Abstract
Recent theories posit that adult neurogenesis supports dentate gyrus (DG) pattern separation and is thereby necessary for some types of discrimination learning. Using an inducible transgenic mouse model, we investigated the contribution of adult-born neurons to spatial and nonspatial touch-screen discriminations of varying levels of difficulty. Arresting neurogenesis caused a modest but statistically significant impairment in a position discrimination task. However, the effect was present only on trials after a learned discrimination was reversed, suggesting that neurogenesis supports cognitive flexibility rather than spatial discrimination per se. The deficit was present 4–10 weeks after the arrest of neurogenesis but not immediately after, consistent with previous evidence that the behavioral effects of arresting neurogenesis arise because of the depletion of adult-born neurons at least one month old. The arrest of neurogenesis failed to affect a nonspatial brightness discrimination task that was equal in difficulty to the spatial task. The data suggest that adult neurogenesis is not strictly necessary for spatial or perceptual discrimination learning and instead implicate adult neurogenesis in factors related to reversal learning, such as cognitive flexibility or proactive interference.
The dentate gyrus (DG) is hypothesized to decorrelate neural inputs into the hippocampus, a process termed pattern separation (Treves and Rolls, 1992; O'Reilly and McClelland, 1994; Gilbert et al., 2001). The DG’s capacity to pattern separate arises from the sparse activity of granule cells (O'Reilly and McClelland, 1994), the principal cells of the DG, and perhaps also the ability of granule cells to modulate their firing rate in response to subtle changes in the external environment (Leutgeb et al., 2007). Computational models posit that DG pattern separation reduces memory interference in CA3, a region whose extensive recurrent connections would otherwise render the circuit unable to differentiate similar inputs (O'Reilly and McClelland, 1994).
There is growing interest in understanding how DG pattern separation contributes to behavior. Kesner and colleagues (Gilbert et al., 2001; Rolls and Kesner, 2006) hypothesized that DG pattern separation contributes to behavioral discriminations when the stimuli have similar or shared features. Consistent with this idea, lesions of the DG impair delayed-match-to-place when the target and distracter locations are proximal (and presumably offer access to similar visual cues) but not when the two locations are distal (Gilbert et al., 2001). More recently, adult neurogenesis in the DG has been implicated in spatial and contextual discriminations of this nature(Clelland et al., 2009; Tronel et al., 2010; Sahay et al., 2011; Nakashiba et al., 2012). One landmark study (Clelland et al., 2009) used touch screen-equipped operant chambers to evaluate the ability of mice to discriminate between spatial locations. Neurogenesis-arrested mice were impaired when the target and distracter locations were proximal, but performed normally when the stimuli were farther apart. Data like these have been viewed as supporting the idea that adult neurogenesis in the DG is necessary for effective pattern separation (but cf., Santoro, 2013).
The studies implicating adult hippocampal neurogenesis in discrimination learning leave several key questions unanswered. First, what is the domain of discrimination tasks that require adult neurogenesis? Is neurogenesis required for difficult discriminations generally or only for those in the spatial or contextual domain? Second, at which cell- developmental stage do adult-born neurons contribute to discrimination learning? Studies investigating the role of neurogenesis in discrimination learning have typically arrested neurogenesis irreversibly and then assessed behavior weeks to months later (Clelland et al., 2009; Tronel et al., 2010). Because adult-born neurons display unique functional properties as they mature (Schmidt-Hieber et al., 2004; Espósito et al., 2005; Ge et al., 2007), ablation effects may arise because immature neurons at a particular developmental stage were depleted (e.g., Denny et al., 2012). Finally, pattern discrimination tasks are often complex, recruiting multiple psychological processes in addition to the ability to make perceptual discriminations. For instance, some tasks (e.g., Clelland et al., 2009) involve reversals, in which the target and distracter locations are swapped after a criterion number of correct responses, thus creating ambiguity about whether neurogenesis is necessary for discriminating between locations or for adapting to changes in the task contingencies. There is some evidence that tasks involving reversals or other changes in task contingencies are especially sensitive to the arrest of neurogenesis (Burghardt et al., 2012; Kalm et al., 2013; Garthe et al., 2014).
Here we investigate the contribution of adult-born neurons to discrimination learning using touch-screen operant tasks modeled after those of Clelland et al. (2009). Using an inducible transgenic mouse model, we ask whether adult neurogenesis is required for spatial and nonspatial discriminations of varying levels of difficulty. Our results suggest that adult neurogenesis influences spatial discrimination performance but only after reversals occur. Furthermore, deficits were present 4–10 weeks after the arrest of neurogenesis but not at earlier time points, suggesting that discrimination learning recruits adult-born neurons at an intermediate level of maturity.
Methods
Subjects
Male GFAP-TK transgenic mice (n = 60) and their wild-type (WT) littermates (n = 67) were used. Mice were 6–8 weeks old at the start of the experiments. GFAP-TK mice express herpes simplex virus thymidine kinase (TK) under the control of the glial fibrillary acidic protein (GFAP) promoter and have been described previously (Garcia et al., 2004) see also (Saxe et al., 2006; 2007; Burghardt et al., 2012; Denny et al., 2012). Mitotic, but not post-mitotic TK-expressing cells are ablated when the prodrug gancyclovir (GCV) is administered systemically (Garcia et al., 2004). The ablation is specific to dividing GFAP+ stem/progenitor cells; quiescent GFAP+ astrocytes are not ablated (Garcia et al., 2004). The GFAP-TK line was backcrossed to the c57bl/6J background for at least 10 generations. Breedings consisted of a WT c57BL/6J male (Jackson Laboratory, Bar Harbor, ME) with a GFAP-TK female.
Mice were housed with littermates in groups no larger than four with a 12-hour light-dark schedule in standard polycarbonate cages with water available ad libitum. During behavioral testing mice were fed 2–3g of chow per day, which maintained them at 90% body weight during the testing week (chow was available ad libitum Friday evenings through Sunday afternoons). Two mice were removed from the study due to complications following surgery. All procedures were performed in accord with the NIH Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin Institutional Animal Care and Use Committee.
Administration of GCV via subcutaneous osmotic minipumps
GCV was administered to GFAP-TK and WT mice for 4 weeks via subcutaneous osmotic minipumps (Alzet Model 1004, 0.11µL/h). The timing of GCV administration with respect to behavioral testing is described below. Mice were anesthetized with isofluorane (2–4%). A small incision was made on the upper dorsum. A hemostat was inserted to open a subcutaneous pocket for the osmotic minipump, which was inserted parasagitally on the back. The incision was closed with sutures. Pumps were filled with 60mg/mL GCV in sterile 0.9% saline. To prevent necrosis at drug infusion site, the pumps were gently rotated within the subcutaneous space every other day. The pumps were removed under isoflurane anesthesia 4 weeks after implantation.
Immunohistochemistry
Mice were deeply anesthetized with ketamine/xylazine (150/15 mg/kg) and transcardially perfused with cold 0.1 M phosphate buffered saline (PBS), followed by 25mL of cold 4% paraformaldehyde in PBS. Brains were postfixed overnight in 4% paraformaldehyde at 4°C, then cryoprotected in 30% sucrose in PBS, and stored at 4°C. Coronal sections (35 µm) were cut through the entire hippocampus on a cryostat and stored in PBS with 0.1% NaN3.
For doublecortin (DCX) immunohistochemistry, sections were blocked in 10% normal donkey serum in 0.1M phosphate-buffered saline (PBS) with 0.25% Triton X-100 for 2h at room temperature. After overnight incubation with primary antibodies (goat anti-DCX, 1:500, Santa Cruz #SC 8066; mouse anti-NeuN 1:500, Millipore MAB377), sections were washed in PBS and incubated with secondary antibodies (Cy3-conjugated donkey anti-goat and Cy5-conjugated donkey anti-Mouse, 1:500, Jackson ImmunoResearch) for 2h at room temperature. Sections were mounted on slides, dried, rinsed in water, and cover slipped using aqueous mounting medium.
Quantification of DCX+ Cells
DCX+ cells in the granule cell layer and subgranular zone were quantified using StereoInvestigator software (MBF Bioscience). Two sections (one anterior and one posterior) were analyzed per mouse using a 40× objective, counting frame of 75×75µm, sampling grids of 150×100µm, and 1µm guard zones. At least 200 cells were counted per WT mouse.
Apparatus
Operant chambers (25 cm×20 cm×14 cm high; Lafayette Instruments) contained a touch screen (25 cm×15 cm) on one wall and reward port on the opposite wall. A black plastic mask in front of the touch screen had 5 openings (4×4 cm each, spaced 1 cm apart), through which stimuli were presented. The reward (a drop of evaporated milk) was delivered using a peristaltic pump, whose operation was signaled by a click (50ms, 85dB). Head entries into the reward port were recorded using infrared detectors. The only illumination in the chamber was provided by the touch screen, which emitted dim light even when stimuli were not explicitly presented on the screen.
Pre-Training
Prior to surgery, all mice were pre-trained to touch illuminated boxes on the screen and drink milk from the reward receptacle. Pre-training commenced at 6–8 weeks of age. First, mice were acclimated to the testing chambers and trained to drink from the reward port in daily 30min sessions. At the outset of the session, the reward port was filled with evaporated milk, and mice were allowed to drink freely. Sessions continued until mice consumed all the available milk on two consecutive days.
Next mice received magazine training in which the click was paired with milk delivery, with an intertrial interval averaging 160s. Milk delivery was not contingent on a behavioral response. Mice received 50 trials per day until they consumed all rewards in one session with a mean latency of less than 5s.
Mice were then shaped to touch illuminated positions on the screen. On each trial, all five positions on the screen were illuminated. Milk was delivered on a 160-s variable-time schedule, but, at any time, a screen touch caused immediate milk delivery. The session ended after 50 rewards were delivered. Mice received daily sessions until they touched an illuminated position within 20s on each trial on at least two testing days. In the final phase of training only a single position was illuminated on each trial, with the location varying randomly among the 5 positions on the screen. Mice continued on this task until the screen-touch latency was less than 15s on each of the 50 trials on one testing day.
Position Discrimination
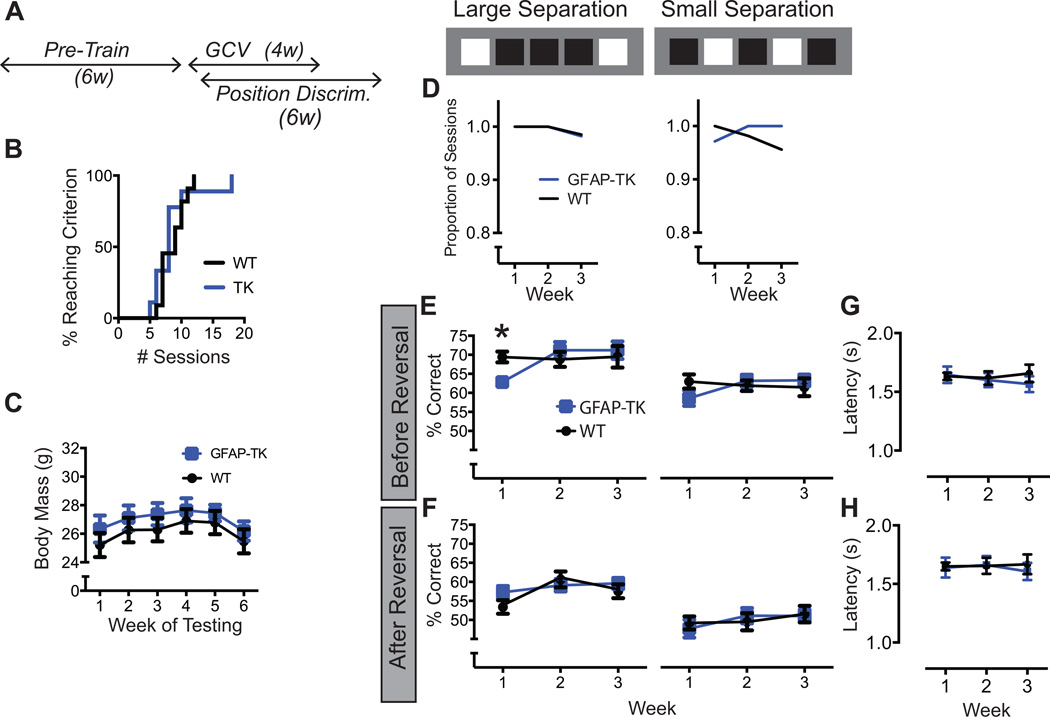
Two positions on the screen were illuminated in white on each trial. The illuminated positions were separated by either 1 (low separation) or 3 (high separation) empty positions (see Figures 2C, 3C). At the start of each session, one position was randomly selected as correct. Upon a nose poke to either the correct or incorrect position, the illuminated positions disappeared. A nose poke to the correct position produced a milk reward, whereas a nose poke to the incorrect position produced a 15-s time out during which no stimuli were presented. A new trial was initiated after the time out (following an incorrect trial) or immediately after the reward was retrieved (following a correct trial). After a mouse made 7 correct responses in 8 trials, the correct and incorrect positions were reversed. Sessions ended after 81 trials. The mean session duration was 25 min. There was no explicit limit on the number of reversals that could occur in a session. Mice were tested on a single separation for an entire testing week. The order of separations was counterbalanced between subjects. Mice were tested 5 days per week for 6 weeks (3 weeks on each separation).
Brightness Discrimination
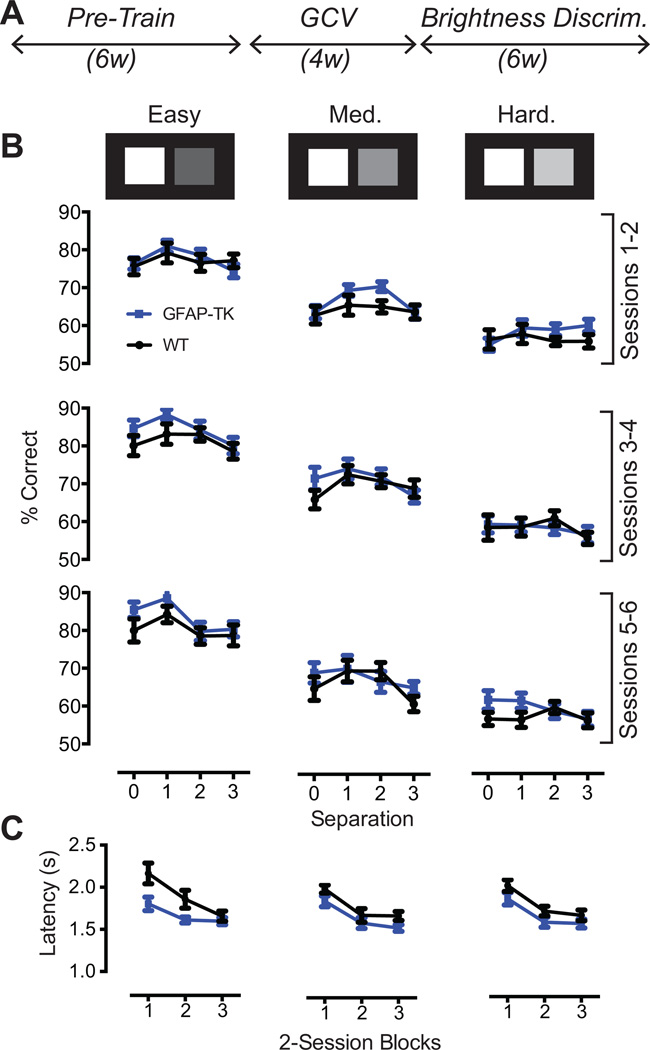
Two positions on the screen were illuminated on each trial. One position was illuminated in bright white (100% brightness), the other at 25%, 50%, or 75% brightness. The location and separation of the illuminated positions varied randomly between trials (see Figure 6B,C), except each location and separation was represented equally in each session. Touches to the either illuminated position on the screen caused both illuminated positions to disappear. Touches to the 100% bright position were reinforced with milk. Touches to the other position led to a 15-s time out. A new trial was initiated after the time out (following an incorrect trial) or immediately after the reward was retrieved (following a correct trial). Mice received 150 trials per day. The mean session duration was 63 min. The brightness of the incorrect square was held constant within each session and randomized between sessions. Mice were tested 5 days per week for 4 weeks for a total of 6 sessions at each contrast level. Reversals were not implemented in this task, because pilot studies revealed the following asymmetry: trials in which the brighter stimulus was correct were significantly easier than trials in which the darker stimulus was correct, regardless of the reversal status. Because this asymmetry would complicate interpretation of reversal performance, we elected to maintain the 100% bright stimulus as the correct stimulus on all trials.
Data Analysis
Correct and incorrect screen touches were recorded throughout each session. The main measure of interest was choice accuracy, defined as the number of correct responses as a percentage of total responses. Relative to other commonly used measures such as number of reversals and trials-to-criterion, choice accuracy has the advantages that (1) all responses can be included in the analysis (incomplete reversals need not be excluded), and (2) it is likely to be more sensitive than other measures (e.g., number of reversals) that discretize performance into larger bins. Choice accuracy was calculated separately for the pre- and post-reversal phases. The pre-reversal phase included all trials in each session prior to the first reversal. The post-reversal phase included all trials after the first reversal. Effects were analyzed using mixed-effects restricted maximum likelihood (REML) model implemented in JMP (SAS Institute), except where noted. Significant higher-order interactions were probed using REML at each level of the interacting variables. Two-way interactions were probed using protected LSD tests. Alpha was set to 0.05 in all tests.
Results
Ablation of adult hippocampal neurogenesis
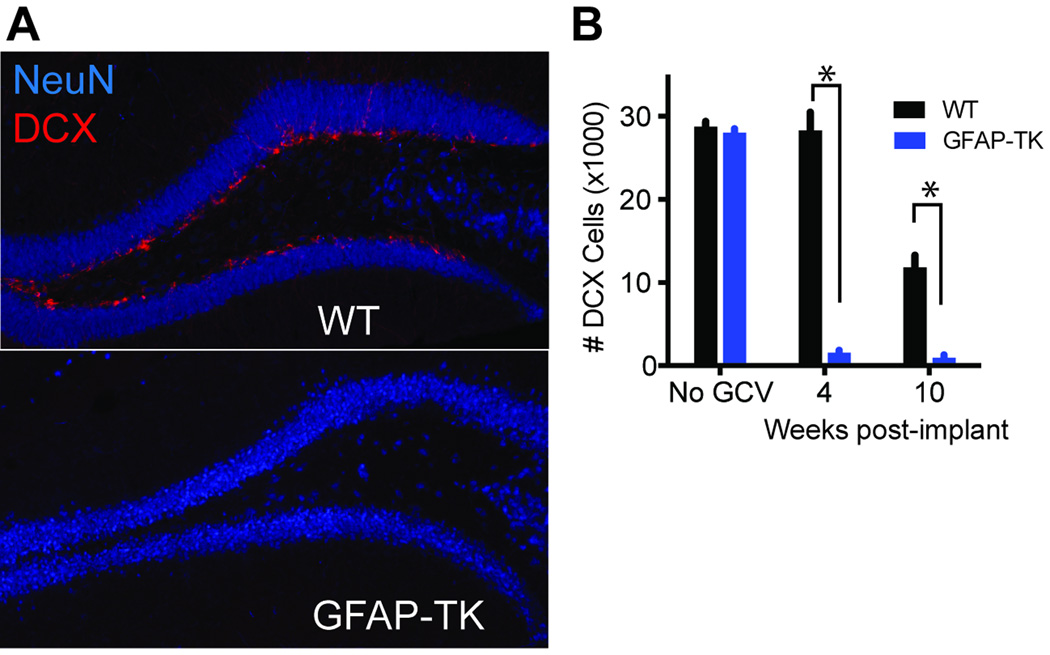
To confirm neurogenesis ablation, GFAP-TK+ (n = 12) and WT (n = 14) mice were euthanized either without prior GCV treatment, or 4 or 10 weeks after the start of a 4-week GCV treatment. Consistent with earlier studies (Garcia et al., 2004; Saxe et al., 2006; Burghardt et al., 2012; Denny et al., 2012) GCV treatment greatly reduced the DCX+ population in GFAP-TK but not WT mice (Figure 1A,B). The number of DCX+ cells was significanly reduced in GFAPTK mice compared to WT mice at 4 weeks (t10 = 10.1, p < .001) and 10 weeks (t6 = 7.2, p < .01) after the start of GCV, confirming a lasting arrest of neurogenesis in GFAP-TK mice. The number of DCX+ cells did not differ between GFAP-TK and WT mice in the absence of GCV (t4 = 0.9, p = .43). Treating GFAP-TK mice with high-dose GCV (100 mg/kg/day) can cause intestinal defects (Bush et al., 1998), but no such defects are observed at the lower dose (~ 6 mg/kg/day) used here (Garcia et al., 2004; Saxe et al., 2006; 2007; Denny et al., 2012). Indeed, the behavior and appearance of GFAP-TK mice were grossly normal, and the weights of GFAPTK and WT mice did not differ during or after GCV treatment (weight data described below and in Figures 2B, 5B).
Figure 1.
A, Immunohistochemical labeling of DCX+ cells in GFAP-TK and WT mice treated with GCV for 4 weeks. DCX+ cells are absent in GFAP-TK mice, confirming that neurogenesis was arrested. B, Quantification of DCX+ cells in GFAP-TK (n = 12) and WT (n = 14) mice. Mice were euthanized without GCV treatment, or they were euthanized 4 or 10 weeks after the start of a 4-week GCV treatment. GFAP-TK mice had significantly fewer DCX+ cells than WT mice at 4 and 10 weeks, but the genotypes did not differ prior to GCV treatment. Error bars represent SEM. *t-test p < .01
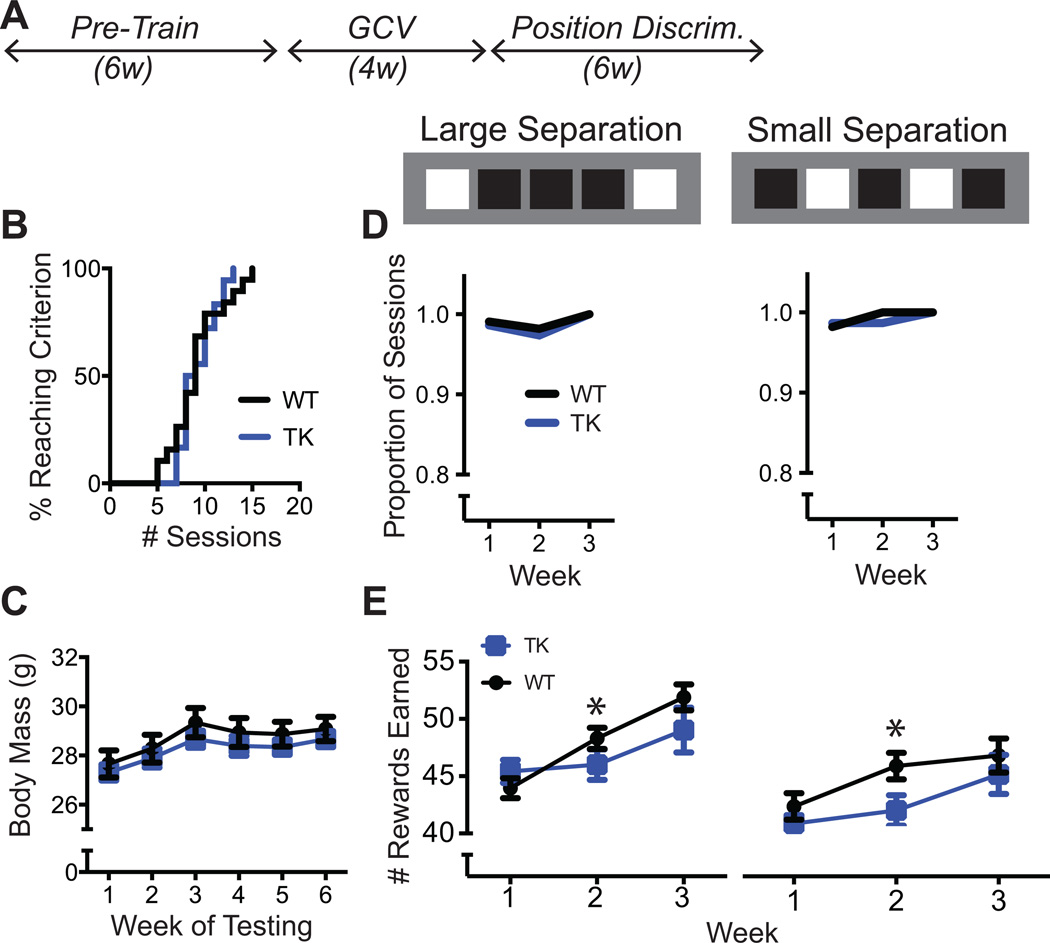
Figure 2.
A, In Experiment 1, position discrimination testing began following a 4-week treatment with GCV. B, Cumulative distribution functions showing the number of sessions required to complete touch screen pre-training. GFAP-TK (n = 15) and WT (n = 22) distributions did not differ. C, Body mass of GFAP-TK and WT mice did not differ during the 6 weeks of discrimination testing. D, Both GFAP-TK and WT mice completed nearly all position discrimination sessions, and the proportion of sessions completed did not differ between genotypes (p = .77, Log-rank test). E, Number of rewards earned per 81-trial session. GFAP-TK mice earned slightly but significantly fewer rewards than WT mice. Error bars represent +/−1 SEM. *p<.05 (statistical tests described in Results)
Role of neurogenesis in position discrimination
In this task, mice were required to discriminate between two illuminated positions on the screen. The correct position changed each time a mouse achieved a sequence of 7 correct responses in 8 trials. Task difficulty was manipulated by varying the spatial separation between the correct and incorrect positions.
Experiment 1: Delayed Testing
In accord with earlier studies indicating that the effects of arresting neurogenesis have a delayed onset (Denny et al., 2012), position discrimination commenced 4 weeks after the start of GCV(Figure 2A). Both GFAP-TK and WT mice were treated with GCV. GFAP-TK (n = 15) and WT (n = 22) mice were pre-trained to touch the touch screens prior to administration of GCV. The proportion of subjects successfully completing pre-training was plotted as function of the cumulative number of pre-training sessions (Figure 2B). The effect of Genotype on the distribution was analyzed using the Mantel-Cox Log-rank test, which failed to reach significance (χ2 = .09, p = .77).
The position discrimination task began 2 days after removal of the GCV pumps. Mice were tested for 6 weeks, including 3 weeks on each of the two spatial separations. Body weight was monitored throughout behavioral testing (Figure 2C). Weight increased over time (F5, 175 = 44.49, p < .001), but there was no effect of Genotype (F1, 35 < 1) or the Genotype X Week interaction (F5, 175 < 1).
Both GFAP-TK+ and WT mice completed nearly all the testing sessions, each of which terminated after 81 trials with a touch screen response (Figure 2D). There was no effect of genotype on the probability of completing a session during any of the weeks of testing (χ2’s < 1, p’s > .05). However, GFAP-TK+ mice exhibited a modest but statistically significant decrease in the number of correct responses (and, thus, rewards earned) per session (Figure 2E). The Week X Genotype interaction was significant (F2,56 = 4.4, p = .045). Simple effects analysis at each level of Week revealed that GFAP-TK+ and WT differed in Week 2 (F1,35 = 4.5, p = .041) but not in Weeks 1 or 3 (p’s > .1).
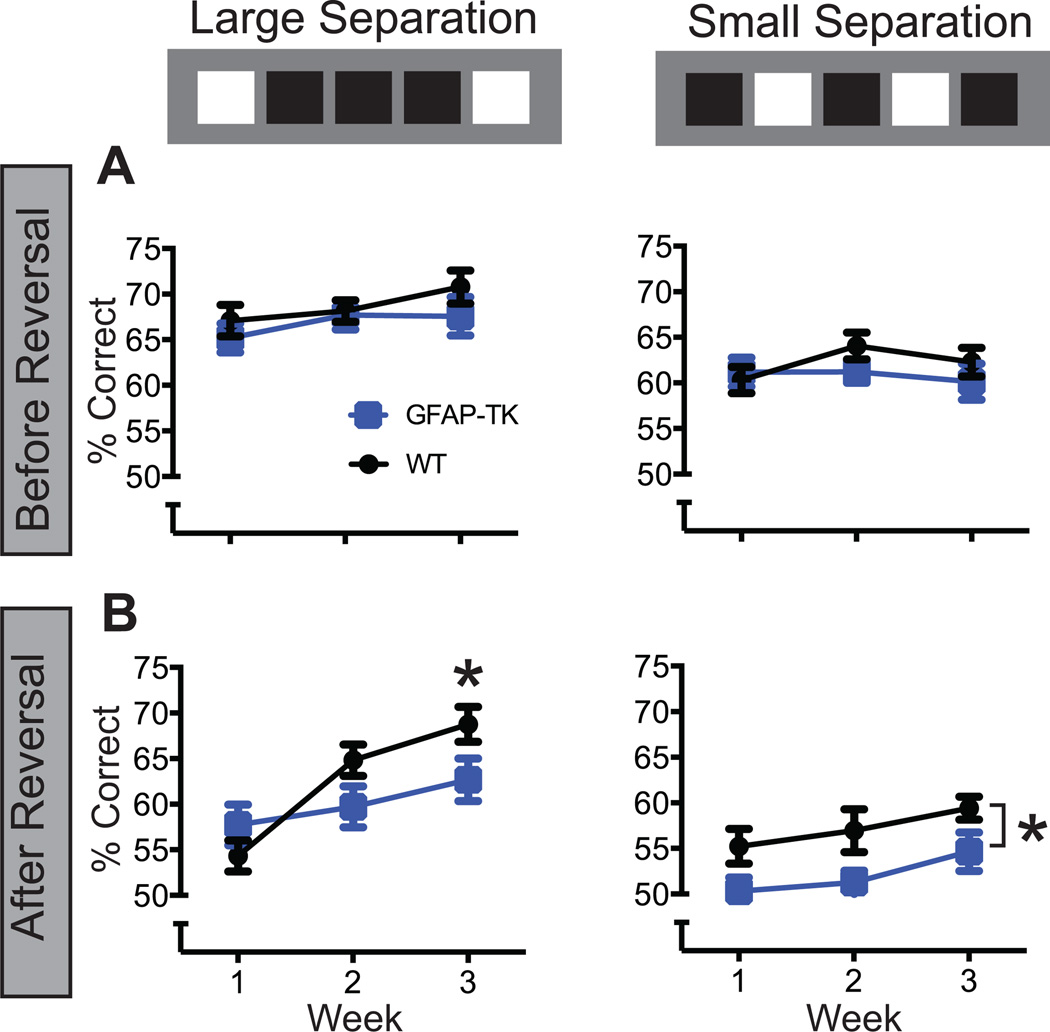
The reduction in rewards earned indicates that choice accuracy was impaired in GFAP-TK mice. Our pilot studies indicated that choice accuracy varies significantly between pre- and post-reversal phases, and previous studies suggest that the pre- and post-reversal performance recruit distinct neural mechanisms (e.g., Bannerman et al., 2012). Thus, we investigated whether the choice accuracy impairment in GFAP-TK mice occurred in the pre- or post-reversal phases of the session. The pre-reversal phase included all trials before the first reversal, and the post reversal phase included all trials after the first reversal. GFAP-TK and WT mice performed comparably on pre-reversal trials at both the small and large separation (Figure 3A). However, GFAP-TK mice were impaired at both separations on post-reversal trials (Figure 3B). The Genotype X Separation X Week X Phase (pre- versus post-reversal) interaction reached significance (F2,70 =3.01, p = 0.02). The interaction was probed using a simple effects approach in which the effects of Week and Genotype (and their interaction) were evaluated at each level of the other two variables. On pre-reversal trials, the effect of Genotype failed to reach significance at either the large separation or the small separation [Genotype: F’s1,35 < 1.7, p’s > .20; Interaction: F’s2,70 < 1, p’s > .50]. The effect of Week also failed to reach significance, F’s2,70 < 2.4, p’s > .09. On post-reversal trials, however, there were significant effects of both Genotype and Week. At the small separation (post-reversal), there were significant effects of Genotype (F1,35 = 5.6, p = .020) and Week (F2,70 = 4.61, p = .013), but not of the interaction [F2,35 < 1, p = .939]. At the large separation (post-reversal) there was a significant Genotype X Week interaction (F2,35 = 7.3, p = .001). Pairwise comparisons of the large separation data (WT versus GFAP-TK) reached significance at week 3 (t105 = 2.13, p = .036) but not weeks 1 and 2 (p’s = 0.236 and .077).
Figure 3.
Pre- and post-reversal choice accuracy in Experiment 1. The pre-reversal phase includes all trials prior to the first reversal; Post-reversal includes all trials after. A, GFAP-TK and WT mice performed comparably on pre-reversal trials at both the large and small separations. B, However, on post-reversal trials, GFAP-TK mice were impaired relative to WT mice at both separations. The impairment reached significance only in week 3 at the large separation but was significant in all weeks at the small separation. Error bars represent +/−1 SEM. *p < .05
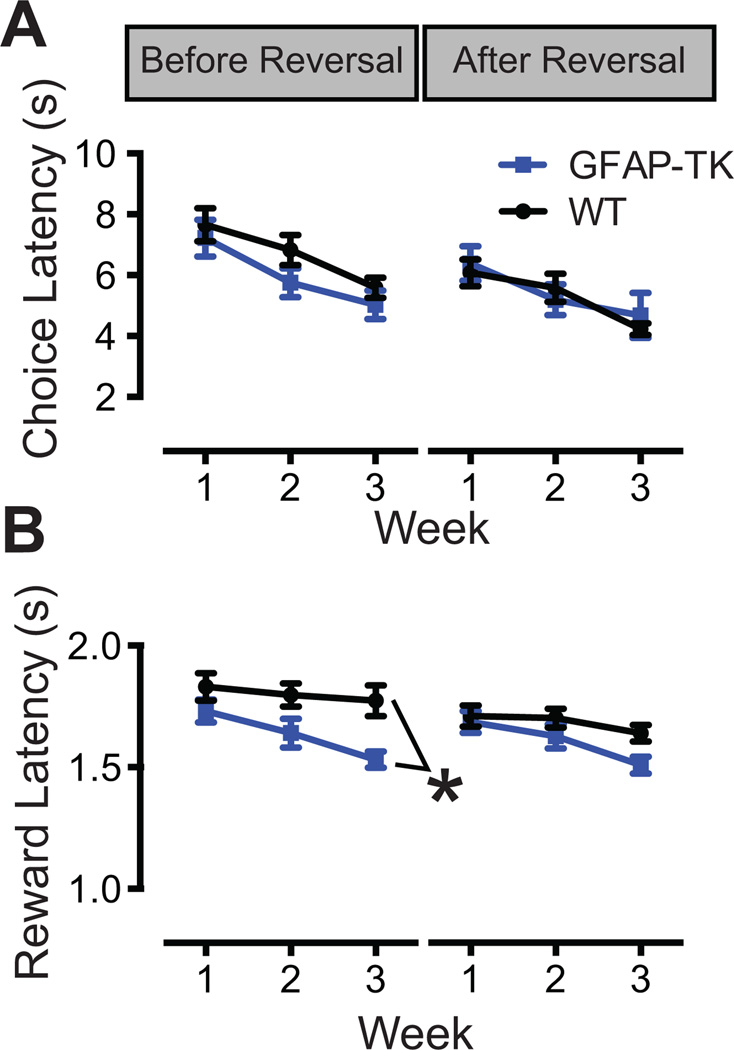
This effect may indicate that arrest of neurogenesis specifically impairs the ability to reverse a learned discrimination. However, because post-reversal trials necessarily occur after pre-reversal trials, there is also the possibility that the data reflect subtle differences in attention or motivation that become more pronounced over the course of a session. To test for differences in motivation and attention, we evaluated choice latency (latency from stimulus presentation to screen touch; Figure 4A) and reward latency (time between reward presentation and the first head entry into the reward port; Figure 4B). Latencies did not differ between the two separations, so the data are collapsed across this variable. For choice latency, the Genotype X Phase interaction reached significance (F1,35 = 4.9, p = .033), but simple effects analysis failed to detect an effect of Genotype at either level of Phase (F’s1,35 < 1.4, p’s > .2). For reward latency, the Genotype X Phase interaction again reached significance (F1,34 = 4.2, p = .048). Simple effects analysis revealed a significant effect of Genotype for the pre-reversal phase (F1,35 = 7.2, p = .011) but not the post-reversal phase (F1,35 = 2.2, p = .143). In summary, reward (but not choice) latencies were shorter in GFAP-TK mice, and this effect was more pronounced on pre-reversal trials. The latency data suggest that the reduced choice accuracy in GFAP-TK mice does not reflect impairment in motivation or in attention to the task.
Figure 4.
Response latency measures for Experiment 1. A, GFAP-TK and WT mice exhibited similar choice latencies, defined as the latency between appearance of the target stimuli and the touch screen response. B, Latency to collect the rewards was significantly shorter in GFAP-TK mice on pre-reversal trials. GFAP-TK and WT mice did not differ on post- reversal trials. Error bars represent +/− 1 SEM. *Main effect of Genotype, p < .05
Experiment 2: Immediate Testing
To test for possible effects of genotype independent of the suppression of neurogenesis, we tested a second cohort of GFAP-TK (n = 9) and WT (n = 11) mice on the position discrimination task but commenced testing 2d after starting GCV administration (Figure 5A). As in Experiment 1, both GFAP-TK and WT were treated with GCV. Previous findings indicate that the arrest of neuronal proliferation does not cause an immediate disruption of behavior (Denny et al., 2012). If the performance impairment observed in Experiment 1 related specifically to the ablation of relatively mature adult born neurons, then no impairment would be observed in mice tested immediately after ablation.
Figure 5.
A, In Experiment 2, GFAP-TK (n = 9) and WT (n = 11) mice began position discrimination testing 2d after the start of GCV administration. B, Cumulative distribution functions showing the number of sessions required to complete touch screen pre-training. GFAP-TK and WT distributions did not differ. C, There was no effect of genotype on body mass during the 6 weeks of behavioral testing. D, GFAP-TK and WT mice completed nearly all discrimination testing sessions and did not differ on the proportion of sessions completed (p > .2, Log-rank test). In contrast to Experiment 1, GFAP-TK and WT choice accuracy was largely comparable on both pre-reversal (E) and post-reversal (F) trials. The only significant effect of genotype on choice accuracy occurred in the pre-reversal phase of week 1. The latency to retrieve rewards did not differ between GFAP-TK and WT mice in either the pre-reversal (G) or post-reversal phase (H). (Error bars represent +/−1 SEM.*Post-hoc LSD test p <.05
As in Experiment 1 there was no effect of genotype on pre-training performance (Figure 5B; (χ2 = .06, p = .81) or body weight (Figure 5C; Genotype effect: F1,22 < 1). Both GFAP-TK+ and WT mice completed nearly all the testing sessions (Figure 5D). There was no effect of genotype on the probability of completing a session during any of the weeks of testing (χ2’s < 1.6, p’s > .2). However, in contrast to Experiment 1, Genotype had only a transient effect on choice accuracy on pre-reversal trials (Figure 5E) on no effect on post-reversal trials (Figure 5F). REML analysis revealed significant effects of Separation (F1,19 = 104.5, p < .001) and of the Genotype X Week X Phase interaction (F2,38 = 7.3, p = .002). The interaction was probed with pairwise (GFAP-TK versus WT) comparisons at each level of Week×Phase. GFAP-TK choice accuracy was impaired relative to WT on pre-reversal trials in week 1 (F1,19 = 11.0, p = .004) but not at any other comparison point (F’s < 1.6, p’s > .20). The latency to retrieve rewards did not differ between GFAP-TK and WT mice on pre-reversal (Figure 5G) or post-reversal trials (Figure 5H). The latency data were subjected to Genotype X Week X Phase REML. The effect of Genotype was not significant (F1,19 < 1), nor were any of the interaction effects (F’s < 1). In summary, GFAP-TK and WT performed largely comparably when position discrimination testing began at the start of GCV treatment.
Experiment 3: Role of Neurogenesis in a Non-Spatial Discrimination
Next we examined the effects of arresting neurogenesis in a non-spatial brightness discrimination task. The task had two purposes. First, we sought to test whether arrest of neurogenesis would impair performance on a nonspatial discrimination that was equal in difficulty to the position discrimination task described above. Second, we sought to rule out an alternative, motoric explanation for the effects of spatial separation on choice accuracy: that decreasing the separation between target and distracter increases the likelihood of accidental touches to the distracter location. If the motoric explanation is correct, then spatial separation should affect performance even in the nonspatial task.
GFAP-TK (n = 24) and WT (n = 20) mice were tested on the brightness discrimination task for 6 sessions at each of 3 levels of contrast between target and distractor. As in Experiment 1, testing commenced 2d after a 4-week GCV treatment (Figure 6A). Spatial separation between target and distractor was varied randomly between trials. As expected, contrast strongly affected choice accuracy, but spatial separation had little effect (Figure 6B). At all levels of choice accuracy the GFAP-TK and WT mice performed comparably. The data were subjected to REML with Sessions (2-session blocks), Genotype, Separation, and Contrast Level as factors. There was no effect of Genotype (F1,42 = 1.5, p = .225) nor did Genotype interact with any other variable (F’s < 1.7, p’s > .1). However, as in Experiment 1, GFAP-TK mice were faster to retrieve rewards than WT mice (Figure 6D). The reward latency data were subjected to REML with Sessions, Genotype, and Contrast as factors. The analysis confirmed a significant effect of Genotype (F1,42 = 5.1, p = .028). The interaction effects were not significant (F’s < 1.1, p’s > .3)
Figure 6.
A, The brightness discrimination task began after completion of a 4-week GCV treatment. B, Task difficulty was manipulated by varying the contrast between the target and distracter stimuli. GFAP-TK (n = 24) and WT (n = 20) mice performed comparably at all difficulty levels and all spatial separations. Spatial separation had little effect on choice accuracy, suggesting that decreases in spatial separation do not increase the incidence of accidental incorrect screen touches. C, As in Experiment 1, GFAP-TK mice were faster to retrieve rewards than WT mice (main effect of Genotype, p = .028) Error bars represent +/− 1 SEM.
Discussion
We examined the effects of arresting adult neurogenesis in two touch screen discrimination tasks. There were two main results. First, the arrest of neurogenesis impaired choice accuracy in a position discrimination task, but the impairment appeared to relate not to the difficulty of the position discrimination per se but rather to the presence of reversals. Neurogenesis-arrested and control mice performed comparably on trials prior to the first reversal, but neurogenesis-arrested mice were impaired on trials after the first reversal. Second, the deficit was present when testing began about 4 weeks after the arrest of neurogenesis but not when testing began immediately after the arrest of neurogenesis. Arrest of neuronal proliferation by itself was not sufficient to impair behavior. Impairments in the position discrimination task arose either because adult-born neurons at an intermediate level of maturity were gradually depleted (e.g., Denny et al., 2012) or because there was a cumulative effect of arresting proliferation over an extended period of time (e.g., Imayoshi et al., 2008).
Arrest of neurogenesis had no effect in a non-spatial brightness discrimination task. The brightness and position tasks were equally difficult, in that they produced similar levels of choice accuracy. Thus, the observation that arresting neurogenesis affected only the position discrimination task indicates that neurogenesis is not strictly required for difficult discriminations. Furthermore, the spatial separation between the target and distracter stimuli had little effect on performance in the brightness discrimination task, which confirms that the effect of spatial separation in the position discrimination task reflected spatial difficulty, not an increased incidence of accidental incorrect responses.
We found that neurogenesis-arrested GFAP-TK mice were not impaired on the first discrimination of the session. However, after the first reversal, the performance of GFAP-TK mice declined below that of WT mice. This finding is consistent with recent literature implicating the hippocampus, and adult-born neurons specifically, in spatial reversal learning. Hippocampal knockout of NMDAR1, a requisite NMDA receptor subunit, affects spatial reversal learning much more than initial acquisition (Bannerman et al., 2012). Computational models suggest that neurogenesis could contribute to reversal learning by reducing proactive interference via two mechanisms. The integration of newborn neurons may weaken existing memories (Meltzer et al., 2005; Kitamura et al., 2009; Weisz and Argibay, 2012; Frankland et al., 2013). In addition, newborn neurons may allow memories encoded at different times to have more distinct neural codes (Aimone et al., 2009). Consistent with these ideas, Burghardt and colleagues (2012) showed that arrest of adult hippocampal neurogenesis impaired reversal learning but not initial acquisition of a highly hippocampus-dependent spatial avoidance task. Conversely, increasing neurogenesis via wheel running impairs retention of previously acquired memories (Akers et al., 2014). In the current study, arrest of neurogenesis may have impaired performance because it strengthened the retention or retrieval of memories from earlier discrimination trials (Saxe et al., 2007).
In addition to impairing discrimination performance, the arrest of neurogenesis was associated with a reduced latency to retrieve rewards. The effects was present in both GFAP-TK groups tested 4 weeks after the start of GCV (Experiments 1 and 3), but it was not present in the group tested immediately after the start of GCV (Experiment 2). The pattern of results leads us to conclude that the decrease in latency is a reliable, delayed-onset effect of arresting neurogenesis. Reduced reward latency is suggestive of an increase in appetitive motivation. We can offer two speculative explanations for this effect. First,Noonan et al. (2010) reported that arrest of hippocampal neurogenesis via x-irradiation increased operant responding for intravenous cocaine, suggesting that arrest of neurogenesis may increase the hedonic impact or incentive value of rewards. However, in the Noonan et al. study, arrest of neurogenesis failed to affect operant responding for sucrose pellets, leading the authors to conclude that neurogenesis modulates motivation for drugs of abuse but not for natural rewards. A second possibility is that the apparent motivational phenotype of GFAP-TK mice relates to suppression of neurogenesis in the hypothalamus. Neurogenesis occurs in the adult rodent hypothalamus, and selective ablation of hypothalamic neural progenitors alters appetite and/or metabolism(Kokoeva, 2005; Haan et al., 2013). Although the neural progenitors implicated in regulation of appetite/metabolism do not express GFAP (Haan et al., 2013), proliferative GFAP+ cells do reside in hypothalamus and appear to be neurogenic (Robins et al., 2013). We were not able to assess hypothalamic neurogenesis in the present study, because the systemic BrdU injections used here produce very little BrdU labeling in the hypothalamus (Kokoeva et al., 2007). However, another study using a similar GFAP-TK mouse line reported no change in hypothalamic cell proliferation in GFAP-TK mice treated with GCV (Snyder et al., 2011). Whatever the mechanism, the apparent increase in motivation in GFAP-TK mice allows us to rule out decreased motivation as an explanation for their performance deficits in the position discrimination task.
Our data appear to conflict with several other recent studies showing that the arrest of neurogenesis impairs spatial and contextual discrimination learning even when reversals are not explicitly required. For instance, irradiation-induced arrest of neurogenesis impairs acquisition of contextual fear discrimination (Sahay et al., 2011; Nakashiba et al., 2012). Perhaps these context discrimination tasks are sensitive to the arrest of neurogenesis because they contain an embedded reversal-like process. A classic feature of discrimination learning is that, at the start of training, the subject generalizes between the CS+ and CS-, in effect treating both as a CS+; then, with continued training responding to the CS- diminishes (Pavlov, 1927). Adult hippocampal neurogenesis may be necessary specifically for the attenuation (“reversal”) of CS- responding that occurs with extended training. Consistent with this idea, arrest of neurogenesis impairs acquisition of trained context discriminations but typically does not affect spontaneous generalization between contexts (Drew et al., 2010; Tronel et al., 2010; Sahay et al., 2011; Cushman et al., 2012; Nakashiba et al., 2012).
Our results should be interpreted with several caveats in mind. First, the magnitude of the impairment in GFAP-TK mice was small, and, although statistically significant, the functional significance may be questioned. Indeed, our results suggest that neurogenesis is not strictly required for spatial discrimination or reversals, and the effects of arresting neurogenesis on performance most likely relate to subtle differences in the content of memory rather than the complete absence of a mnemonic capability such as pattern separation. Second, the GFAP-TK-mediated arrest of neurogenesis is not specific to the hippocampus. Although subventricular zone neurogenesis is also affected in GFAP-TK mice, the behavioral impairments reported here most likely relate to the arrest of hippocampal neurogenesis, as previous studies indicate that hippocampal manipulations are sufficient to affect performance in this task (Clelland et al., 2009; McTighe et al., 2009) . Finally, although we predicted that GFAP-TK mice would perform comparably to WT mice when tested immediately after the start of GCV, GFAP-TK mice were, in fact, impaired at one comparison point during the first week after the start of GCV (on pre-reversal “large” trials, Figure 5E). This impairment is puzzling given that the same mice performed at WT levels on the more difficult post-reversal and small separation trials. The impairment may indicate that the GFAP-TK transgene has mild off-target effects (Groves et al., 2013).
Our results differ from, but do not contradict, the results of Clelland et al. (2009), who employed a task similar, if not identical, to that used here. First, Clelland et al. found that the effects of arresting neurogenesis are limited to the “small” touch-screen separation, whereas in our study GFAP-TK mice were impaired on both the small and large separations. In our study, the impairment in the large separation was time-dependent: GFAP-TK and WT mice performed comparably in week 1, but GFAP-TK mice were impaired in week 3. In the Clelland et al. study, the effect of testing week was not assessed, which could have prevented detection of time-dependent effects. A second difference is that in our study, the impairments in GFAP-TK mice were specific to reversal trials, whereas Clelland et al. did not explicitly compare reversal and non-reversal trials. Finally, while the Clelland et al. study used trials-to-criterion as the measure of performance, we used percent correct, for reasons described above (see Methods). In pilot studies, we found that percent correct was sometimes more sensitive to small group differences in performance than was trials-to-criterion. Consistent with this observation, when we analyzed trials-to- criterion for Experiment 1, there was a trend toward poorer performance in the GFAP-TK mice at both separations, but the effect of Genotype failed to reach significance (p = .08).
In conclusion, our results indicate that adult neurogenesis is not strictly necessary for spatial or nonspatial discrimination learning. Mice without neurogenesis were able to perform very difficult spatial and perceptual discriminations as well as control mice. Effects of arresting neurogenesis arose only when the mice were required to discriminate between a new spatial contingency and a remembered one. Thus, if adult neurogenesis contributes to “pattern separation” in the DG, its role most likely relates to the separation of competing memories rather than to the discrimination of similar perceptual stimuli.
References
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Bus T, Taylor A, Sanderson DJ, Schwarz I, Jensen V, Hvalby Ø, Rawlins JNP, Seeburg PH, Sprengel R. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012;15:1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JD, Maldonado J, Kwon EE, Garcia AD, Fan G, Imura T, Sofroniew MV, Fanselow MS. Juvenile neurogenesis makes essential contributions to adult brain structure and plays a sex-dependent role in fear memories. Front Behav Neurosci. 2012;6:3. doi: 10.3389/fnbeh.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4-to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. Journal of Neuroscience. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Kohler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36:497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garthe A, Huang Z, Kaczmarek L, Filipkowski RK, Kempermann G. Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav. 2014;13:357–364. doi: 10.1111/gbb.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Groves JO, Leslie I, Huang G-J, McHugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model. PLoS Genet. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, Agha El E, Bellusci S, Hajihosseini MK. Fgf10-Expressing Tanycytes Add New Neurons to the Appetite/Energy- Balance Regulating Centers of the Postnatal and Adult Hypothalamus. Journal of Neuroscience. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Kalm M, Karlsson N, Nilsson MKL, Blomgren K. Loss of hippocampal neurogenesis, increased novelty-induced activity, decreased home cage activity, and impaired reversal learning one year after irradiation of the young mouse brain. Exp Neurol. 2013;247:402–409. doi: 10.1016/j.expneurol.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV. Neurogenesis in the Hypothalamus of Adult Mice: Potential Role in Energy Balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Meltzer LA, Yabaluri R, Deisseroth K. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 2005;28:653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, Mchugh TJ, Barrera VR, Chittajallu R, Iwamoto KS, Mcbain CJ, Fanselow MS, Tonegawa S. Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell. 2012:1–14. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. Journal of Neuroscience. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Dover Publications; 1927. [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nature Communications. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A. Reassessing pattern separation in the dentate gyrus. Front Behav Neurosci. 2013;7:96. doi: 10.3389/fnbeh.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest J-M, Piazza P-V, Koehl M, Abrous DN. Adult- born neurons are necessary for extended contextual discrimination. Hippocampus. 2010;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Weisz VI, Argibay PF. Neurogenesis interferes with the retrieval of remote memories: Forgetting in neurocomputational terms. Cognition. 2012;125:13–25. doi: 10.1016/j.cognition.2012.07.002. [DOI] [PubMed] [Google Scholar]