency than duration, suggesting that sleep quality as well as duration influences blood pressure levels.35
Our longitudinal results indicate that curtailed sleep is associated with higher insulin and HOMA-IR, and that associations are explained by mid-childhood BMI. By contrast, cross-sectional evidence in adolescents links both shorter 36 and longer 37 sleep duration with higher HOMA-IR independent of BMI. Additionally, we observed a non-significant trend towards higher hsCRP and IL-6 with more curtailed sleep while cross-sectional studies in schoolchildren and adolescents linked more irregular 8 and shorter sleep 38 with higher hsCRP plasma concentrations independent of BMI. Finally, our study also did not find any significant associations with lipid levels though cross-sectional studies of healthy schoolchildren in Hong Kong 39 and Kentucky 8 have found inverse associations of sleep duration with total and LDL-cholesterol.
This study makes an innovative contribution to the literature through its repeated measures of sleep duration and the availability of cardio-metabolic biomarkers from a cohort of children followed from infancy to mid-childhood. Though prospective evidence that shorter sleep leads to adiposity in children is accumulating, most research including additional markers of cardio-metabolic health has been cross-sectional and has examined individual markers rather than a summary metabolic risk score as used in this study.
There are also important limitations to this study: first, sleep measures were from maternal report. In a recent validation study, though parental report of sleep duration in children 4–6 years old correlated well with actigraphy among healthy controls (ρ =0.85), there were significant discrepancies between reported sleep times and actigraphy. 40 Measurement error in sleep is most likely non-differential with respect to metabolic risk and would attenuate observed effects. Second, we did not have information about sleep quality, which may influence both sleep duration and metabolic health. 7, 11 Third, ideally blood samples would be collected on multiple occasions at identical times of day to minimize the influence of circadian rhythms and random fluctuations. Though collected bloods at one morning occasion, and not at identical times of day, adjustment for time of day did not alter point estimates or conclusions in multivariable models. Fourth, the majority of mothers in our study were non-Hispanic white and college-educated with household incomes ≥$70,000/year; it is possible that results may not be generalizable to populations with different socioeconomic or racial/ethnic compositions where household routines and cultural contexts surrounding children’s sleep might modify sleep’s impact on metabolic health. However, analyses stratified on race/ethnicity showed similar results. Finally, as in all observational studies, unmeasured confounding by aspects of the home or neighborhood environment not controlled for through adjustment for the characteristics included in our models is possible.
Conclusions
Our study suggests that chronic insufficient sleep in childhood may have lasting effects on adiposity, and, as a consequence, on metabolic health. Improving sleep duration and quality in childhood may be potential intervention targets to promote cardio-metabolic health.
Supplementary Material
What is already known about this subject
Shorter sleep in children is associated cross-sectionally with many cardiovascular disease risk factors including obesity.
Few longitudinal studies have examined chronic insufficient sleep or biomarkers of cardio-metabolic health in mid-childhood.
What this study adds
In this cohort of children with cardio-metabolic markers including waist circumference, blood pressure, lipids, glucose, insulin, and inflammation, chronic insufficient sleep from infancy to mid-childhood is associated with greater metabolic risk.
Associations are explained by mid-childhood adiposity.
Acknowledgments
Funding Source: This work was supported by the National Cancer Institute’s Centers for Transdisciplinary Research on Energetics and Cancer (TREC) (U54CA116847) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R37HD034568). Elizabeth Cespedes is supported by the National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007703.
EMC conducted all data analysis, drafted the manuscript, and completed all revisions. EMT conceptualized the analysis. All authors advised in presentation of analysis results, revised drafts critically for important intellectual content and approved a final version.
Footnotes
Competing interests: The authors have no competing interests.
References
- 1.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ (Clinical research ed) 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touchette E, Petit D, Tremblay RE, Boivin M, Falissard B, Genolini C, et al. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep. 2008;31(11):1507–1514. doi: 10.1093/sleep/31.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Annals of the New York Academy of Sciences. 2014 doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 5.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Archiv : European journal of physiology. 2012;463(1):139–160. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep medicine. 2007;8(6):668–680. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Van Cauter E. Sleep disturbances and insulin resistance. Diabetic medicine : a journal of the British Diabetic Association. 2011;28(12):1455–1462. doi: 10.1111/j.1464-5491.2011.03459.x. [DOI] [PubMed] [Google Scholar]
- 8.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127(2):e345–352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung V, Beebe DW, Vandyke R, Fenchel MC, Crimmins NA, Kirk S, et al. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep. 2011;34(7):891–898. doi: 10.5665/SLEEP.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Plachta-Danielzik S, Pfeuffer M, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. European journal of clinical nutrition. 2009;63(6):739–746. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 11.Narang I, Manlhiot C, Davies-Shaw J, Gibson D, Chahal N, Stearne K, et al. Sleep disturbance and cardiovascular risk in adolescents. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2012;184(17):E913–920. doi: 10.1503/cmaj.111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Zheng L, Li Y, Yu S, Liu S, Zhou X, et al. Association between sleep duration and hypertension among Chinese children and adolescents. Clinical cardiology. 2011;34(12):774–781. doi: 10.1002/clc.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taveras EMGM, Peña MM, Redline S, Rifas-Shiman SL. Chronic Sleep Curtailment and Adiposity. Pediatrics. 2014;133(6):1013–1022. doi: 10.1542/peds.2013-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort Profile: Project Viva. International journal of epidemiology. 2014 doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart CN, Cairns A, Jelalian E. Sleep and obesity in children and adolescents. Pediatr Clin North Am. 2011;58(3):715–733. doi: 10.1016/j.pcl.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neiberg RH, Wing RR, Bray GA, Reboussin DM, Rickman AD, Johnson KC, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring, Md) 2012;20(10):2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. CDC Growth Charts. United States: 2000. Available at: http://wwwcdcgov/growthcharts/ [Google Scholar]
- 18.Shorr IJ. How to weigh and measure children. New York: U.N; 1986. [Google Scholar]
- 19.Mueller WH, Martorell R. Reliability and accuracy of measurement. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 20.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. The Journal of clinical endocrinology and metabolism. 2003;88(6):2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 21.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. The Journal of clinical endocrinology and metabolism. 2003;88(10):4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 22.De Ferranti SD, Osganian SK. Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2007;4(4):285–296. doi: 10.3132/dvdr.2007.055. [DOI] [PubMed] [Google Scholar]
- 23.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetology & metabolic syndrome. 2010;2:8. doi: 10.1186/1758-5996-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viitasalo A, Lakka TA, Laaksonen DE, Savonen K, Lakka HM, Hassinen M, et al. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia. 2014;57(5):940–949. doi: 10.1007/s00125-014-3172-5. [DOI] [PubMed] [Google Scholar]
- 25.Hurtado-Alvarado G, Pavon L, Castillo-Garcia SA, Hernandez ME, Dominguez-Salazar E, Velazquez-Moctezuma J, et al. Sleep loss as a factor to induce cellular and molecular inflammatory variations. Clinical & developmental immunology. 2013;2013:801341. doi: 10.1155/2013/801341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MA, Cappuccio FP. Biomarkers of cardiovascular risk in sleep-deprived people. Journal of human hypertension. 2013;27(10):583–588. doi: 10.1038/jhh.2013.27. [DOI] [PubMed] [Google Scholar]
- 27.Tatone-Tokuda F, Dubois L, Ramsay T, Girard M, Touchette E, Petit D, et al. Sex differences in the association between sleep duration, diet and body mass index: a birth cohort study. Journal of sleep research. 2012;21(4):448–460. doi: 10.1111/j.1365-2869.2011.00989.x. [DOI] [PubMed] [Google Scholar]
- 28.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. The Journal of pediatrics. 2005;147(6):830–834. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 30.Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61(1):79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham SA, Kramer MR, Narayan KMV. Incidence of Childhood Obesity in the United States. New England Journal of Medicine. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best practice & research Clinical endocrinology & metabolism. 2010;24(5):687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson SE, Whitaker RC. Household routines and obesity in US preschool-aged children. Pediatrics. 2010;125(3):420–428. doi: 10.1542/peds.2009-0417. [DOI] [PubMed] [Google Scholar]
- 35.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35(10):1353–1358. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. The Journal of pediatrics. 2011;158(4):617–623. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep medicine. 2011;12(10):997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Kong AP, Wing YK, Choi KC, Li AM, Ko GT, Ma RC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep medicine. 2011;12(7):659–665. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Kushnir J, Sadeh A. Correspondence between reported and actigraphic sleep measures in preschool children: the role of a clinical context. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013;9(11):1147–1151. doi: 10.5664/jcsm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


