Abstract
Anorexia nervosa (AN) is a psychiatric illness characterized by restricted eating and irrational fears of gaining weight. There is no accepted pharmacological treatment for AN, and AN has the highest mortality rate among psychiatric illnesses. Anorexia nervosa most commonly affects females during adolescence, suggesting an effect of sex and hormones on vulnerability to the disease. Activity-based anorexia (ABA) is a rodent model of AN that shares symptoms with AN, including over-exercise, elevation of stress hormones, and genetic links to anxiety traits. We previously reported that ABA in adolescent female rats results in increased apical dendritic branching in CA1 pyramidal cells of the ventral hippocampus at postnatal day 44 (P44). To examine the long-term effects of adolescent ABA (P44) in female rats, we compared the apical branching in the ventral hippocampal CA1 after recovery from ABA (P51) and after a relapse of ABA (P55) with age-matched controls. To examine the age-dependence of the hippocampal plasticity, we examined the effect of ABA during adulthood (P67). We found that while ABA at P44 resulted in increased branching of ventral hippocampal pyramidal cells, relapse of ABA at P55 resulted in decreased branching. Activity-based anorexia induced during adulthood did not have an effect on dendritic branching, suggesting an age-dependence of the vulnerability to structural plasticity. Cells from control animals were found to exhibit a dramatic increase in branching, more than doubling from P44 to P51, followed by pruning from P51 to P55. The proportion of mature spines on dendrites from the P44-ABA animals is similar to that on dendrites from P55-CON animals. These results suggest that the experience of ABA may cause precocious anatomical development of the ventral hippocampus. Importantly, we found that adolescence is a period of continued development of the hippocampus, and increased vulnerability to mental disorders during adolescence may be due to insults during this developmentally critical period.
Keywords: apical dendrites, dendritic spines, stratum radiatum, exercise, food restriction
INTRODUCTION
Anorexia nervosa (AN) is an eating disorder characterized by severe self-starvation, high levels of physical activity, and an irrational fear of gaining weight. AN is often coupled with anxiety disorders (Bulik et al., 2006), over-exercise (Yates et al., 1983), and disordered representation of one’s own body (Hartmann et al., 2013; Keizer et al., 2013). The onset of AN most commonly occurs in females at the time of puberty, especially among those who suffered from anxiety disorders during childhood (Kaye et al., 2004). This epidemiologic pattern suggests that the onset of AN may be triggered by hormonal modulation during puberty, which alters the way the brain handles stressful input long after onset. AN patients face a life-long risk of relapse (Pike, 1998), suggesting that the brain retains a ‘memory’ of AN. Though relapse may occur later in life, the initial onset of AN rarely occurs after adolescence (Bulik et al., 2006).
The hippocampus is involved in spatial and cognitive functions and also plays a key role in regulating anxiety (Bannerman et al., 2002; McHugh et al., 2011). The hippocampus responds to the synchronous exposure to stress and hormonal changes (McLaughlin, Wilson et al., 2010) in ways that are distinct from the exposures to stress alone (McEwen, 1999) or hormonal changes alone (Gould et al., 1990; McCarthy and Milner, 2003; Woolley et al., 1990; Woolley and McEwen, 1992). As such, the hippocampus plays a key role in stress-induced anxiety during puberty (Sabaliauskas et al., 2012; Shen et al., 2007).
Adolescence is a point of vulnerability not only to eating disorders but many other psychiatric illnesses, including addictions, anxiety disorders, major depression, and schizophrenia. A significant body of evidence suggests that the hippocampus may be involved in the vulnerability of the adolescent brain to these disorders. Post-pubertal changes in hippocampal axonal innervation as well as spine density have been reported, suggesting that the hippocampus is not fully mature by puberty (Andersen and Teicher, 2004; Yildirim et al., 2008). Early-life stress has been shown to have a delayed effect on post-pubertal development of the hippocampus (Andersen and Teicher, 2004) and to result in anxiety during adulthood (Sarro et al., 2013). Additionally, neonatal lesion of the ventral hippocampus, the region shown to preferentially mediate anxiety behaviors (Bannerman et al., 2002; Barkus et al., 2010; McHugh et al., 2011), results in increased anxiety after puberty onset (Lipska et al., 1993). These studies suggest that adolescence is an important developmental stage for the hippocampus, especially for the ventral region, and for the development of hippocampus-dependent behaviors but that knowledge of the neuroanatomical substrates underlying the behavioral changes during adolescence is far from complete. Moreover, these studies suggest that early-life experiences or inherent genetic predispositions interact with the neuro-maturational processes of adolescence to cause developmental and behavioral deficits that are revealed in adulthood.
To study the effects of pubertal experience on the development of the ventral hippocampus, we have used a rat model of anorexia nervosa called activity-based anorexia (ABA). In this model, rats are given free access to a running wheel for three days before they are subjected to a severely restricted feeding schedule. Paradoxically, the animals become hyperactive after the onset of food restriction, running more than they had been running before food restriction and more than the free-fed animals with access to a wheel. We have previously shown that pubertal induction of ABA affects the morphology of hippocampal neurons. Activity-based anorexia results in decreased branching of CA1 hippocampal cells in the dorsal hippocampus, while causing increased branching in the ventral hippocampus (Chowdhury et al., 2013). However, hippocampal neurons have been shown to recover from stress-induced dendritic changes (Conrad, 2008). In the current study, we analyzed the impact of ABA induction upon subsequent development of the hippocampus. To this end, we analyzed the morphology of CA1 pyramidal cells of the ventral hippocampus immediately after ABA induction, after a seven-day period of recovery, and after a second induction, or “relapse,” of ABA. To assess whether the anatomical effects we previously found are specific to puberty, we also examined the effects of ABA in adult rats.
METHODS
Animals and ABA induction
All experiments were approved by the Institutional Animal Care and Use Committees of the New York State Psychiatric Institute, Columbia University (Animal Welfare Assurance No. A3007-01), and New York University (Animal Welfare Assurance No. A3317-01).
Thirty Sprague-Dawley female rats were purchased from Taconic Farms and delivered to New York State Psychiatric Institute’s animal facility on postnatal day 21 (P21).
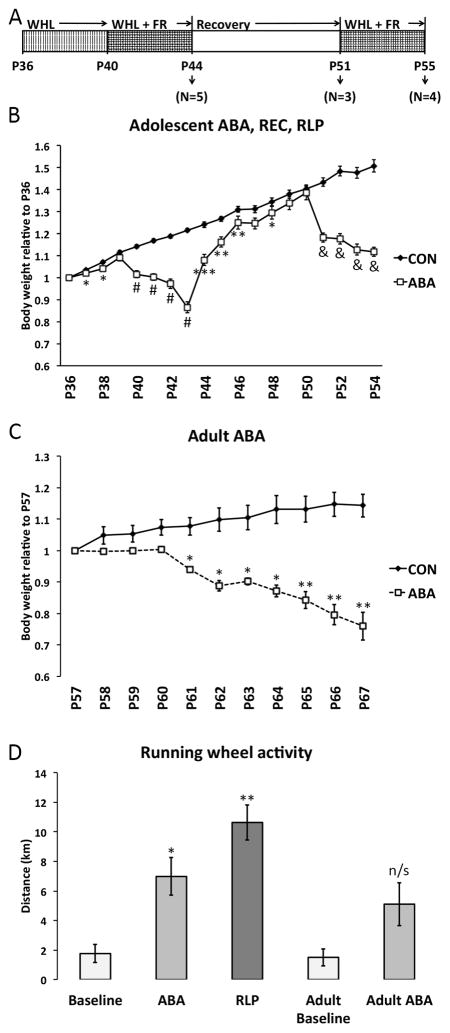
Twenty-four of these animals were assigned to the adolescent ABA group, while six animals were reserved for the adult ABA group. Figure 1A shows a schematic of the experimental timeline for the adolescent ABA, recovery, and relapse. At P36, the adolescent group was divided into two groups of 12 rats each. Twelve rats, assigned to the ABA group, were housed in cages with access to a running wheel (Med Associates, Inc., St. Albans VT), while 12 controls (CON) were housed in standard home cages. All animals had ad libitum access to food at this point. At P40, the beginning of food restriction (FR), all food was removed from the animals with wheel access. From P41 to P43, food was returned to the cages for one hour per day (the first hour of the dark cycle). On P44, at the end of the last day of food restriction, 3 control (P44-CON) animals and 5 ABA (P44-ABA) animals were euthanized. The 7 remaining ABA animals were housed for seven days in a recovery environment, without a running wheel and with free access to food. At P51, 3 of the recovered ABA animals (P51-REC) and 4 controls (P51-CON) were euthanized. The last 4 ABA-recovered animals were returned to the ABA-inducing environment, with free access to the running wheel and 1 hour/day of food access, for an additional 4 days. At P55, these animals (P55-RLP) and 5 controls (P55-CON) were euthanized.
Figure 1.
Adult ABA animals (P67-ABA; N=3) were housed with a running wheel beginning P57, while P67-CON (N=3) animals were housed without access to a running wheel. P67-ABA animals had food access restricted to 1 hour/day for seven days beginning on P60. All 6 adult animals were euthanized on P67.
Body weight, food intake, and wheel activity (where applicable) were measured daily, starting at P36 and ending when the rats were euthanized.
Perfusion and Golgi staining
Animals were euthanasized by being deeply anesthetized by an intraperitoneal urethane injection (34%; 0.65–0.85 mL/g body weight) prior to transcardial perfusion with phosphate-buffered saline containing heparin (1000 U per 500 ml) followed by 10 minutes of perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer.
A 3 mm-thick coronal section of the caudal right hemisphere was collected for Golgi impregnation using the FD Rapid GolgiStain kit (FD Neurotechnologies, Inc., MD, USA). All staining was done in parallel and in accordance with the provided “Staining Procedure” of the kit, with the exception of Step 4, cresyl violet or thionin counterstain, which was eliminated. Coronal sections 250 μm thick were prepared using a vibratome.
Neuron tracing and analysis
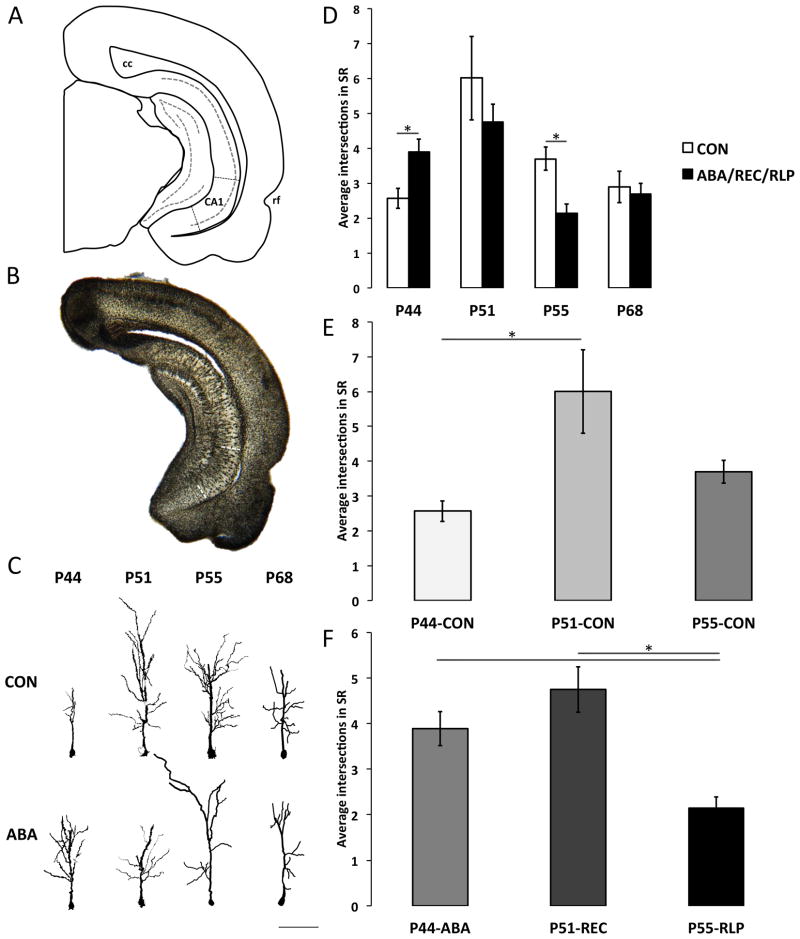
Cells selected for analysis met the following criteria: intense Golgi staining and good visibility within a single slide. Ventral hippocampal CA1 pyramidal cells were chosen from coronal sections approximately −5.60 to −6.3 mm from Bregma and 6.00–7.00 mm from the dorsal surface, close to the rhinal fissure as a landmark (Figure 2A, B). Cells, including dendritic spines, were drawn by hand using a camera lucida extension on a light microscope and were analyzed following standard Sholl analysis, quantifying the number of intersections that the apical arbor makes with concentric circles drawn in radial increments of 20 μm from the center of the cell body. After determining that both hand-drawn and digital Sholl analysis using Neurolucida Standard software (MicroBrightField, Inc., VT, USA) yielded similar results for the same neurons, the remaining cells were traced and analyzed using Neurolucida’s software. Neurons were digitally traced onto a computer file and later analyzed using Neurolucida Explorer’s built-in Sholl analysis software.
Figure 2.
Sholl analysis was performed to quantify the density of dendritic branches from a specified range of distances from the cell body. Two cells were analyzed from each animal, except for some cases in which only one cell met the criteria to be analyzed. The number of cells measured (N) were as follows: CON-P44 N=6; ABA-P44 N=8; CON-P51 N=8; REC-P51 N=6; CON-P55 N=7; RLP-P55 N=10; CON-P67 N=6; ABA-P67 N=6.
Statistical analyses
All statistical analyses for significance were computed using the StatSoft STATISTICA software. Student’s t-test was used to assess the difference when two groups were being compared. One-way ANOVA was used when three or more groups were being compared. Significant pairs were determined using Tukey’s HSD post hoc tests. Two-way ANOVA was used when comparing the effects of two factors across groups.
RESULTS
1. Animal weight & wheel activity
Body weight and wheel activity were measured daily for adolescent ABA and adult ABA experiments. To represent the daily change in body weight, normalized daily body weights were calculated for each animal relative to the animal’s weight on the first experimental day, i.e., P36 for the adolescent group and P57 for the adult group. Daily wheel activity was measured as the number of wheel rotations per day and converted into distance in km. All results are reported as mean±standard error and are displayed in Figure 1.
1.1 Weight of adolescent CON and ABA animals
To study the effects of ABA on adolescent female rats, we began the study soon after the onset of puberty as defined by the average age of vaginal opening in rats, P35. Animals entered the experiment at the end of their 36th postnatal day.
CON animals’ body weights steadily increased throughout the duration of the experiment (Table 1, Figure 1B). Animals in the ABA groups (P44-ABA, P51-REC, and P55-RLP) were acclimated to a running wheel beginning on P37 and were food restricted from P40-P44. On average, CON animals gained slightly more weight than their ABA counterparts on days P37 and P38, likely due to the presence of the wheel in the cage, which gave the rats an opportunity to exercise voluntarily. Relative weight was slightly higher in CON than ABA on P38 and P39, but equalized by P40 at the onset of ABA.
Table 1.
Comparison of body weight changes, relative to P37, between CON and age-matched ABA animals during the first ABA (P41 to P44), recovery (P45 to P51), and second ABA (P52 to P55).
| Age | Mean ± Standard Error (N) | t (df) | p | |
|---|---|---|---|---|
| CON | ABA | |||
| P37 | 1.000 (12) | 1.000 (11) | ||
| P38 | 1.036±0.005 (12) | 1.019±0.006 (11) | 2.244 (21) | 0.04 |
| P39 | 1.071±0.007 (12) | 1.040±0.012 (11) | 2.379 (21) | 0.03 |
| P40 | 1.115±0.010 (12) | 1.091±0.015 (11) | 1.356 (21) | 0.2 |
| P41 | 1.142±0.008 (12) | 1.016±0.015 (11) | 7.471 (21) | <0.000001 |
| P42 | 1.169±0.009 (12) | 1.003±0.014 (11) | 10.359 (21) | <0.000001 |
| P43 | 1.188±0.008 (12) | 0.973±0.022 (11) | 9.656 (21) | <0.000001 |
| P44 | 1.216±0.007 (12) | 0.865±0.025 (11) | 14.103 (21) | <0.000001 |
| P45 | 1.240±0.012 (9) | 1.081±0.025 (7) | 6.147 (14) | 0.00003 |
| P46 | 1.267±0.011 (9) | 1.163±0.023 (7) | 4.417 (14) | 0.0006 |
| P47 | 1.311±0.013 (9) | 1.250±0.030 (7) | 2.049 (14) | 0.06 |
| P48 | 1.312±0.017 (9) | 1.247±0.025 (7) | 2.219 (14) | 0.04 |
| P49 | 1.345±0.018 (9) | 1.295±0.029 (7) | 1.546 (14) | 0.1 |
| P50 | 1.380±0.019 (9) | 1.339±0.029 (7) | 1.226 (14) | 0.2 |
| P51 | 1.403±0.019 (9) | 1.385±0.031 (7) | 0.522 (14) | 0.6 |
| P52 | 1.434±0.019 (5) | 1.182±0.020 (4) | 9.140 (7) | 0.00004 |
| P53 | 1.484±0.022 (5) | 1.176±0.024 (4) | 9.427 (7) | 0.00003 |
| P54 | 1.476±0.024 (5) | 1.128±0.024 (4) | 9.990 (7) | 0.00002 |
| P55 | 1.508±0.029 (5) | 1.118±0.022 (4) | 10.362 (7) | 0.00002 |
After FR began on P40, ABA animals began to lose weight. ABA relative weight was significantly less than CON on days P41, P42, P43, and P44. By the end of the FR period, ABA animals had dropped to 87% of their initial body weight while CON had increased in weight by about 22%. P44-CON and P44-ABA animals were euthanized at this time point, while P51-CON, P51-REC, P55-CON, and P55-RLP animals continued to be weighed daily.
Wheel access was blocked and food restriction was discontinued for seven days for P51-REC and P55-RLP animals. REC and RLP animals gradually regained body weight, approaching that of CON animals by P49, P50, and P51. At this point, P51-CON and P51-REC animals were euthanized.
During the second induction of ABA from P51 to P55, the running wheel was returned and FR began again for RLP animals. RLP animals again lost weight relative to CON on days P52, P53, P54, and P55.
1.2 Wheel activity of adolescent ABA animals
Running wheel activity results corroborated those obtained previously, namely, that animals exhibit hyperactivity after the onset of FR. During ABA, animals ran an average of 1.8±0.6 km/day (N=11) during the baseline period from P37-40, and this increased more than 3-fold to 7±1 km/day (N=11) after the onset of FR. When the second period of FR began for relapse animals, they immediately began running at high levels, an average of 10±1 km/day (N=4). One-way ANOVA indicated that the activity during baseline, ABA, and relapse were significantly different (F(2,23)=13.79, p=0.00012), with Tukey’s post hoc test indicating that the baseline activity was significantly lower than the activity during FR in ABA (p=0.0009) as well as relapse (p=0.00009), while the activity during relapse was not significantly different from that during ABA (p=0.06) (Figure 1D).
1.3 Weight and wheel activity of adult ABA animals
To study the effects of ABA induction on adult female mice, a second cohort of rats entered the experiment at the end of their 56th postnatal day. CON animals did not have access to a running wheel and did not endure FR. ABA animals were given access to a running wheel beginning on P57. FR began on P60 and continued for 7 days.
From P57 to P60, the relative weight of CON and ABA animals was not significantly different, although animals with access to a running wheel were slightly lower in body weight (Figure 1C). By P60, CON animals had gained approximately 7% of their P57 weight, which was not significantly different from animals with running wheel access (CON: 1.07±0.02, N=3; ABA: 1.004±0.007, N=3). Once FR began, the ABA animals began to lose weight, albeit more gradually than the adolescent animals, and they continued to do so for the duration of the seven days of FR. By P67, ABA animals had dropped to approximately 76% of their P57 weight, while CON animals had gained an additional 14% (CON: 1.14±0.04, N=3; ABA: 0.76±0.04, N=3).
Wheel activity increased after the onset of FR in adult females (ABA-P67), but it was not significantly greater than baseline activity (pre-FR: 1.5±0.6, N=3; post-FR: 5.1±1.5, N=3; t(4)=2.27, p=0.08) (Figure 1D).
2. Effects of ABA, recovery, and relapse on hippocampal CA1 pyramidal cells’ dendritic branching relative to age-matched controls
Sholl analysis was performed to quantify the density of dendritic branches at incremental distances from the cell body. Example cells from each experimental group are shown in Figure 2C. Due to the plane of the coronal brain section relative to the orientation of the pyramidal neurons, the most distal portions of the apical dendrites were not consistently retained within the thickness of the 250-μm section. Therefore, analysis was performed only on the dendrites between 80–200μm from the cell body.
Cells from P44-ABA animals showed an increase in branching relative to P44-CON (P44-CON: 2.56±0.29 intersections; P44-ABA: 3.89±0.37; t(12)=-2.65, p=0.02; Figure 2A), confirming the results from our previous study (Chowdhury et al., 2013). After seven days of recovery, P51-REC cells were not significantly different from P51-CON (P51-CON: 6.01±1.20; P51-REC: 4.75±0.50; t(12)=0.86, p=0.41). The second induction of ABA affected dendritic branching in the opposite direction as the first. P55-RLP cells had decreased branching relative to age-matched controls (P55-CON: 3.70±0.33; P55-RLP: 2.15±0.25; t(15)=3.49, p=0.003). When ABA was induced for the first time in adulthood, there was no effect on the apical branching of CA1 (P67-CON: M=2.88±0.46; P67-ABA: 2.69±0.30; t(10) = 0.36, p=0.72).
When ABA was induced in adult rats, no significant difference in apical dendritic branching was found relative to age-matched CONs (CON-P67: 2.88±0.46; ABA-P67: 2.69±0.30; t(9)=-0.36, p=0.72; Figure 2A), indicating that the vulnerability to ABA-induced morphological change in the SR of the ventral hippocampus is age-dependent.
3. Development of CA1 pyramidal cells of the ventral hippocampus of control and ABA groups during adolescence
One-way ANOVA revealed a significant effect of age on dendritic branching of CON cells among the three groups: P44-CON, P51-CON, and P55-CON(F(2, 21)=5.04; p=0.02) (Figure 2B). Unequal N HSD post hoc analysis showed that branching was significantly increased from CON-P44 to CON-P51 (CON-P44: 2.56±0.29; CON-P51: 6.00±1.20; p=0.03); the number of branches more than doubled during these seven days of adolescence. CON-P55 cells (3.70±0.33) had fewer branches than CON-P51 (6.00±1.20), but the difference did not reach significance (p=0.10).
The effect of age in the ABA groups was strikingly different from that of controls. We assessed the effect of age on ABA treatment by comparing cells from animals that had undergone ABA (P44), recovery (P51), and relapse (P55). One-way ANOVA showed a significant effect of age on SR branching in ABA for the 3 age groups (F(2, 18)=11.783; p=0.0005), but unequal N HSD post hoc test showed that the increase in branching seen in CON from P44 to P51 did not occur after ABA. In fact, there was no difference in branching from P44 to P51 (P44-ABA: 3.89±0.37; P51-REC: 4.75±0.50), while cells at P55 (2.15±0.25) had significantly fewer branch points than at P44 (p=0.01) and P51 (p=0.0008) (Figure 2C).
4. Dendritic spine analysis
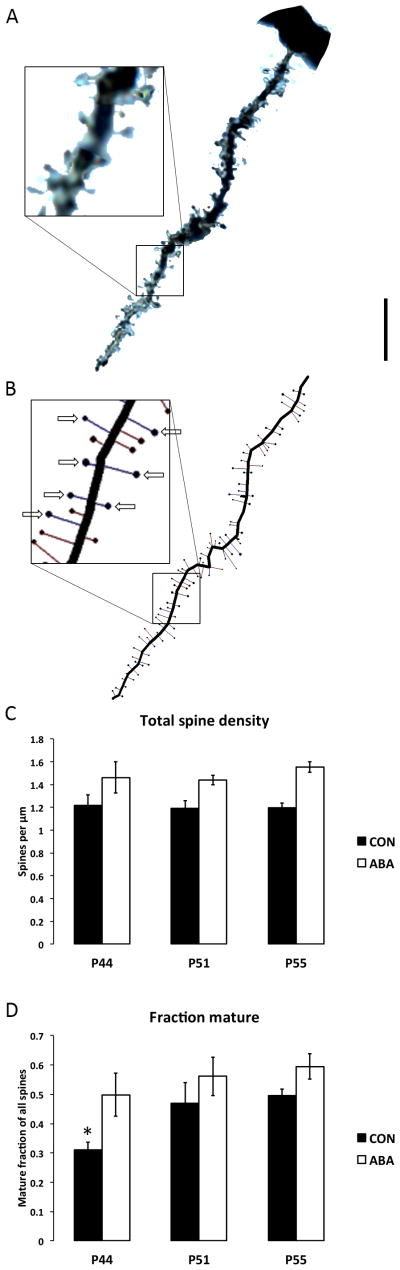
Spine density analysis was conducted at the three time-points of P44, P51, and P55 to study the effects of age on overall spine density as well as the relative densities of immature thin spines and mature spines (mushroom and stubby spines). Two-way ANOVA indicated that there was no difference in the total spine density across ages and between CON and ABA (F(2,16)=0.17, p=0.84). There was a main effect of ABA having greater total spine density than CON when all ages were combined (F(1,16)=9.82, p=0.006).
Dendritic spines were categorized based on their morphological characteristics into three groups: thin (immature), stubby, and mushroom spines. The relative proportion of mature spines was calculated for each dendrite as the ratio of the density of mushroom and stubby spines to the total spine density (Figure 3D). One-way ANOVA of the proportion of mature spines on CON dendrites indicated that mature spines became more abundant with age (P44-CON: 0.31±0.02, N=3; P51-CON: 0.47±0.07, N=4; P55-CON: 0.50±0.02, N=5; F(2,9)-4.4473, p=0.045). Fisher’s LSD post hoc test showed that the proportion of mature spines at P44 was significantly lower than at P51 (p=0.04) and P55 (p=0.02). There was not a significant change in the fraction of mature spines with age in the P44-ABA, P51-REC, and P55-RLPanimals. The average fraction of mature spines at P44, after ABA, was similar to that of the P55 CONs (P44-ABA: 0.50±0.07, N=4; P51-REC: 0.56±0.07, N=3; P55-RLP: 0.60±0.04, N=3).
Figure 3.
DISCUSSION
Development of CA1 pyramidal cells in the ventral hippocampus during adolescence for female rats
In this study, we show that the post-pubertal female hippocampal CA1 pyramidal cells exhibit a transient doubling of apical dendritic branching around P51. This is a previously unreported aspect of normal hippocampal development in the absence of any environmental manipulations. We found that this growth spurt is followed by a decrease in dendritic branching, which is likely the result of pruning branch points as a part of the maturation process. This result is consistent with that of Andersen and Teicher who suggested that a transient overproduction of synapses is a feature of normal adolescent development of the hippocampus (Andersen and Teicher, 2004). The previous study had shown that axonal innervation of CA1 transiently increases in density, and our results confirm that the postsynaptic mechanism for increasing synaptic density is by increasing the number of dendritic branches per cell while maintaining a constant density of spinous protrusions on those dendrites. Our results also indicate that while spine density does not change as the animals approach adulthood, the proportion of mature spines continues to increase. This suggests that after the period of synapse overproduction, the pruning of spines selectively eliminates immature spines while retaining the stronger, more mature, spines. Our analysis was confined to the SR layer of the ventral hippocampus, where CA1 pyramidal cell dendrites receive inputs via the Schaffer collateral pathway from CA3 pyramidal cells (Amaral and Witter, 1989); thus, our results suggest that adolescence is a developmental period responsible for refining at least the ventral CA3-to-CA1 pathway.
Development of the CA1 pyramidal neurons of the ventral hippocampus of adolescent female rats is altered by ABA
At P44, soon after the first experience of ABA, CA1 pyramidal cells of the ventral hippocampus show hypertrophy in ABA animals relative to naïve controls. We had previously hypothesized that the experiences of exercise and FR may cause increased BDNF expression in the ventral hippocampus, inducing the growth of dendritic branches (Gelegen et al., 2008; Gomez-Pinilla et al., 1997; Lee et al., 2002; Neeper et al., 1996; Stranahan et al., 2007; Stranahan et al., 2009). The current results, showing that CON cells exhibit a dramatic proliferation of dendrites, suggest that the hypertrophy in ABA-P44 cells is analogous to the increase in CON from P44 to P51, but occurring at an earlier time-point (from P40 to P44). We additionally showed that after re-exposure to ABA induction from P51 to P55 (RLP-P55), at P55, these cells have decreased branching relative to CON-P55. Relative to the first ABA, the induction of a second ABA at P51 had an effect that was the opposite of that seen at a younger age. We suggest that the reduced dendritic branching in RLP-P55 cells may be the effect of precocious dendritic pruning in animals that have experienced ABA. That is, that the pruning of branches following the transient over production seen at P51 for CON also occurs sooner (by P44) in the animals that experienced ABA. By P55, both ABA and CON dendritic arbors are approaching the average number of branches observed in adult animals. The composition of dendritic spines after ABA at age P44 was similar to the P55CON animals, having a higher proportion of mature spines than age-matched CONs. Again, as was seen for the dendritic branching pattern, the spine morphology results suggest precocious maturation induced by the ABA environment.
The transient increase in dendritic branching with no change in total spine density indicates a net increase in the total number of excitatory synapses onto the pyramidal cells. The increase in the proportion of mature spines suggests that the individual excitatory synapses would be stronger and more persistent (Ziv and Smith, 1996). However, it is not possible to speculate on how the excitability of these cells changes during adolescence without measuring the number, size, and strength of inhibitory synapses as well as the contribution of tonic inhibition via non-synaptic GABA receptors, which increases, at least in the dorsal CA1, from P51 to P55 (Wang, Chowdhury, Barbarich-Marsteller and Aoki, unpublished observations).
We propose that the morphological changes to theCA1 pyramidal cells in response to ABA are the result of an acceleration of the normal developmental course of the hippocampus. The evidence supporting this theory includes events that we see happening at an earlier age in animals that experienced ABA: (1) an increase in branching of CA1 pyramidal cell apical dendrites (overproduction of synapses), (2) a subsequent decrease in branching (pruning), and (3) an increase in the proportion of mature dendritic spines.
Importantly, our results suggest that once this post-pubertal period of developmental growth and pruning of synapses is complete, CA1 pyramidal cells of the ventral hippocampus are no longer vulnerable to the large-scale dendritic changes induced by pubertal ABA. When ABA is induced at P60, no change in the dendritic structure of CA1 cells was found and FR-evoked hyperactivity was much less than during adolescence, despite the extended period of time in the ABA environment, consisting of seven days of food restriction. This approach was taken because adult ABA animals develop more severe levels of running and weight loss when maintained in a more chronic environment (e.g., at least 7–10 days; Barbarich-Marsteller, unpublished data). Thus, we suggest that (1) adolescence is a period of robust development of the CA1 hippocampus, (2) puberty opens a critical period for extensive, experience-dependent modifications in the dendritic structure of theCA1 hippocampus; and that (3) ABA induction, consisting of food restriction and excessive exercise, accelerates the closure of the critical period.
Acknowledgments
Grant Sponsor: National Institutes for Health; Grant Number: UL1TR000038
Grant Sponsor: National Institutes for Health; Grant Number: R21MH091445-01
Grant Sponsor: National Institutes for Health; Grant Number: R01NS066019-01A1
Grant Sponsor: National Institutes for Health; Grant Number: R01NS047557-07A1
Grant Sponsor: National Institutes for Health; Grant Number: EY13079
Grant Sponsor: National Institutes for Health; Grant Number: R25GM097634-01
Grant Sponsor: National Institutes for Health; Grant Number: 1R25GM097634-01
The authors would like to thank Kevin Laurino for his technical assistance and GauriWable, Nicole Sabaliauskas, and Yi-Wen Chen for helpful discussions. This work was supported by the following grants: The Klarman Foundation Grant Program in Eating Disorders Research to C. A. and N. B. M.; National Institutes for Health Grants R21MH091445-01 to C. A. and N. B. M.; R01NS066019-01A1 to C. A.; R01NS047557-07A1 to C. A.; N.E.I. Core Grant EY13079 to C. A.; R25GM097634-01 to C. A.; NYU CTSA grant UL1TR000038 from the National Center for Advancing Translational Sciences (NCATS), NIH to T. G. C.; and NYU’s Research Challenge Fund to C.A.; BP-ENDURE 1R25GM097634-01 to M. R. and C. A.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116(5):884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626(1):49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63(3):305–12. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- Chowdhury TG, Barbarich-Marsteller NC, Chan TE, Aoki C. Activity-based anorexia has differential effects on apical dendritic branching in dorsal and ventral hippocampal CA1. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19(6):395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelegen C, van den Heuvel J, Collier DA, Campbell IC, Oppelaar H, Hessel E, Kas MJ. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008;7(5):552–9. doi: 10.1111/j.1601-183X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764(1–2):1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann AS, Greenberg JL, Wilhelm S. The relationship between anorexia nervosa and body dysmorphic disorder. Clin Psychol Rev. 2013;33(5):675–85. doi: 10.1016/j.cpr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215–21. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Keizer A, Smeets MA, Dijkerman HC, Uzunbajakau SA, van Elburg A, Postma A. Too fat to fit through the door: first evidence for disturbed body-scaled action in anorexia nervosa during locomotion. PLoS One. 2013;8(5):e64602. doi: 10.1371/journal.pone.0064602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9(1):67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Milner TA. Dendritic ribosomes suggest local protein synthesis during estrous synaptogenesis. Neuroreport. 2003;14(10):1357–60. doi: 10.1097/01.wnr.0000078380.40088.99. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and the aging hippocampus. Front Neuroendocrinol. 1999;20(1):49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Fillenz M, Lowry JP, Rawlins JN, Bannerman DM. Brain tissue oxygen amperometry in behaving rats demonstrates functional dissociation of dorsal and ventral hippocampus during spatial processing and anxiety. Eur J Neurosci. 2011;33(2):322–37. doi: 10.1111/j.1460-9568.2010.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- Pike KM. Long-term course of anorexia nervosa: response, relapse, remission, and recovery. Clin Psychol Rev. 1998;18(4):447–75. doi: 10.1016/s0272-7358(98)00014-2. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extra synaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10(4):469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–61. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531(1–2):225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12(7):2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A, Leehey K, Shisslak CM. Running--an analogue of anorexia? N Engl J Med. 1983;308(5):251–5. doi: 10.1056/NEJM198302033080504. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Mapp OM, Janssen WG, Yin W, Morrison JH, Gore AC. Postpubertal decrease in hippocampal dendritic spines of female rats. Exp Neurol. 2008;210(2):339–48. doi: 10.1016/j.expneurol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17(1):91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]