Abstract
Objective
Despite recent advancements in post-cardiac arrest resuscitation, the optimal measurement of post-arrest outcome remains unclear. We hypothesized that cerebral performance category (CPC) score can predict the long-term outcome of post- arrest survivors who received targeted temperature management (TTM) during their post-arrest hospital care.
Design
Retrospective chart review.
Setting
Two academic medical centers from May, 2005 to December, 2012.
Patients
The medical records of 2,417 out-of-hospital and in-hospital post-cardiac arrest patients were reviewed to identify 140 out of 582 survivors who received TTM.
Interventions
None.
Measurements and Main Results
The CPC scores at hospital discharge were determined by three independent abstractors. The 1-month, 6-month, and 12-month survival of these patients were determined by reviewing hospital records, querying the Social Security Death Index, and follow-up telephone calls. The unadjusted long-term survival and adjusted survival association with CPC were calculated. Of the 2,417 identified cardiac arrest patients, 24.1% (582/2417) were successfully resuscitated, of whom 24.1% (140/582) received post-arrest TTM. Overall, 42.9% (60/140) were discharged with CPC 1, 27.1% (38/140) with CPC 2, 18.6% (26/140) with CPC 3, and 11.4% (16/140) with CPC 4. CPC 1 survivors had the highest long-term survival followed by CPC 2 and 3, with CPC 4 having the lowest long-term survival (p < 0.001, log-rank test). We found that CPC 3 (hazard ratio = 3.62, p < 0.05) and CPC 4 (hazard ratio = 12.73, p <0.001) remained associated with worse survival after adjusting for age, gender, race, shockable rhythm, time to TTM initiation, total duration of resuscitation, withdrawal of care, and location of arrest.
Conclusion
Patients with different CPC scores at discharge have significantly different survival trajectories. Favorable CPC at hospital discharge predicts better long-term outcomes of cardiac arrest survivors who received TTM than those with less favorable CPC scores.
Keywords: Cerebral Performance Category, targeted temperature management, therapeutic hypothermia, cardiac arrest, survival, resuscitation
BACKGROUND
Cardiac arrest remains one of the leading causes of death in the United States, affecting approximately 350,000 individuals each year with about half of them occurring in-hospital (1). Despite recent advancements in cardiopulmonary resuscitation, post-arrest survival to hospital discharge remains low for both out-of-hospital cardiac arrest (OHCA) (2) and in-hospital cardiac arrest (IHCA) (3, 4). Moreover, previous studies have suggested that approximately two-thirds of the OHCA patients and a quarter of the IHCA patients died from neurological injury (5).
Post-cardiac arrest targeted temperature management (TTM) has been shown to improve survival and neurologic outcome of cardiac arrest patients (6–9) and is now a Class I and IIb recommendation for patients resuscitated from shockable and non-shockable rhythms, respectively (10). Recent data from the multicenter, international, randomized TTM trial found no difference in the survival and neurologic outcome of OHCA survivors who were maintained at 33°C compared to 36°C, but emphasized the importance of active post-arrest temperature management to prevent pyrexia (11, 12). Many measurement tools have been utilized in attempt to describe post-arrest neurologic functional outcome (13–25). However, little is known regarding the parameter that best predicts long-term survival of both OHCA and IHCA patients. Large retrospective cohort studies thus far have focused on in-hospital survival (2–4,26–28). A few smaller studies have examined predictors of long-term survival (29–32) and even fewer studies specifically evaluated the long-term prognosis of patients who received TTM (11, 15,20, 21, 25, 33, 34). As such, cardiac arrest survivors and their families are often faced with many uncertainties regarding their survival duration and neurologic outcome upon hospital discharge.
Cerebral Performance Category (CPC) is one of the more widely used assessments of the functional status of cardiac arrest patients. Its score ranges from 1 (good cerebral performance) to 5 (brain death), with CPC 1 and 2 generally categorized in prior investigations as good neurological outcome and 3 to 5 as poor outcome (35–37). Recent studies have demonstrated that CPC at hospital discharge can predict the long-term survival outcome of OHCA (38) and elderly survivors of IHCA (39). However, little is known regarding the correlation between CPC scores and long-term survival of patients who received TTM following cardiac arrest resuscitation. We hypothesize that CPC score could be a useful predictor of long-term survival of both OHCA and IHCA patients who received post-arrest TTM.
MATERIALS AND METHODS
We performed a retrospective analysis of 2,417 cardiac arrest patients cared for at two academic medical centers between May, 2005 and December, 2012. We identified the patients who received post-arrest care after return of spontaneous circulation (ROSC) and survived to hospital discharge and the subset of them who received TTM. All TTM was performed with an external cooling device to achieve a targeted temperature of 33 to 34°C. The CPC scores at hospital discharge were determined by one of three independent abstractors. The 1-month, 6-month, and 12-month survival of these patients were determined by reviewing hospital records, querying the Social Security Death Index, and follow-up telephone calls. Study cohort characteristics were analyzed using ANOVA to test the equivalence in means of continuous variables between CPC groups. Chi-square tests and Fisher's exact tests were used to test for equivalence in proportions for the categorical variables. This study was approved by the University of Pennsylvania Institutional Review Board with waiver of informed consent.
Survival time was calculated as the difference between date of death and discharge date or between May 4, 2013 and discharge date for those still alive. Non-parametric Kaplan-Meier survival analysis was used to compare survival among four CPC categories of the overall cohort then within the OHCA and IHCA subgroups. Log-rank test was used to determine whether differences in survival were statistically significant. Cox proportional hazards regression models were used to estimate survival associated with CPC, with and without adjusting for relevant covariates. For the adjusted model, we adjusted for age, gender, race, shockable rhythm, time to TTM initiation, total duration of resuscitation, withdrawal of care, and location of arrest. Both hazard ratios from unadjusted and adjusted Cox proportional hazard model were determined. Patients for whom adjusted variables were not available were removed from the hazard ratio analysis. ANOVA was used to determine whether there were significant differences between the missing data from the different CPC groups. All analyses were conducted using R Version 2.15.
RESULTS
Of the 2,417 identified patients with cardiac arrest, 24.1% (582/2417) survived to hospital discharge, and 24.1% (140/582) of them received post-arrest TTM (Figure 1). The mean age was 54.4±15.7 years; 65% (91/140) were male, 49.6% (64/140) had initial shockable rhythms, and 64.8% (83/140) were OHCA events (Table 1). Overall, 42.9% (60/140) were discharged with CPC 1, 27.1% (38/140) with CPC 2, 18.6% (26/140) with CPC 3, and 11.4% (16/140) with CPC 4 (Figure 1). There were significant differences between the mean age, percentage of shockable initial rhythm, withdrawal of care, and location of arrest between the CPC groups (Table 1). CPC 1 had the most patients with shockable rhythm (66.7%) and OHCA (82.4%), while CPC 4 had the most withdrawal of care (37.5%).
Figure 1.
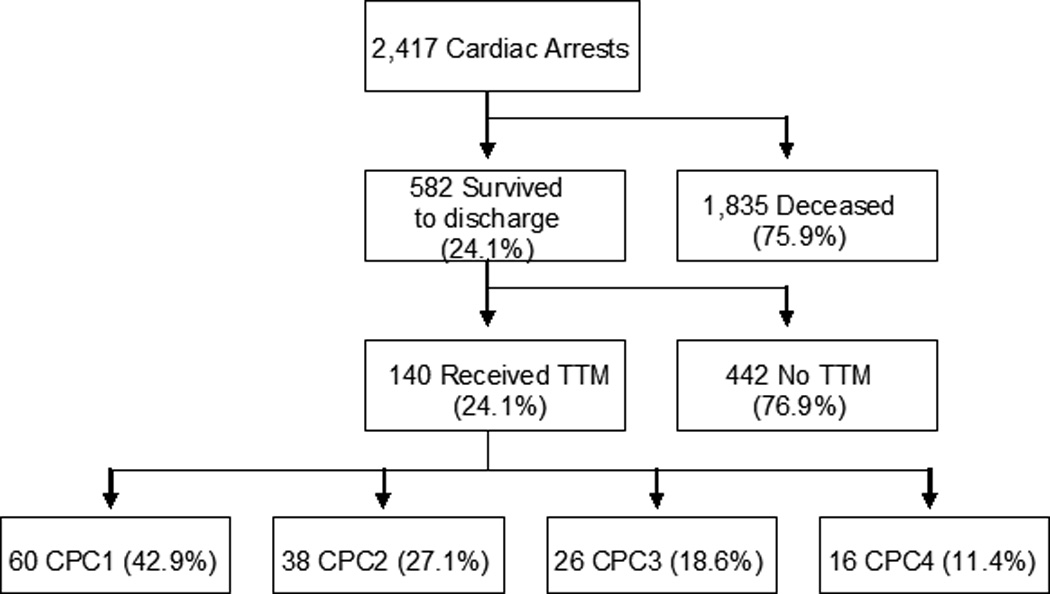
Flow Diagram of Study Population. Of the 2,417 identified patients with cardiac arrest, 582 survived to hospital discharge and 140 received post-arrest TTM. Of the TTM survivors, 42.9% (60/140) was discharged with CPC 1, 27.1% (38/140) with CPC 2, 18.6% (26/140) with CPC 3, and 11.4% (16/140) with CPC 4. CPC = Cerebral Performance Category; TTM = Targeted Temperature Management
Table 1.
Patient Characteristics According to CPC Scores. CPC = Cerebral Performance Category; TTM = targeted temperature management; OHCA = out-ofhospital cardiac arrest; IHCA = in-hospital cardiac arrest; sd = standard deviation
| Characteristics | Overall | CPC1 | CPC2 | CPC3 | CPC4 | p-value | |
|---|---|---|---|---|---|---|---|
| N=140 | N=60 | N=38 | N=26 | N=16 | |||
| Mean age, yr (sd) | 54.4 (15.7) | 50.2 (14.5) | 58.1 (16.9) | 59.7 (14.6) | 53.1 (15.1) | <0.05 | |
| Female, % (n) | 35.0 (49) | 40.0 (24) | 28.9 (11) | 30.8 (8) | 37.5 (6) | 0.677 | |
| Race | White, % (n) | 46.4 (65) | 46.7 (28) | 52.6 (20) | 42.3 (11) | 37.5 (6) | 0.309 |
| Black, % (n) | 40 (56) | 40 (24) | 34.2 (13) | 34.6 (9) | 62.5 (10) | ||
| Other, % (n) | 13.6 (19) | 13.3 (8) | 13.2 (5) | 23.1 (6) | 0 (0) | ||
| Shockable initial rhythm, % (n) | 49.6 (64) | 66.7 (36) | 52.9 (18) | 28.0 (7) | 18.8 (3) | <0.001 | |
| Mean time to TTM initiation, hours | 3.3 (4.6) | 2.3 (3.4) | 3.2 (4.0) | 5.3 (7.1) | 4.4 (4.1) | 0.084 | |
| Mean total duration of resuscitation in minutes (sd) | 42.3 (113.0) | 46.9 (119.4) | 21.8 (44.5) | 73.0 (184.6) | 27.1 (18.1) | 0.360 | |
| Withdrawal of care, % (n) | 5.8 (8) | 0 (0) | 0 (0) | 7.7 (2) | 37.5 (6) | <0.001 | |
| Location of arrest | OHCA, % (n) | 64.8 (83) | 82.4 (42) | 54.1 (20) | 42.3 (11) | 71.4 (10) | <0.05 |
| IHCA, % (n) | 32.1 (45) | 15 (9) | 44.7 (17) | 57.7 (15) | 25 (4) | ||
When non-parametric Kaplan-Meier survival analysis was used to compare survival among four CPC categories (Figure 2A), the estimated survival rates of each CPC group and of the overall cohort showed variability. CPC 1 survivors had the highest long-term survival followed by CPC 2 and 3, with CPC 4 having the lowest long-term survival (p < 0.001, log-rank test). The one-year survival estimate was 0.915 for CPC 1, 0.736 for CPC 2, 0.536 for CPC 3, and 0.125 for CPC 4 (Table 2A). We also performed separate Kaplan-Meier survival analysis for the OHCA and IHCA subgroups. We found that the CPC 1 and CPC 2 OHCA survivors had similar unadjusted survival trajectories (Figure and Table 2B), while IHCA CPC 2 survivors had worse unadjusted survival and no IHCA CPC 4 patients survived to 6 months (Figure and Table 2C).
Figure 2.
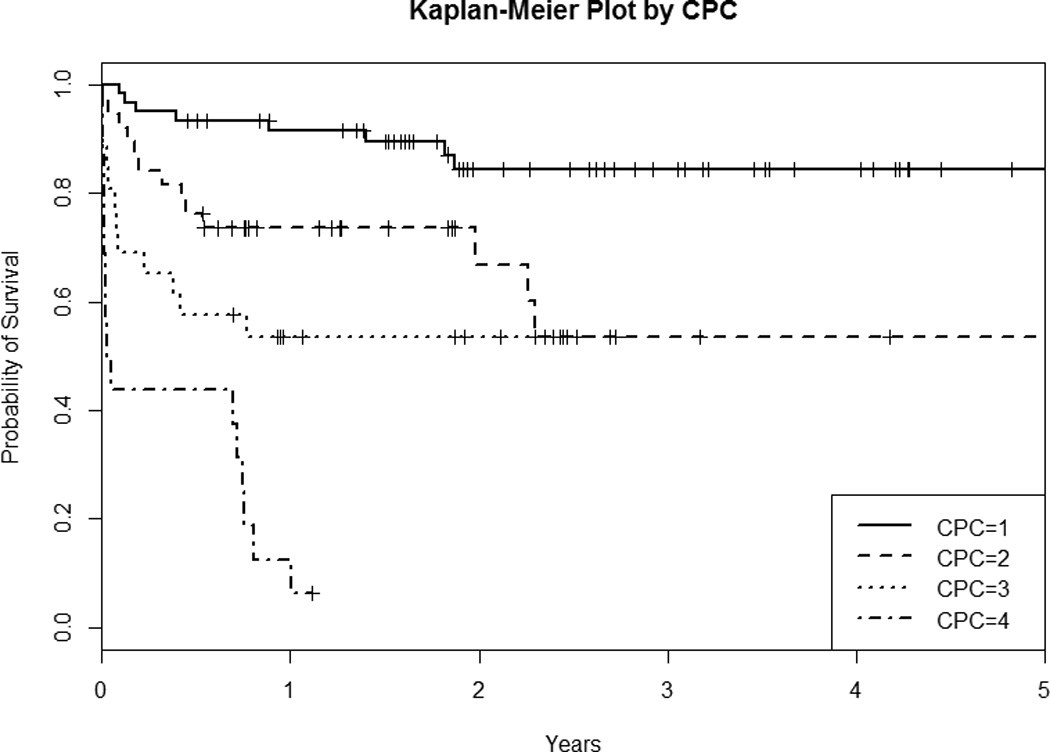
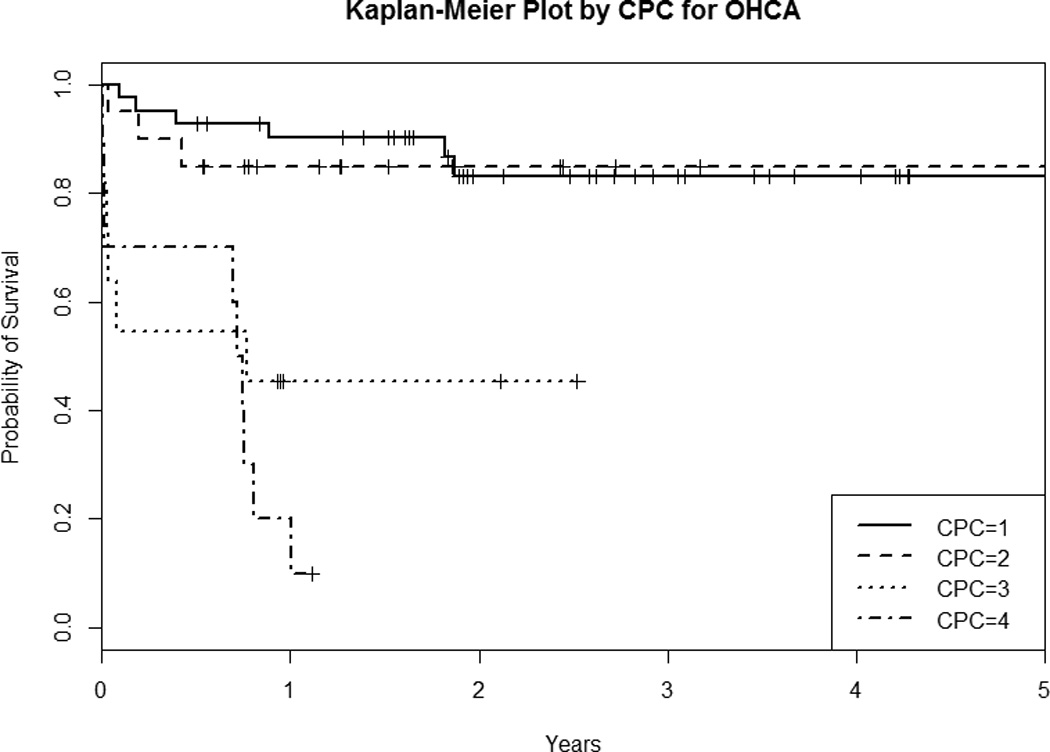
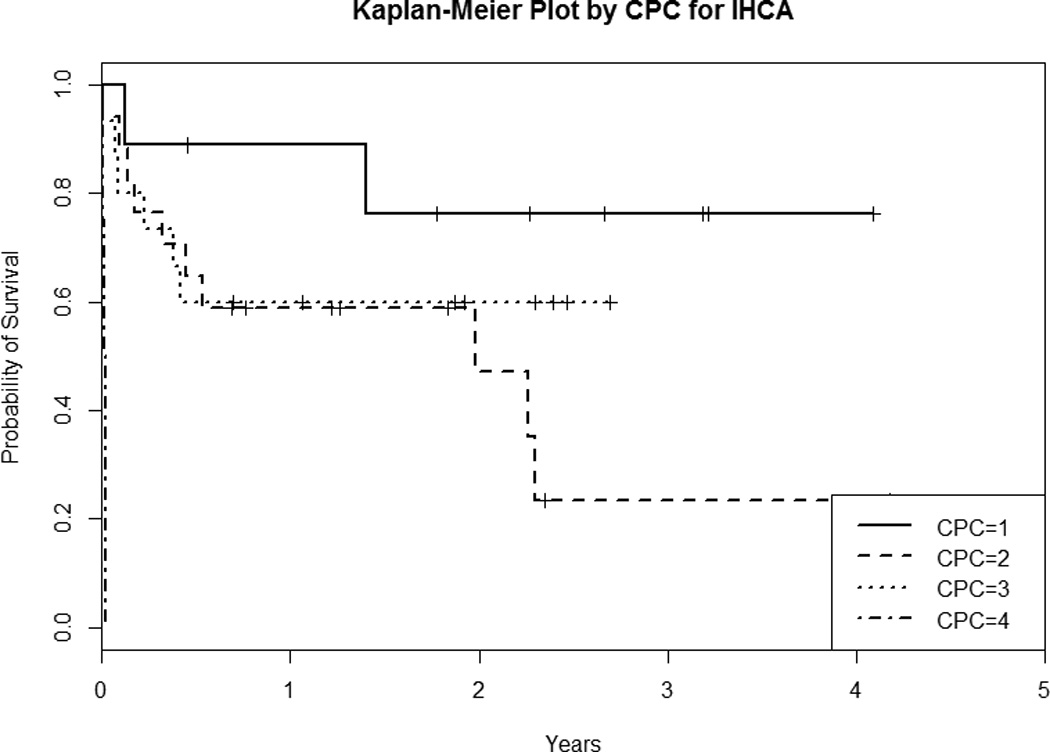
Kaplan-Meier Survival Plot By CPC of the A) Overall Cohort, B) OHCA Subgroup, and C) IHCA Subgroup. CPC = Cerebral Performance Category; OHCA = out-of-hospital cardiac arrest; IHCA = in-hospital cardiac arrest
Table 2.
Survival Estimates by CPC of the A) Overall Cohort, B) OHCA Subgroup, and C) IHCA Subgroup. The 6-month to 3-year survival estimates are presented with 95% confidence intervals. CPC = Cerebral Performance Category; OHCA = out-of-hospital cardiac arrest; IHCA = in-hospital cardiac arrest
| 2A) Overall | ||||
|---|---|---|---|---|
| CPC1 | CPC2 | CPC3 | CPC4 | |
| 6 month | 0.933 (0.872 – 0.999) | 0.763 (0.639 – 0.911) | 0.577 (0.415 – 0.802) | 0.438 (0.251– 0.763) |
| 1 year | 0.915 (0.846 – 0.989) | 0.736 (0.608 – 0.891) | 0.536 (0.374 – 0.768) | 0.125 (0.034 – 0.457) |
| 2 year | 0.845 (0.750 – 0.953) | 0.669 (0.512 – 0.874) | 0.536 (0.374 – 0.768) | NA |
| 3 year | 0.845 (0.750 – 0.953) | 0.535 (0.355 – 0.806) | NA | NA |
| 2B) OHCA | ||||
|---|---|---|---|---|
| CPC1 | CPC2 | CPC3 | CPC4 | |
| 6 month | 0.929 (0.854 – 1.000) | 0.850 (0.707 – 1.000) | 0.545 (0.318 – 0.936) | 0.700 (0.467 – 1.000) |
| 1 year | 0.903 (0.817 – 0.998) | 0.850 (0.707 – 1.000) | 0.455 (0.238 – 0.868) | 0.200 (0.058 – 0.691) |
| 2 year | 0.832 (0.715 – 0.968) | 0.850 (0.707 – 1.000) | 0.455 (0.238 – 0.868) | 0.100 (0.016 – 0.642) |
| 3 year | 0.832 (0.715 – 0.968) | 0.850 (0.707 – 1.000) | NA | NA |
| 2C) IHCA | ||||
|---|---|---|---|---|
| CPC1 | CPC2 | CPC3 | CPC4 | |
| 6 month | 0.889 (0.706 – 1.000) | 0.647 (0.456 – 0.919) | 0.600 (0.397 – 0.907) | NA |
| 1 year | 0.762 (0.521 – 1.000) | 0.588 (0.395 – 0.876) | 0.600 (0.397 – 0.907) | NA |
| 2 year | 0.762 (0.521 – 1.000) | 0.471 (0.260 – 0.850) | 0.600 (0.397 – 0.907) | NA |
| 3 year | 0.762 (0.521 – 1.000) | 0.235 (0.075 – 0.739) | NA | NA |
Table 3 provides estimated hazard ratios from both unadjusted and adjusted Cox Proportional Hazards Models. For the adjusted model, we adjusted for age, gender, race, shockable rhythm, time to TTM initiation, total duration of resuscitation, withdrawal of care, and location of arrest. We found that worse CPC scores were associated with higher hazards of death (p<0.001). Compared to CPC 1, CPC 3 (hazard ratio = 3.62, p < 0.05) and CPC 4 (hazard ratio = 12.73, p <0.001) remained associated with worse survival after adjusting for all the aforementioned variables (Table 3). In the adjusted model, 49 out of 140 (35%) observations were eliminated from the hazard ratio analysis due to unavailable data. The distribution and percentage of missing data were similar across all CPC groups (Table 4).
Table 3.
Hazard Ratios from Unadjusted and Adjusted Cox Proportional Hazard Model. HR = hazard ratio compared to CPC 1; Unadjusted and adjusted hazard ratio with 95% confidence interval and p-value
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | CI | p-value | HR | CI | p-value | |
| CPC1 | 1 | - | - | 1 | - | - |
| CPC2 | 3.24 | (1.34, 7.84) | <0.01 | 0.87 | (0.24, 3.12) | 0.834 |
| CPC3 | 5.12 | (2.09, 12.57) | <0.001 | 3.62 | (1.06, 12.35) | <0.05 |
| CPC4 | 18.56 | (7.61, 45.25) | <0.001 | 11.99 | (2.98, 48.22) | <0.001 |
Table 4.
Distribution and Percentage of Missing Data by CPC. CPC = Cerebral Performance Category
| % Missing (n) | p-value | |||||
|---|---|---|---|---|---|---|
| Variables | Overall | CPC1 | CPC2 | CPC3 | CPC4 | |
| Shockable initial rhythm | 7.9 (11) | 10.0 (6) | 10.5 (4) | 3.8 (1) | 0.0 (0) | 0.448 |
| Time to TTM initiation | 20.7 (29) | 18.3 (11) | 23.7 (9) | 26.9 (7) | 12.5 (2) | 0.649 |
| Total duration of resuscitation | 7.9 (11) | 8.3 (5) | 7.9 (3) | 11.5 (3) | 0.0 (0) | 0.607 |
| Location of arrest | 8.6 (12) | 0.15 (9) | 3.6 (1) | 0.0 (0) | 12.5 (2) | 0.054 |
DISCUSSION
In this study, we found evidence that CPC scores at hospital discharge are associated with long-term survival of cardiac arrest patients who received TTM. Specifically, CPC scores of 3 and 4 at hospital discharge were associated with worse long-term survival after adjusting for age, gender, race, shockable initial rhythm, time to TTM initiation, total duration of resuscitation, withdrawal of care, and location of arrest. In addition, patients with worse CPC at discharge had larger decrement in their unadjusted long-term survival over time. The data from our study may help to determine the duration of resources such as medical assessment, home-care assistance, and physical and occupational therapy required for post-arrest survivors after their hospital discharge.
Our findings are consistent with recent studies on the association between CPC at hospital discharge and long-term survival of cardiac arrest patients. Phelps et al (38) found that 62% of OHCA patients over a 9-year period in King County, WA, had CPC 1 at discharge, 23% had CPC 2, 10% had CPC 3, and 5% had CPC 4. They found that a more favorable CPC was associated with better long-term prognosis, with 5 year survival of 74% for CPC 1, 55% for CPC 2, 44% for CPC 3, and 22% for CPC 4. Our study showed a similar survival distribution of CPC groups but differed from Phelps et al. in that we focused on patients who received TTM but included both OHCA and IHCA patients. Our study cohort had higher percentages of patients with CPC 3 and CPC 4 at discharge compared to that of Phelps et al, and we did not see a difference between the adjusted hazard ratio for the CPC 1 and CPC 2 groups. Interestingly, our subgroup Kaplan-Meier survival analysis of the OHCA TTM survivors also showed that CPC 1 and CPC 2 groups had similar unadjusted survival trajectories (Table and Figure 2B), although this finding was from much smaller sample sizes compared to that of Phelps et al.
Chan et al (39) linked the IHCA database of Get with the Guidelines-Resuscitation (GWTG-R) with Medicare claim files to identify elderly patients greater than 65 years of age who survived to hospital discharge after IHCA from 2000 to 2008. They found that 59% survived for at least 1 year and 50% survived to 2 years. Their study cohort consisted of 48.1% with CPC 1 at discharge, 34.3% with CPC 2, 14.4% with CPC 3, and 3.2% with CPC 4. Our 1-year survival distribution was similar to that of Chan et al where the risk adjusted 1-year survival of CPC 1 was 72.8%, of CPC 2 was 61.1%, of CPC 3 was 42.2%, and of CPC 4 was 10.2%. They also found 1-year survival to be lower for older patients, men, and black patients. Although Chan et al examined a much larger cohort of elderly IHCA patients than included in our study, it was not clear how many patients in that study received TTM. However, Mikkelsen et al recently reported that TTM was underutilized in the GWTG-R cohort, with TTM applied to only 2% of patients from 2003 to 2009 (40). Our subgroup Kaplan-Meier survival analysis of the IHCA TTM survivors suggested that IHCA CPC 2 survivors might have worse unadjusted survival and no IHCA CPC 4 TTM patients survived to 6 months, although the individual sample sizes of the IHCA CPC groups were small.
Our study has several limitations. First, this study was conducted at two tertiary centers that shared the same TTM protocol, which may limit the applicability of our findings to other settings. Both Phelps et al (38) and Chan et al (39) saw differences between the long-term survival of CPC 1 and CPC 2, whereas we did not see any differences in hazard ratio after adjusting for age, gender, race, shockable rhythm, time to TTM, total duration of resuscitation, withdrawal of care, and OHCA. This could be explained by several factors. Our smaller sample size of the post-arrest TTM patients and the higher percentage of missing data (35%) for the adjusted variables might have contributed to the insufficient power seen in the adjusted hazard ratio for CPC 1–3. However, we found that the distribution and percentage of missing data were consistent in all variables between the different CPC groups. We also did not adjust for other comorbidities or the cause of death. The CPC score at discharge was determined either by reviewing medical records for explicitly stated CPC scores or extrapolated from clinical notes by one of three different abstractors. Thus, it is possible that inter-rater reliability may vary for the study cohort (41–43). Finally, as we focused only on the patients who received TTM, it is possible that the baseline differences between the TTM and non-TTM groups could affect the prognosticative accuracy of the discharge CPC score.
There are inherent limitations associated with the use of CPC as the sole prognosticative tool. Although we obtained valuable information regarding the long-term survival rate of cardiac arrest patients based on their CPC at discharge, we did not study their long-term neurologic or functional status after discharge. Thus, it is possible that differences exist between the functional status of CPC1 and 2 patients after hospital discharge despite no significant differences in their long-term survival. CPC has been criticized as a fairly crude measurement of neurological recovery and functional status due to its assessment of multiple functional domains with variable reliability and subjectivity. Prior studies have suggested that CPC can underestimate the level of neuropsychologic and cognitive impairments (43–50) as well as societal participation in cardiac arrest survivors (46). In addition to CPC, other global assessment tools such as the Glasgow Coma Scale, Glasgow Outcome Scale, Glasgow Outcome Scale–Extended, and modified Rankin scores as well as predictive models that specifically addresses the outcome TTM patients such as the 5-R score (34) have been proposed as alternative classification schemata and may have greater predicative value (36, 42, 50). To address the aforementioned limitations, we hope to examine the differences in the functional status between the CPC groups in the future by utilizing neurocognitive tests.
CONCLUSIONS
Post-arrest TTM patients with different CPC score at discharge have significantly different survival trajectories. Favorable CPC at hospital discharge predicts better long-term outcomes of cardiac arrest survivors who received TTM than those with less favorable CPC scores. Specifically, CPC 3 and CPC 4 scores are associated with worse long-term outcome in post-arrest patients. As TTM becomes more widely utilized in post-arrest care, CPC at discharge could serve as a useful prognostic assessment for providers and survivors. Further studies and neurocognitive testing of post-arrest survivors may help to predict the duration and types of outpatient resources necessary after their hospital discharge.
Acknowledgments
Copyright form disclosures: Dr. Leary consulted for Stryker Medical and disclosed ownership (Resuscor, LLC - no financial income currently). Dr. Abella consulted for Velomedix Corp and Heartsine Corp, has stock options with Resuscor, and received support from Medivance and Stryker (Honorarium). His institution received grant support from NIH NHLBI, Philips Healthcare, Medtronic, and Stryker.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Travers AH, Rea TD, Bobrow BJ, et al. Part 4: CPR overview: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) Suppl 3:S676–S684. doi: 10.1161/CIRCULATIONAHA.110.970913. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 4.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 6.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 8.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Sagalyn E, Band RA, Gaieski DF, et al. Therapeutic hypothermia after cardiac arrest in clinical practice: review and compilation of recent experiences. Crit Care Med. 2009;37(7 Suppl):S223–S226. doi: 10.1097/CCM.0b013e3181aa5c7c. [DOI] [PubMed] [Google Scholar]
- 10.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) Suppl 3:S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted Temperature Management at 33 degrees C versus 36 degrees C after Cardiac Arrest. N Engl J Med. 2013 [Google Scholar]
- 12.Leary M, Grossestreuer AV, Iannacone S, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84(8):1056–1061. doi: 10.1016/j.resuscitation.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Levy DE, Caronna JJ, Singer BH, et al. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253(10):1420–1426. [PubMed] [Google Scholar]
- 14.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 15.Stammet P, Wagner DR, Gilson G, et al. Modeling serum level of s100beta and bispectral index to predict outcome after cardiac arrest. J Am Coll Cardiol. 2013;62(9):851–858. doi: 10.1016/j.jacc.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Al Thenayan E, Savard M, Sharpe M, et al. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008;71(19):1535–1537. doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 17.Rittenberger JC, Sangl J, Wheeler M, et al. Association between clinical examination and outcome after cardiac arrest. Resuscitation. 2010;81(9):1128–1132. doi: 10.1016/j.resuscitation.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leary M, Fried DA, Gaieski DF, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation. 2010;81(9):1133–1137. doi: 10.1016/j.resuscitation.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Steffen IG, Hasper D, Ploner CJ, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69. doi: 10.1186/cc8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiainen M, Kovala TT, Takkunen OS, et al. Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med. 2005;33(8):1736–1740. doi: 10.1097/01.ccm.0000171536.63641.d9. [DOI] [PubMed] [Google Scholar]
- 21.Tiainen M, Roine RO, Pettila V, et al. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 22.Zandbergen EG, de Haan RJ, Stoutenbeek CP, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998;352(9143):1808–1812. doi: 10.1016/S0140-6736(98)04076-8. [DOI] [PubMed] [Google Scholar]
- 23.Nair SU, Lundbye JB. The occurrence of shivering in cardiac arrest survivors undergoing therapeutic hypothermia is associated with a good neurologic outcome. Resuscitation. 2013;84(5):626–629. doi: 10.1016/j.resuscitation.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Robinson LR, Micklesen PJ, Tirschwell DL, et al. Predictive value of somatosensory evoked potentials for awakening from coma. Crit Care Med. 2003;31(3):960–967. doi: 10.1097/01.CCM.0000053643.21751.3B. [DOI] [PubMed] [Google Scholar]
- 25.Cloostermans MC, van Meulen FB, Eertman CJ, et al. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40(10):2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 26.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 27.Chan PS, Krumholz HM, Nichol G, et al. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 28.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Kalbag A, Kotyra Z, Richards M, et al. Long-term survival and residual hazard after in-hospital cardiac arrest. Resuscitation. 2006;68(1):79–83. doi: 10.1016/j.resuscitation.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Kutsogiannis DJ, Bagshaw SM, Laing B, et al. Predictors of survival after cardiac or respiratory arrest in critical care units. CMAJ. 2011;183(14):1589–1595. doi: 10.1503/cmaj.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloom HL, Shukrullah I, Cuellar JR, et al. Long-term survival after successful inhospital cardiac arrest resuscitation. Am Heart J. 2007;153(5):831–836. doi: 10.1016/j.ahj.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoch TW, Desbiens NA, DeStefano F, et al. Short- and long-term survival after cardiopulmonary resuscitation. Arch Intern Med. 2000;160(13):1969–1973. doi: 10.1001/archinte.160.13.1969. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 34.Okada K, Ohde S, Otani N, et al. Prediction protocol for neurological outcome for survivors of out-of-hospital cardiac arrest treated with targeted temperature management. Resuscitation. 2012;83(6):734–739. doi: 10.1016/j.resuscitation.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Edgren E, Hedstrand U, Kelsey S, et al. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet. 1994;343(8905):1055–1059. doi: 10.1016/s0140-6736(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 36.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 37.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84(2):960–975. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 38.Phelps R, Dumas F, Maynard C, et al. Cerebral Performance Category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med. 2013;41(5):1252–1257. doi: 10.1097/CCM.0b013e31827ca975. [DOI] [PubMed] [Google Scholar]
- 39.Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368(11):1019–1026. doi: 10.1056/NEJMoa1200657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikkelsen ME, Christie JD, Abella BS, et al. Use of therapeutic hypothermia after in-hospital cardiac arrest. Crit Care Med. 2013;41(6):1385–1395. doi: 10.1097/CCM.0b013e318287f2c4. [DOI] [PubMed] [Google Scholar]
- 41.Ajam K, Gold LS, Beck SS, et al. Reliability of the Cerebral Performance Category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med. 2011;19:38. doi: 10.1186/1757-7241-19-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittenberger JC, Raina K, Holm MB, et al. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–1040. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raina KD, Callaway C, Rittenberger JC, et al. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79(2):249–256. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiainen M, Poutiainen E, Kovala T, et al. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke. 2007;38(8):2303–2308. doi: 10.1161/STROKEAHA.107.483867. [DOI] [PubMed] [Google Scholar]
- 45.Moulaert VR, Verbunt JA, van Heugten CM, et al. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Wachelder EM, Moulaert VR, van Heugten C, et al. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80(5):517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 47.van Alem AP, de Vos R, Schmand B, et al. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J. 2004;148(3):416–421. doi: 10.1016/j.ahj.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Sauve MJ, Doolittle N, Walker JA, et al. Factors associated with cognitive recovery after cardiopulmonary resuscitation. Am J Crit Care. 1996;5(2):127–139. [PubMed] [Google Scholar]
- 49.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269(2):237–242. [PubMed] [Google Scholar]
- 50.Becker LB, Aufderheide TP, Geocadin RG, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]


