Abstract
Acute liver failure (ALF) is a fatal syndrome associated with massive hepatocyte death. There is no cure for ALF except liver transplantation. Protein farnesylation is a lipid modification of cysteine residues that is catalyzed by farnesyltransferase (FTase) and has been proposed as an integral component of acute inflammation. Previously, we have demonstrated that FTase inhibitors improve survival in mouse models of endotoxemia and sepsis. Here we studied the effects of FTase inhibitor, tipifarnib, on galactosamine (GalN) /lipopolysaccharide (LPS)-induced acute liver failure. The effects of tipifarnib (10 mg/kg, IP) were studied in GalN (400 mg/kg, IP) and LPS (3 μg/kg)-challenged mice by histological and biochemical analyses.
GalN/LPS administration caused prominent liver injury characterized by the increased plasma alanine aminotransferase (ALT) and aspartic aminotransferase (AST) levels leading to significant mortality in mice. Tipifarnib inhibited GalN/LPS-induced caspase 3 activation, inflammatory cytokine production, and c-Jun N-terminal Kinase (JNK) phosphorylation in the liver. On the other hand, Tipifarnib upregulated anti-apoptotic protein, Bcl-xL, in the liver after GalN/LPS challenge. Tipifarnib also protected primary hepatocytes from GalN/tumor necrosis factor-α (TNF-α)-induced cell death by inhibiting caspase 3 activation and upregulating anti-apoptotic proteins. GalN/LPS-induced liver injury was associated with increased protein farnesylation in the liver. Tipifarnib prevented protein farnesylation in the liver and markedly attenuated liver injury and mortality in GalN/LPS-challenged mice. These results suggest that protein farnesylation is a novel potential molecular target to prevent hepatocyte death and acute inflammatory liver failure in fulminant hepatitis.
Introduction
Acute liver failure (ALF) is a fatal syndrome attributed to massive hepatocyte death. Although a variety of insults including viral infection and drugs can cause ALF, the resulting clinical picture is remarkably similar across the different etiologies, reflecting common patterns of response of the innate immune system and the resulting inflammation in the liver. Management of severe ALF continues to be one of the most challenging problems in clinical medicine. Liver transplantation has been shown to be the most effective therapy, but the procedure is limited by shortage of donor organs combined with the disadvantage of required immunosuppressant treatment (1, 2). New preventive and/or therapeutic strategies need to be developed to improve the clinical outcome of patients with ALF.
Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. However, statins have been shown to exert pleiotropic beneficial effects independent of their cholesterol-lowering effects. For example, statins have been shown to prevent septic shock in animal models (3). Statins reduce the biosynthesis of not only cholesterol but also farnesyl pyrophosphate, which is the precursor of cholesterol as well as the substrate of protein farnesylation. Protein farnesylation is a lipid modification of cysteine residues in the CAAX motif located in the carboxyl terminus of proteins (where C is cysteine, A is aliphatic amino acid, and X is any amino acid, but usually serine, methionine, glutamine, or alanine). Farnesyltransferase (FTase) is an enzyme that catalyzes the covalent attachment of a farnesyl group, from farnesyl pyrophosphate to the cysteine thiols in the C-terminal CAXX consensus sequences. In many proteins, farnesylation serves as a critical regulatory mechanism of protein function, such as maturation, activation, protein-protein interaction, and membrane localization. Proteins that are known to be affected by farnesylation include the Ras family small G-proteins, lamin A, the nuclear protein, and the CENP, the centrometric protein (4).
We have previously shown that FTase inhibitors reduced mortality after endotoxin shock or polymicrobial sepsis induced by cecum ligation and puncture in mice (4, 5). Of note, FTase inhibitors prevented LPS-induced caspase 3 cleavage and activation of JNK in the mouse liver (5). These observations indicate that protein farnesylation plays a role in LPS-induced liver damage. Nonetheless, a role of protein farnesylation in ALF has not yet been studied. The safety and tolerability of the FTase inhibitors, including tipifarnib, have been confirmed in the clinical studies (6), while the mixed results were reported about the effectiveness of the FTase inhibitors in cancer and hematologic malignancies (7).
To elucidate the role of protein farnesylation in ALF, we examined the effects of tipifarnib, a FTase inhibitor, in a mouse model of ALF induced by GalN/lipopolysaccharide (LPS). GalN sensitizes the liver towards other stimuli in part reflecting the role of uridine containing compounds in hepatic biotransformation. Co-administration of LPS and GalN causes hepatocyte apoptosis, leading to ALF (8, 9). Here, we show that tipifarnib, a FTase inhibitor, prevented apoptotic hepatocyte death and reduced mortality in mice subjected to GalN/LPS-induced ALF.
Materials and Methods
Animals and acute liver failure model
After approval by the Massachusetts general Hospital Subcommittee on Research Animal Care, all animal experiments were performed in accordance with the guidelines of the National Institutes of Health. Male C57BL/6J mice at 8-9 weeks of age purchased from the Jackson Laboratory (Bar Harbor, ME) were used in this study. These mice were given access to food and water ad libitum in our animal facility until the time of experiments. To induce acute liver failure, mice were challenged with D-Galactosamine (GalN, 400mg/kg, Sigma-Aldrich, St. Louis, MO) and LPS (3 μg/kg, Escherichia coli, Sigma) intraperitoneally. Tipifarnib (10 mg/kg, Selleckchem, Houston, TX) or saline (vehicle) was administered intraperitoneally at 60 minutes before the GalN/LPS challenge in mice.
Survival analysis after GalN/LPS challenge
Survival after challenge with GalN/LPS was studied in 8-9 week old male mice. To avoid dehydration, normal saline (1 ml) was given intraperitoneally to all mice at times 0, 6, and 24 h after the GalN/LPS challenge. We determined the dose of GalN/LPS based on our preliminary experiments in which 40% of C57BL/6J mice died. Mice were kept in a room in which ambient temperature was controlled at 25°C.
Plasma liver enzyme
Blood was drawn from IVC and centrifuged to collect the plasma. Plasma alanine aminotransferase (ALT) and aspartic aminotransferase (AST) levels, as markers of hepatic damage, at baseline and 5 h after GalN/LPS injections were measured using an Infinity™ ALT and AST assay regent (Thermo Fisher Scientific Inc., Middletown, VA) as previously reported (10).
Histology and Immunohistochemistry
Liver tissues were dissected and fixed in 4% paraformaldehyde and embedded in paraffin. Five μm sections were stained with hematoxylin and eosin (H&E) using a standard protocol, and then analyzed with a microscopy (Nikon ECLIPSE-TE2000). Images were scored by an investigator blinded as to the identity of the samples. Sinusoidal congestion, cytoplasmic vacuolization, and parenchymal necrosis were scored according to the criteria described by Suzuki and colleagues (11, 12). Parraffin-embedded sections were also stained with polyclonal antibodies for farnesylated proteins (AB4073; Millipore) and visualized using diaminobenzidine substrate kit for peroxidase (Vector Laboratories). Staining with normal IgG in place of anti-farnesylated proteins antibody served as a negative control. The sections were counter stained by Methyl Green solution (Sigma-Aldrich).
TNF-α and IL-6 secretion by primary peritoneal macrophages
Peritoneal macrophages were prepared from un-stimulated naïve mice, as previously described (10). The cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100U/ml penicillin and streptomycin, and 2 mM glutamine. After overnight incubation, the macrophages were treated with LPS (0.5 μg/ml), and TNF-α and IL-6 levels in the medium were measured by ELISA kit (R&D system, Minneapolis, MN) according to the manufacturer’s instructions. TNF-α and IL-6 levels were compared between macrophages treated with and without tipifarnib (100 nM or 1 μM). We measured TNF-α and IL-6 after 6-h incubation with LPS since both cytokine production reached a plateau after 6 h in macrophages (10). Tipifarnib was added at 30 min before the LPS challenge.
Hepatocyte isolation and evaluation of cell viability
Primary hepatocytes were isolated from naïve mice using a modified two-step collagenase perfusion method (13). Briefly, after the cannulation into the portal vein, liver was perfused with Krebs-Ringer bicarbonate (KRB) solution containing EDTA (1.7 mM), and followed by perfusion with KRB solution containing collagenase (Worthington) and CaCl2 (7.4 mM). Isolated cells were filtered through 100 and 70 μm nylon cell strainer (BD Falcon). Hepatocytes were purified by centrifugation at 600 rpm for 10 min in 50% Percoll (GE Healthcare Bioscience) solution. The Trypan Blue method was applied to confirm cell viability (>85%). The viable primary hepatocytes were suspended in High Glucose DMEM with 10% heat-inactivated FBS. To measure hepatocyte viability after GalN (5mM)/TNF-α (10 ng/ml) challenge, we used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and Crystal Violet (CV) assay, as described previously (14). Tipifarnib was added to the cell culture at 30 min before GalN/TNF-α challenge.
Measurements of gene expression
Total RNA was extracted from the liver of mice 5 h after saline or GalN/LPS challenge using the RNAspin Mini kit (GE Healthcare, Piscataway, NJ) and cDNA was synthesized using MMLV-RT (Promega, Madison, WI). TNF-α, interleukin (IL)-6, IL-1β, IL-10 and 18S ribosomal RNA transcript levels were measured by real-time PCR using a Realplex 2 system (Eppendorf North America, Westbury, NY) as previously described (15, 16). mRNA levels were normalized to that of 18S rRNA.
Western blotting
Liver tissue was dissected and frozen 5 h after saline or GalN/LPS injections. Liver homogenates were prepared as previously described (17). Protein levels in liver homogenates were determined using standard immunoblot techniques using primary antibodies (1:10,000, Cell Signaling Technology Inc., Danvers, MA, unless otherwise noted) against phosphorylated JNK at threonine183 (Thr183) and tyrosine185 (Tyr185) (1:1,000, Cell Signaling Technology Inc), JNK (1:5,000, Cell Signaling Technology Inc), Bcl-2 (1:1,000, Cell Signaling Technology Inc), BclxL (1:1,000, Cell Signaling Technology Inc), caspase 3, cleaved PARP, GAPDH and Vinculin. Bound antibody was detected with a horseradish peroxidase-linked antibody directed against rabbit IgG (1:10,000, Cell Signaling Technology Inc.) or mouse IgG (1: 5,000, GE healthcare) and was visualized using chemiluminescence with ECL Advance kit (GE healthcare).
Measurement of histone H3 and cyclophilin A in plasma
Protein levels in plasma were determined using standard immunoblot techniques using primary antibodies (1:10,000, Cell Signaling Technology Inc., Danvers, MA) against histone H3 and cyclophilin A. The other methods are same as mentioned above in western blotting.
Measurement of High-Mobility Group Protein Box 1 (HMGB1)
Concentrations of HMGB1 in the plasma and the culture medium were measured by ELISA kit (IBL International, Toronto, ON, Canada) according to the manufacturer’s instructions.
Statistics
All data are presented as means±SD. Data were analyzed by ANOVA using Prism 5 software package (GraphPad Software, La Jolla, CA). Newman-Keuls multiple comparison post hoc test or Bonferroni post hoc test were performed for One-way ANOVA or Two-way ANOVA, respectively. Kaplan-Meier survival analysis was performed with Log-rank test. P values smaller than 0.05 were considered significant.
Results
Tipifarnib improved survival rate in mice after GalN/LPS challenge
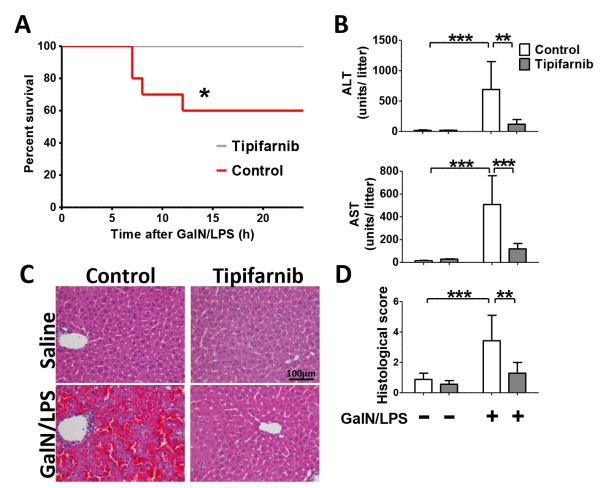
Four out of ten mice died within 24 h after challenge with GalN (400 mg/kg) and LPS (3 μg/kg) when treated with vehicle alone. In contrast, when treated with tipifarnib, no mice died after GalN/LPS challenge (Fig. 1A, p=0.0289 by log-rank test, n=10 each group). No mice of either group died thereafter up to 48 h after GalN/LPS challenge.
Fig. 1. Effects of tipifarnib on GalN/LPS-induced mortality and ALF in mice.
(A) Kaplan-Meier survival curve of mice challenged with GalN/LPS with or without tipifarnib. *p=0.0289 vs. control, n=10 per group. (B) White and grey columns depict control (vehicle-treated) and tipifarnib-treated groups, respectively; **p<0.01, ***p<0.001, n=3 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice. All were measured at 5h after saline or GalN/LPS challenge. (C) Representative liver histology (H/E staining at 5h after saline or GalN/LPS) by microscopic images are shown. (D) Histology score with or without GalN/LPS challenge in control or Tipifarnib group. N=3 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice; **p<0.01, ***p<0.001.
Tipifarnib attenuated liver injury after GalN/LPS challenge
Plasma alanine aminotransferase (ALT) and aspartic aminotransferase (AST) levels at 5 h after GalN/LPS challenge were measured as markers of liver injury. GalN/LPS challenge markedly increased ALT and AST levels in vehicle-treated mice. Treatment with tipifarnib prevented GalN/LPS-induced increase of plasma ALT and AST levels (Fig. 1B). We found that hepatic architecture in vehicle-treated mice was disrupted with hemorrhage, necrosis and neutrophil infiltration at 5 h after GalN/LPS challenge. In contrast, tipifarnib treatment markedly ameliorated liver injury as reflected by histological alterations after GalN/LPS challenge (Fig. 1, C and D).
Tipifarnib inhibited GalN/LPS-induced hepatic inflammation and circulating damage-associated molecular patterns (DAMPs)
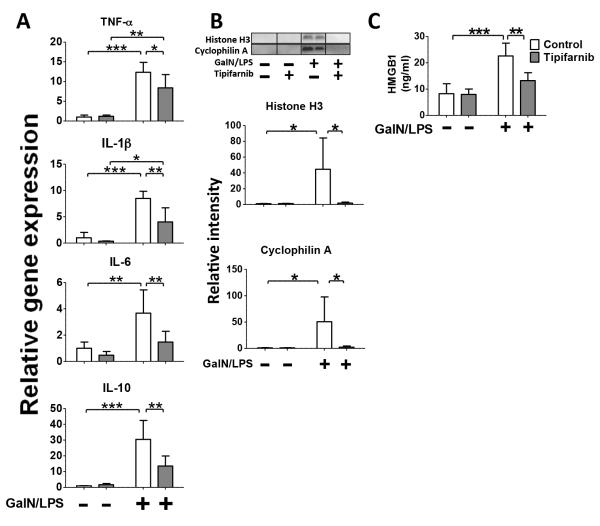
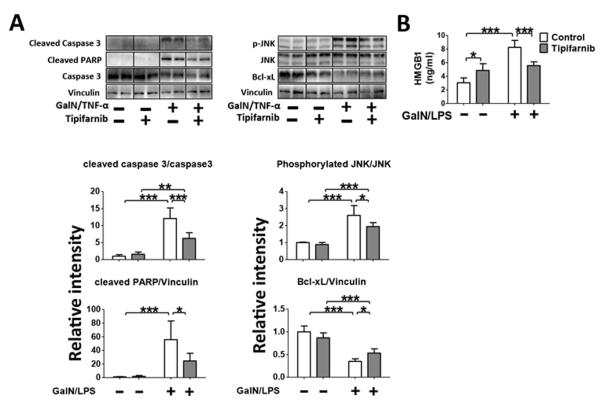
Levels of TNF-α, interleukin (IL)-6, IL-1β, and IL-10 mRNA in the liver were increased at 5 h after GalN/LPS challenge in vehicle-treated GalN/LPS-challenged mice. Tipifarnib significantly attenuated GalN/LPS-induced up-regulation of inflammatory cytokines (Fig. 2A). Next, we evaluated circulating levels of histone H3, a chromatin protein in the nucleus, cyclophilin A, a cytosolic peptidly-prolyl cis-trans isomerase, and HMBG1, the intranuclear nonhistone DNA binding protein, all of which are major DAMPs and biomarkers of cytotoxicity in ALF (18, 19). GalN/LPS challenge markedly increased plasma histone H3 and cyclophilin A levels in vehicle-treated mice, while histone H3 and cyclophilin A were undetectable in plasma of GalN/LPS-naïve mice. Tipifarnib markedly prevented GalN/LPS-induced increases in histone H3 and cyclophilin A (Fig. 2B). Plasma HMBG1 was also significantly increased after GalN/LPS challenge and tipifarnib attenuated the GalN/LPS-induced increase of HMBG1 (Fig. 2C). These results suggest that GalN/LPS-induced systemic inflammatory response as well as hepatotoxicity may be prevented by tipifarnib.
Fig. 2. Effects of tipifarnib on GalN/LPS-induced cytokine expression in the liver and circulating histone H3, cyclophilin A, and HMBG1.
(A) mRNA expression levels of inflammatory cytokines in the liver were measured at 5 h after GalN/LPS challenge or saline injection in mice treated with (grey column) or without (white column, control group) tipifarnib. *p<0.05, **p<0.01, ***p<0.001, n=3 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice. (B) Plasma levels of histon H3 and cyclophilin A, as evaluated by immunoblotting at 5 h after GalN/LPS or saline injection. *p<0.05, n=3 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice. (C) Plasma levels of HMGB1 at 5 h after GalN/LPS-challenge without or with tipifarnib. **p<0.01, ***p<0.001, n=4 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice.
Tipifarnib prevented GalN/LPS-induced apoptotic change in the liver
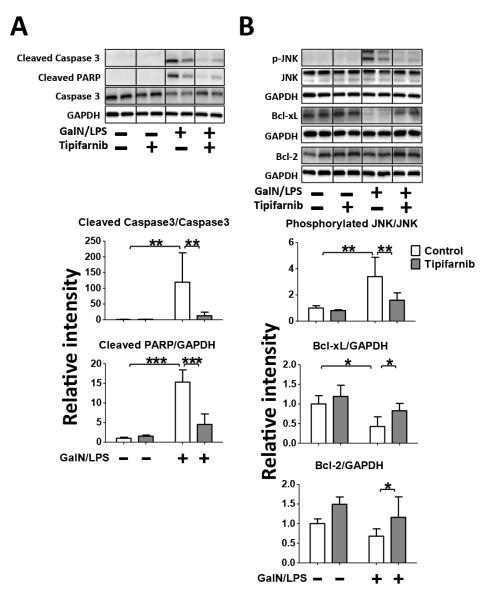
To study the effects of tipifarnib in GalN/LPS-induced apoptotic change, we evaluated cleavage of caspase-3 and PARP in the liver. Consistent with previous studies (10), GalN/LPS induced cleavage of caspase-3 and PARP in vehicle-treated mice. Tipifarnib markedly prevented GalN/LPS-induced cleavage of caspase-3 and PARP (Fig. 3A).
Fig. 3. Effects of Tipifarnib on GalN/LPS-induced apoptotic change in the liver.
Representative immunoblots and densitometric analyses of (A) cleaved caspase 3, cleaved PARP, (B) phosphorylated JNK at Thr183 and Tyr185, Bcl-xL and Bcl-2 in the liver at 5h after saline or GalN/LPS challenge with or without tipifarnib. Relative intensity was normalized to uncleaved caspase 3 (for cleaved caspase 3), JNK (for phosphorylated JNK) and GAPDH (for cleaved PARP, Bcl-2, Bcl-xL) expression levels, respectively, and mean value for control group challenged with saline was set to 1. N=3 per group for GalN/LPS-naïve mice, n=6 per group for GalN/LPS-challenged mice; *p<0.05, **p<0.01, ***p<0.001.
To further investigate the anti-apoptotic effects of tipifarnib, we examined phosphorylation (activation) status of JNK and protein expression of Bcl-xL and Bcl-2 in the liver. GalN/LPS increased JNK phosphorylation, which was inhibited by tipifarnib. GalN/LPS significantly suppressed the protein expression of Bcl-xL, which was prevented by tipifarnib. Tipifarnib significantly increased the protein expression of Bcl-2 in GalN/LPS-challenged mice. In GalN/LPS-naïve mice, there was a trend of increased Bcl-2 expression by tipifarnib, but there was no statistical difference (Fig. 3B). Total JNK and GAPDH protein expression were not affected by GalN/LPS or tipifarnib. These results indicate that the protective effects of tipifarnib were associated with inhibition of JNK activation and up-regulation of Bcl-xL and Bcl protein expression in the liver.
Tipifarnib did not prevent TNF-α and IL-6 secretion from LPS-challenged primary peritoneal macrophage in vitro
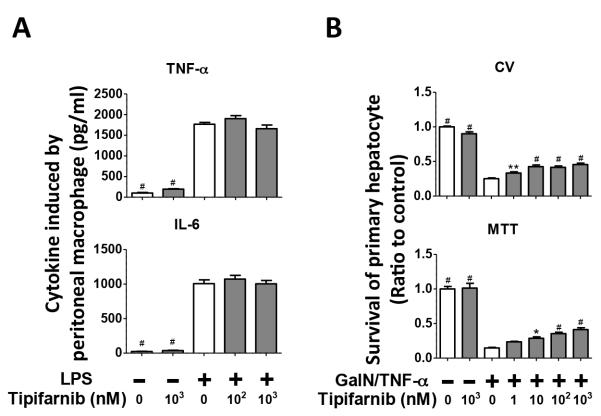
Since GalN/LPS-induced hepatotoxicity is mediated, at least in part, by LPS-induced TNF-α production in macrophages (20), we examined the impact of tipifarnib on TNF-α and IL-6 secretion in primary murine peritoneal macrophages. In our recent study, the concentrations of TNF-α and IL-6 in the culture media of LPS-stimulated murine primary peritoneal macrophages increased in a time-dependent manner, reaching the plateau levels at 6 h (10). We, therefore, measured cytokine levels after 6-h incubation with LPS. Unexpectedly, tipifarnib (100 nM or 1 μM) did not alter LPS-stimulated secretion of TNF-α or IL-6 by primary peritoneal macrophages (Fig. 4A). Tipifarnib did not alter TNF-α or IL-6 secretion from GalN/LPS-naïve macrophages, either. These results raise the possibility that the protective effects of tipifarnib against GalN/LPS-induced ALF may not be attributable to direct inhibition of pro-inflammatory cytokine production by macrophages.
Fig. 4. Effects of in vitro treatment with tipifarnib on cytokine production in primary peritoneal macrophage and cell viability in primary hepatocytes after GalN/ TNF-α incubation.
(A) TNF-α and IL-6 levels secretion by cultured primary mouse peritoneal macrophage at 6h after incubation with saline or LPS with or without tipifarnib. N=7-8 in each group; #p<0.001 vs. group incubated with LPS alone without tipifarnib. (B) Survival of primary hepatocytes assessed by cristal violet (CV) method and MTT assays at 20h after incubation with GalN/TNF-α with or without tipifarnib. Mean value for group incubated with saline and without tipifarnib was set to 1. N=6-7 in each group; *p<0.05, **p<0.01, #p<0.001 vs. group incubated with GalN/TNF-α alone without tipifarnib.
Tipifarnib improved survival of primary culture of hepatocytes after GalN/ TNF-α challenge in vitro
Since tipifarnib did not affect LPS-induced TNF-α secretion by peritoneal macrophages, we examined whether tipifarnib protects hepatocytes from GalN/TNF-α-induced cell death. Tipifarnib significantly improved cell viability of primary hepatocytes at 20 h after GalN (5 mM)/TFN-α (10 ng/ml) challenge in a dose-dependent manner, as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and crystal violet (CV) assays (Fig. 4B). The protective effects of tipifarnib were observed starting at 1 or 10 nM in cultured hepatocytes. Tipifarnib did not affect cell viability of GalN/LPS-naïve hepatocytes. These observations suggest that tipifarnib can directly protect hepatocytes from GalN/TNF-α-induced cytotoxicity.
Tipifarnib protected from GalN/TNF-α-induced apoptosis and cell death in cultured hepatocytes
GalN/TNF-α induced activation of caspase-3 in cultured hepatocytes, as indicated by cleavage of caspase-3 and PARP. Tipifarnib (1 μM) significantly inhibited GalN/TNF-α-induced caspase-3 activation. Likewise, GalN/TNF-α increased phosphorylation (activation) of JNK, which was significantly inhibited by tipifarnib. GalN/TNF-α decreased Bcl-xL expression, which was significantly ameliorated by tipifarnib (Fig. 5A). These results indicate that the protective effects of tipifarnib in cultured hepatocyte are associated with inhibition of caspase-3 and activation of JNK and downregulation of anti-apoptotic molecule, Bcl-xL in GalN/TNF-α-challenged hepatocytes. Moreover, GalN/TNF-α-induced increase of HMGB1 in cultured hepatocytes was significantly attenuated by tipifarnib (Fig. 5B). These findings are consistent with the in vivo observation in GalN/LPS-challenged mice (Fig. 2 and 3).
Fig. 5. Effects of in vitro treatment with tipifarnib on GalN/ TNF-α-induced apoptotic changes and HMBG1 in primary hepatocytes.
(A) Representative immunoblots and densitometric analyses of cleaved caspase 3, cleaved PARP, phosphorylated JNK at Thr183 and Tyr185and Bcl-xL in the hepatocytes at 20h after incubation with saline or GalN/ TNF-α with or without tipifarnib. Relative intensity was normalized to uncleaved caspase 3 (for cleaved caspase 3), JNK (for phosphorylated JNK) and Vinculin (for cleaved PARP, Bcl-xL) expression levels, respectively, and mean value for control mice challenged with saline was set to 1. N=4-5 in each group; *p<0.05, **p<0.01, ***p<0.001. (B) HMGB1 levels in culture medium of primary hepatocytes assessd by ELISA. N=4 per group for GalN/LPS-naïve mice, n=5 per group for GalN/LPS-challenged mice; *p<0.05, ***p<0.001.
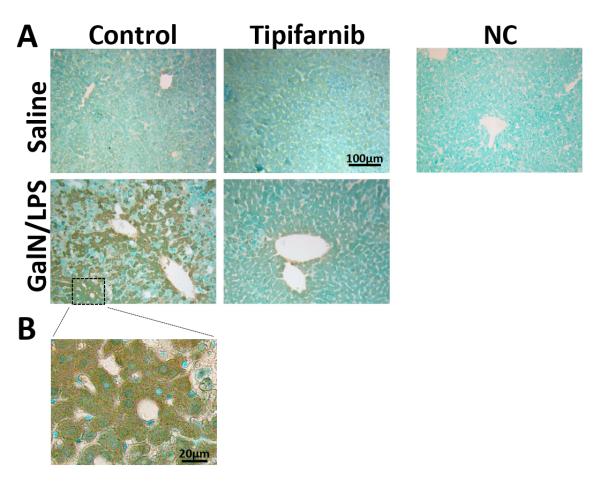
Tipifarnib inhibited GalN/LPS-induced increase in farnesylated proteins in mouse liver
Farnesylated proteins were increased in the liver at 5 h after GalN/LPS challenge, as judged by immunohistochemistry. Elevated protein farnesylation in the liver was attenuated by tipifarnib (Fig. 6A). On the other hand, if any, there was little effect of tipifarnib in farnesylated proteins in the liver of GalN/LPS-naïve mice. Increased farnesylated proteins were observed primarily in hepatocytes of GalN/LPS-challenged mice (Fig. 6B).
Fig. 6. Effects of GalN/LPS and tipifarnib on farnesylated proteins in the liver.
Representative liver histology and amounts of farnesylated proteins. farnesylated proteins in the liver were evaluated by immunohistochemical analysis. Representative microscopic images are shown and fig. 6B is magnified from control mice with GalN/LPS. NC, negative control.
Discussion
In the current study, we demonstrated that a FTase inhibitor tipifarnib prevented GalN/LPS-induced ALF as indicated by lower mortality, attenuated increase of serum ALT and AST, better histological score, and inhibited apoptotic change in the liver. GalN/LPS-induced increase of protein farnesylation in the liver was markedly attenuated by tipifarnib. The protective effects of tipifarnib were associated with attenuated inflammation, inhibition of JNK activation and upregulation of anti-apoptotic molecules, Bcl-2 and BCl-xL in the liver. Taken together, these observations suggest important protective effects of FTase inhibition against inflammatory ALF.
Previous study showed that a major target of the protective effects of FTase inhibitor is the immune system in the mouse model of sepsis (4). In a mouse model of GalN/LPS-induced ALF, LPS-induced TNF-α secretion by macrophages has been considered as an important contributor to cellular demise of hepatocytes (21). We, therefore, examined the effects of tipifarnib on LPS-induced TNF-α secretion in cultured macrophages. However, tipifarnib failed to decrease TNF-α secretion from macrophages (Fig. 4A). In contrast, tipifarnib improved viability of primary hepatocytes incubated with GalN/TNF-α (Fig. 4B). We also observed that GalN/LPS increased the expression of farnesylated proteins primarily in the hepatocytes while the protein farnesylation was markedly inhibited by tipifarnib (Fig. 6). It was reported that the farnesylated proteins induce JNK/SAPK activation, and apoptotic changes (5). These findings raise the possibility that tibifarnib prevented GalN/LPS-induced ALF in mice by attenuating GalN/TNF-α-induced hepatocytes death, but not by inhibiting the LPS-induced TNF-α production by macrophages.
Of note, tipifarnib markedly prevented the GalN/LPS-induced increase in circulating histone H3 and cyclophilin A. When these intracellular proteins are released from dead cells into the circulation, they may cause and/or exacerbate inflammation by functioning as damage-associated molecular patterns (DAMPs) in a variety of inflammatory conditions including ALF (22, 23). HMGB1, which belongs to group of DAMPs, is also released to extracellular milieu from necrotic or injured cells (24). Elevated HMGB1 concentrations were attenuated by tipifarnib not only in plasma of GalN/LPS-challenged mice (Fig. 2C) but also in GalN/ TNF-α-induced culture medium of primary hepatocytes (Fig. 5B). This observation is consistent with our finding that tipifarnib markedly prevented GalN/TNF-α-induced hepatocyte cell death, since the release of DAMPs is reduced by the attenuation of hepatic cell death by tipifarnib. A previous study has shown that administration of neutralizing antibody for HMGB1 protects rats from GalN-induced acute liver failure (25). Together, it is conceivable that the suppression of increased circulating levels of HMGB1 as well as histone H3 and cyclophilin A may play a role in the protective effect of tipifarnib in of GalN/LPS-challenged mice.
Although tipifarnib failed to inhibit LPS-induced TNF-α and IL-6 secretion by macrophages in vitro (Fig. 4A), tipifarnib significantly attenuated TNF-α and IL-6 expression in the liver of GalN/LPS-challenged mice (Fig. 2). This apparent discrepancy could be explained, at least in part, by the effects of tipifarnib on circulating DAMPs (i.e., histone H3, cyclophilin A, HMGB1). It is reasonable to speculate that the reduction of amount of circulating DAMPs by tipifarnib may contribute to the attenuated inflammatory gene expression in the liver of tipifarnib-treated GalN/LPS-challenged mice, as compared with vehicle alone. Alternatively, it is also possible that peritoneal macrophages and circulating monocyte-derived macrophages and resident macrophages in the liver (Kupffer cells) react differently to LPS (or GalN/LPS). For example, tipifarnib may inhibit production of pro-inflammatory cytokines from resident macrophages but not from monocyte-derived macrophages. Impact of GalN/LPS with or without tipifarnib on circulating and resident macrophage in the liver remains to be determined in the future studies.
Interestingly, we observed that the area stained as farnesylated proteins are predominantly hepatocytes. GalN/LPS-induced increase of farnesylated proteins were inhibited by tipifarnib in the hepatocytes. Although the current study design does not enable us to define the protein farnesylation as the cause of hepatocyte injury after GalN/LPS challenge, our results suggest a strong association between excessive protein farnesylation and hepatocyte death in the GalN/LPS-induced ALF. On the other hand, it is of note that compared with negative controls, GalN/LPS-naïve healthy livers also express low levels of farnesylated proteins. These findings suggest that protein farnesylation may have physiological roles and that complete inhibition of protein farnesylation may have negative impact on cellular function and viability. It is important to note that unlike in GalN/LPS-challenged mice, we did not find decrease in farnesylated proteins by the tipifarnib treatment in the liver of GalN/LPS-naïve mice. This could be explained, at least in part, by the fact that FTase inhibitor does not alter farnesylation status of proteins that have been already farnresylated prior to FTase inhibitor administration. Limitation--In this study, we did not examine the effects of tipifarnib administered after GalN/LPS. Because GalN/LPS-induced ALF followed a super-acute time course and many mice died within 7h after administration of GalN/LPS. Additionally, the time to peak concentration of tipifarnib in plasma is about 2h and half –time is about 3h (26). Therefore, we thought any delay in treatment would be ineffective in this particular model. The goal of this project is to show a proof-of-concept of the effects of FTase inhibition in the treatment of ALF. To maximize the chance of detecting the effects of FTase inhibition, we chose to administer tipifarnib prior to GalN/LPS challenge.
In this acute liver failure model using GalN/LPS, LPS stimulates immune cells such as liver residential Kupffer cells and peritoneal macrophage, leading to the secretion of inflammatory cytokines such as TNF-α. However it is not clear which immune cell populations have most important role or whether there is critical differences between Kupffer cells and peritoneal macrophage in this model. It was reported that intraperitoneal administration of an agent that depletes macrophages reduced TNF-α production induced by GalN/LPS (27). Therefore, we believed that peritoneal macrophages had an important role in this model. Nevertheless, our study design does not allow us to determine the role of Kupffer cells in this model and we also recognize this as a limitation.
Our results indicate that protein farnesylation may play an important role in the development of ALF in GalN/LPS-challenged mice. These results suggest that inhibition of hepatocyte death and subsequent release of DAMPs may contribute to the protective effects of tipifarnib against GalN/LPS-induced ALF. These findings identify FTase as a novel potential molecular target to prevent and/or treat ALF. Further studies are warranted on the protective effects of FTase inhibitiors in inflammatory liver injury.
Brief Summary.
Farnesyltransferase inhibitor attenuated galactosamine/lipopolysaccharide-induced hepatocyte apoptosis and prevented acute liver failure and death in mice. These findings indicate that protein farnesylation plays an important role in the development of inflammatory liver failure.
Acknowledgments
Financial Support This work was supported by grants from Shriners Hospitals for Children to M. Kaneki and National Institute of Health grants HL101930 to F. Ichinose.
Footnotes
Author Disclosure No competing financial interests exist.
Reference
- 1.Lee WM. Acute liver failure. N Engl J Med. 1993;329(25):1862–72. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 2.Aminlari M, Li A, Kunanithy V, Scaman CH. Rhodanese distribution in porcine (Sus scrofa) tissues. Comp Biochem Physiol B Biochem Mol Biol. 2002;132(2):309–13. doi: 10.1016/s1096-4959(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Luo L, Wang Y, Rahman M, Lepsenyi M, Syk I, Jeppsson B, Thorlacius H. Simvastatin protects against T cell immune dysfunction in abdominal sepsis. Shock. 2012;38(5):524–31. doi: 10.1097/SHK.0b013e31826fb073. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Yamada M, Tamura Y, Chang K, Mao J, Zou L, Feng Y, Kida K, Scherrer-Crosbie M, Chao W, Ichinose F, Yu YM, Fischman AJ, Tompkins RG, Yao S, Kaneki M. Farnesyltransferase inhibitor FTI-277 reduces mortality of septic mice along with improved bacterial clearance. The Journal of pharmacology and experimental therapeutics. 2011;339(3):832–41. doi: 10.1124/jpet.111.183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinozaki S, Inoue Y, Yang W, Fukaya M, Carter EA, Yu YM, Fischman A, Tompkins R, Kaneki M. Farnesyltransferase inhibitor improved survival following endotoxin challenge in mice. Biochemical and biophysical research communications. 2010;391(3):1459–64. doi: 10.1016/j.bbrc.2009.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(8):1430–8. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 7.Harousseau JL. Farnesyltransferase inihibitors in hematologic malignancies. Blood Rev. 2007;21(4):173–82. doi: 10.1016/j.blre.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Tunon MJ, Alvarez M, Culebras JM, Gonzalez-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15(25):3086–98. doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman TM, Hodgson HJ. Animal models of acute hepatic failure. Int J Exp Pathol. 2000;81(2):145–57. doi: 10.1046/j.1365-2613.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine gamma-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid Redox Signal. 2014;20(2):204–16. doi: 10.1089/ars.2013.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14(3):528–35. doi: 10.1007/s11605-009-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, Fujise Y. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation. 1991;52(6):979–83. doi: 10.1097/00007890-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Seglen PO. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82(2):391–8. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 14.Marutani E, Kosugi S, Tokuda K, Khatri A, Nguyen R, Atochin DN, Kida K, Van Leyen K, Arai K, Ichinose F. A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. The Journal of biological chemistry. 2012;287(38):32124–35. doi: 10.1074/jbc.M112.374124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxidants & redox signaling. 2012;17(1):11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxidants & redox signaling. 2011;15(2):343–52. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinozaki S, Choi CS, Shimizu N, Yamada M, Kim M, Zhang T, Shiota G, Dong HH, Kim YB, Kaneki M. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. The Journal of biological chemistry. 2011;286(40):34959–75. doi: 10.1074/jbc.M110.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christofferson DE, Yuan J. Cyclophilin A release as a biomarker of necrotic cell death. Cell death and differentiation. 2010;17(12):1942–3. doi: 10.1038/cdd.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Liu B, Fukudome EY, Lu J, Chong W, Jin G, Liu Z, Velmahos GC, Demoya M, King DR, Alam HB. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150(3):442–51. doi: 10.1016/j.surg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979;76(11):5939–43. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo Y, Shibazaki M, Yamaguchi K, Kai K, Sugawara S, Takada H, Kikuchi H, Kumagai K. Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment. British journal of pharmacology. 1999;128(1):5–12. doi: 10.1038/sj.bjp.0702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Z, Liu Y, Li F, Ren F, Chen D, Li X, Wen T. Circulating histones exacerbate inflammation in mice with acute liver failure. Journal of cellular biochemistry. 2013;114(10):2384–91. doi: 10.1002/jcb.24588. [DOI] [PubMed] [Google Scholar]
- 23.Dear JW, Simpson KJ, Nicolai MP, Catterson JH, Street J, Huizinga T, Craig DG, Dhaliwal K, Webb S, Bateman DN, Webb DJ. Cyclophilin A is a damage-associated molecular pattern molecule that mediates acetaminophen-induced liver injury. Journal of immunology. 2011;187(6):3347–52. doi: 10.4049/jimmunol.1100165. [DOI] [PubMed] [Google Scholar]
- 24.Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113(3):163–71. doi: 10.4149/bll_2012_039. [DOI] [PubMed] [Google Scholar]
- 25.Takano K, Shinoda M, Tanabe M, Miyasho T, Yamada S, Ono S, Masugi Y, Suda K, Fukunaga K, Hayashida T, Hibi T, Obara H, Takeuchi H, Kawachi S, Kawasako K, Okamoto M, Yokota H, Maruyama I, Kitagawa Y. Protective effect of high-mobility group box 1 blockade on acute liver failure in rats. Shock. 2010;34(6):573–9. doi: 10.1097/SHK.0b013e3181df0433. [DOI] [PubMed] [Google Scholar]
- 26.Cloughesy TF, Kuhn J, Robins HI, Abrey L, Wen P, Fink K, Lieberman FS, Mehta M, Chang S, Yung A, DeAngelis L, Schiff D, Junck L, Groves M, Paquette S, Wright J, Lamborn K, Sebti SM, Prados M. Phase I trial of tipifarnib in patients with recurrent malignant glioma taking enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2005;23(27):6647–56. doi: 10.1200/JCO.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Endo Y, Shibazaki M, Yamaguchi K, Kai K, Sugawara S, Takada H, Kikuchi H, Kumagai K. Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment. Br J Pharmacol. 1999;128(1):5–12. doi: 10.1038/sj.bjp.0702747. [DOI] [PMC free article] [PubMed] [Google Scholar]