Abstract
Objective
Differentiating trajectories of weight change and identifying associated baseline predictors can provide insights for improving behavioral obesity treatment outcomes.
Design and Methods
Secondary, observational analyses using growth mixture models were conducted in pooled data for 604 black American, primarily female adults in three completed clinical trials. Covariates of identified patterns were evaluated.
Results
The best fitting model identified three patterns over 2 years: 1) mean weight loss of approximately 2 kg (n=519); 2) mean weight loss of approximately 3 kg at 1 year, followed by ~ 4 kg regain (n=61); and 3) mean weight loss of approximately 20 kg at 1 year followed by ~ 4 kg regain (n=24, with 23 from one study). In final multivariate analyses, higher BMI predicted having pattern 2 (OR[95% CI]) 1.10[1.03, 1.17]) or 3 (OR[95% CI] 1.42[1.25, 1.63]), and higher dietary fat score was predictive of a lower odds of having patterns 2 (OR[95% CI] 0.37[0.15, 0.94]) or 3 (OR[95% CI] 0.23[0.07, 0.79]).
Conclusions
Findings were consistent with moderate, clinically non-significant weight loss as the predominant pattern across all studies. Results underscore the need to develop novel and more carefully targeted and tailored approaches to facilitating weight loss in black American adults.
Keywords: obesity, weight loss, healthy lifestyle intervention
Introduction
The high prevalence of obesity is a major public health and clinical concern for the entire U.S. population and deserves particular attention among black Americans. National survey data for 2011–2012 indicated that 56.6% of black women were obese, compared with 32.8% of white women (1). Obesity prevalence is also higher in black than white men: 37.1% and 32.4%, respectively. The greater health burden of obesity in black Americans is reflected in relatively higher prevalence of obesity-related conditions such as diabetes and high blood pressure (2). The high prevalence of obesity in black youth (1) suggests that this high burden of obesity will persist and possibly worsen, given that child and adolescent obesity track into adulthood (3).
Clinical trials in diverse populations have demonstrated that weight reduction facilitates prevention and management of diabetes and cardiovascular risk factors (4–7). The beneficial effects on comorbidities are linked to the amount of weight loss. It is therefore of concern that weight loss outcomes for black participants in obesity treatment trials are less favorable than those for white participants in the same trials (8–12). In addition, trials of lifestyle behavior change programs adapted for black Americans report relatively modest mean weight losses, as only a small percent achieve clinically significant weight loss (usually defined as loss of ≥ 5% of baseline weight (13). Additional evidence indicates that patterns of weight loss differ between blacks and whites. For example, weight loss occurs more slowly or to a lesser degree but is more likely sustained in black compared to white participants in the same trial (9, 14, 15). However, the characteristics of weight change trajectories within black American weight loss program participants and factors that might differentiate those who are more or less successful have not been explored.
We conducted an analysis of weight loss patterns in pooled data for black participants in three lifestyle weight loss trials conducted by the same research group between 2000 and 2010. Besides a descriptive analysis of patterns indicative of more or less success in losing weight, we were also interested in characteristics, identifiable at the time of enrollment, of individuals who exhibited these patterns. An ability to identify broad categories of people with likely different program outcomes could help to identify treatment approaches that would better position program enrollees for success.
Methods
Data Sources
Data from three completed randomized controlled trials were pooled to increase the analytic sample size and to enhance generalizability of findings combined across studies with similar eligibility and behavioral counseling content but with different settings. Table 1 summarizes the three trials’characteristics. (1) The Healthy Eating and Lifestyle Program (HELP) used a primarily group counseling approach delivered by research staff on site in a family medicine practice (16). Phase 2, a randomized trial, hypothesized that, following a non-randomized weight loss induction phase, continued HELP Classes or Self-HELP, or both, would result in better long-term weight management than Clinic Visits Only. (2) The SHARE Study (Supporting Healthy Activity and eating Right Everyday) used a combined group and individual counseling approach delivered by staff in a research setting. SHARE recruited and treated participants in two strata of which one involved individuals recruited together with one or two family members and friends and the other involved individuals recruited alone (17). SHARE hypothesized that participants treated with partners participating fully (High Support) would have larger weight loss than those treated with minimal partner involvement (Low Support). Similar High and Low support conditions were created in those who enrolled alone. (3) The Think Health! Study used individual counseling delivered by primary care providers (PCPs) to patients recruited from within their own practices (18–20). Only data for the 169 black participants (from a total sample of 261) were considered in the pooled data set. All patients received counseling every 4 months from their PCPs. A moderate intensity treatment involved additional counseling by an ancillary practice staff member trained to serve as a lifestyle coach (LC), with counseling delivered monthly (year 1) or bimonthly (year 2). Think Health! hypothesized that participants receiving moderate intensity counseling would lose more weight than those in the low-intensity arm.
Table 1.
Key features of randomized controlled trials contributing data for the pooled data set.
| Characteristic | HELP Study | SHARE | Think Health! |
|---|---|---|---|
| Sponsor | American Heart Association National Center |
National Heart Lung, and Blood Institute (HL 69400) |
State of Pennsylvania Tobacco Settlement Funds |
| Time frame | 2000–2002 | 2002–2006 | 2005–2010 |
| Eligible race/ethnicity | Black American | Black American (not required for partners) |
Any, but emphasized Black Americans and Latinos |
| Study setting | Family medicine practice office in university health system |
Research offices in university area |
Primary care practices |
| Recruitment targets | Local residents with primary care provider in university of health system |
Local residents, particularly those who could identify partners to enroll with them |
Only current patients of the participating primary care practices |
| Recruitment method | Newspaper advertisements, referrals, other |
Newspaper advertisements, referrals, other |
Lists generated from practices and referrals |
| Eligible age range | 25 – 70 years | 35 – 70 years for Index 16 – 70 years for Partners |
18 – 70 years |
| Eligible BMI | 30 – 50 kg/m2 | 27 – 55 kg/m2 | 25 – 55 kg/m2 |
| Major clinical exclusions | pregnancy, unstable mental or physical health; weight altering medications |
pregnancy, unstable mental or physical health; weight altering medications |
pregnancy, unstable mental or physical health; weight altering medications |
| Number Black Americans enrolled |
237 in initial program; 128 in RCT |
344; 193 Index Participants and 151 Partners |
169 |
| Study-provided treatments |
After participating in program of 10 weekly group counseling sessions, randomized to: 1) 6 bi-weekly, then monthly group sessions (HELP classes) 2) Facilitated self-help program (Self- HELP) 3) Semi-annual health monitoring (Clinic visits only) |
If enrolled with partner (Family/Friend), randomized to: 1) 23 weekly, 12 bi-weekly, then 12 monthly group sessions including periodic personal counseling; partners attended group sessions 2) as in 1), but, no partners at group sessions If enrolled alone (Individual), randomized to: 3) as in 1), but were assigned partners within the group 4) as in 3), no partners assigned |
Randomized to: 1) brief counseling by primary care provider ~ every 4 months plus 12 monthly counseling sessions with an auxiliary staff member acting as a lifestyle coach, then bi-monthly sessions with the coach 2) brief counseling by primary care provider every 4 months |
| Participant materials | printed manuals; calorie counter; pedometer; educational video; incentives |
printed manuals and handouts; pedometers; resistance band; incentives |
printed manuals; audio versions of first 12 sessions; resistance band; incentives |
| Length of follow up | 3–6, or 11–21 months depending on enrollment date and involvement in Phase 2 |
24 months | 16 –24 months, depending on date of enrollment |
| % (n) with final weight measurements |
57% (134) of initial program 68% (87) of RCT |
63% (215) overall 66% of Index participants |
56% (95) of those randomized |
| % (n) with final BP measurements |
100% of those with final weight measurements; initial and RCT |
97% (208) of those with final weight measurements |
100% (95) of those with final weight measurements |
| Overall weight loss from baseline to end of follow up |
Mean (SD)* 0.8 (4.4) kg -HELP Classes −1.3 (5.5) kg -Self-HELP −1.4 (5.7) kg -Clinic visits only |
Mean (SD)‡ 2.6 (5.7) kg -Family/Friend 1.4 (5.2) kg -Individual |
Mean (SD)§ 0.9 (5.7) kg -moderate intensity 0.5 (5.0) kg -low intensity |
| % with > 5% weight loss | 25%# | 23.9% -Family/Friend 16.1% -Individual |
14% |
based on data for completers; treatment group difference were not statistically significant (p=.90)
data for completers pooled across treatment arms
Based on intention-to-treat analyses within the Family/Friend and Individual strata for Index participants only, i.e., participants who enrolled as partners of other participants are not included; these estimates do not include a participant who had bariatric surgery during the study period. Treatment outcomes were not significantly different between the high and low social support conditions within strata
calculated for the black American participants in Think Health! The treatment group differences was not statistically significant
Analysis Variables
Height and weight measurements were assessed with the same or similar equipment and comparable protocols across studies (16–18). Demographic, medical history, and behavioral characteristics were obtained by questionnaire, with each subsequent study using or adapting the case report forms used in the prior study or studies. Each study measured a “baseline weight” during screening before the start of interventions. “Follow up weights” refers to weights taken directly or from medical records (Think Health!), by research staff not involved in the intervention. In HELP, follow up weights were planned for 3 months (i.e., after the induction phase) and then at 6, 12, and 18 months post randomization or at the time of administrative censoring. In SHARE, follow up weights were planned for 6, 12, 18, and 24 months post randomization. In Think Health! follow up weights were planned for 1 and 2 years post randomization or at the time of administrative censoring. In all studies, actual weights were measured as close to the specified times as possible. The actual measurement times rather than planned times were used in this analysis. Weights taken from those who attended classes (intervention weights) were available for SHARE for all index participants and partners whose randomization assignment permitted class attendance. Intervention weights for Think Health! were taken whenever patients attended a PCP or LC visit. No class or intervention weights were available from HELP.
We defined a variable to represent the lowest intensity or usual care condition in each study: “monitoring only arm” in HELP (n=42); partners assigned to low support in SHARE (n=56); and the low intensity arm in Think Health! (n=85). We reviewed and recoded demographic, medical history, and behavioral variables to achieve comparability across studies. Physical activity required categorization into low, moderate, and high levels based on the measure available, which differed across studies. Positive medical history for obesity-related comorbidities was coded as “yes” or “no,” based on a composite of participants’ answers to questions about whether they had: heart disease (heart disease or heart disease, angina or stroke), lung disease (chronic obstructive pulmonary disease or asthma, or asthma or lung disease); mobility limitations (difficulty walking or orthopedic limitation, or difficulty walking associated with back problems or joint problems); high blood pressure; and diabetes. Self-rated health was taken from the SF-8 (21) or SF-36 (22). A 30-item version of a longer food habits questionnaire was administered in SHARE and Think Health! and a comparable score could be calculated from the original long form of the questionnaire that was administered in HELP (23). Items on this questionnaire reflected primarily dietary-fat related behaviors (food intake and food preparation methods) associated with weight control. All three studies included Cohen’s Perceived Stress measure (24); the 4-item version administered in SHARE and HELP was calculated from the 14-item version used in HELP.
Statistical Analysis
All available weights were included in the analysis except in special cases. Due to departures of actual measurement times from planned measurements, time was measured as a continuous variable in weeks from the baseline weight at week 0. All weights that yielded a weight change of more than 30 kg were reviewed by the team. A few weights that could not be corrected with a kg/lbs conversion were considered obvious data errors (e.g., > 20 kg change within a month) and were deleted. Thirty-five SHARE participants were excluded from the analysis. Two of the 35 excluded participants were not black American, 1 had bariatric surgery, and for 7 participants the next available weight measurement occurred more than 2 years after baseline and thus beyond the range of the current study. The other 25 had only a baseline weight measurement. Two Think Health! Participants were excluded due to only having a baseline weight measurement. Fifteen HELP participants also had enrolled in the SHARE study. For the current analysis, weight measurements from both studies were included and considered to be independent.
Longitudinal data are commonly analyzed using linear mixed effects models that assume individual trajectories of the longitudinal outcome deviate from a mean growth model (random intercepts and slopes), but carry the assumption that the sample is composed of individuals from one population. Growth mixture modeling (GMM) (25, 26) allows for the assumption of more than one population resulting in more than one mean growth model. The GMM combines elements of the linear mixed effects model and the latent class model. The model simultaneously estimates longitudinal patterns of weight loss within each unobserved group (latent class). The parameters from the linear mixed effects model define the shape of the average longitudinal patterns within each class. Random intercepts and slopes allow individuals to vary randomly around the average pattern. The parameters from the latent class component yield individual probabilities of class membership into each class (e.g. 0.95, 0.02, and 0.05 for classes 1, 2 and 3 respectively). Individuals are classified into groups based on the largest probability of class membership (class 1 in the example).
We used GMM to estimate latent classes corresponding to patterns of weight loss across the follow up period for the combined studies. The outcome measures were repeated weight (kg) over time for each individual. The model for the pattern of weight loss included random intercepts and slopes to account for within-subject correlations and separate fixed effect intercepts and fixed effects slopes for time within each latent class of weight loss. Since weight loss patterns depended highly on baseline weight, the random intercepts and slopes were allowed to depend on baseline covariates to adjust for baseline weight and study membership. Using a standard likelihood ratio test, we compared the results of this longitudinal linear model to models that accommodated nonlinear weight loss over time, specifically a quadratic trend in time. The choice of the number of classes was determined through examination of fit indices as well as the clinical interpretation of the results. The Bayesian Information Criteria (BIC) (27) were used to assess the model goodness of fit, and the Rubin-Lo-Mendell test (28) was used in selecting the number of classes. Results are reported in terms of weight loss as estimated by the model.
The preliminary examination of predictors of latent class membership was carried out in two ways. First, each covariate was included in separate growth mixture models and evaluated for significance using a Wald Chi-square test. Secondly, each respondent was assigned to his or her most likely class under the growth mixture model as indicated by the predicted probabilities of class membership. Then the relationship between class membership and baseline covariates was explored using one-way ANOVA for continuous covariates and Chi-square test for categorical covariates (29). The second approach has the potential of inducing measurement error; therefore the first approach is preferred. Due to the large number of potential covariates and to avoid over-fitting, we selected predictors that had p-value<0.2 in the preliminary examination. A final growth mixture model was fit with selected baseline predictors of class membership. The relationships between baseline covariates and the latent classes were expressed as odds ratios and confidence intervals. All analyses were performed using Mplus (v 6.1) and R (v 2.15).
Results
Table 2 summarizes baseline characteristics of participants by weight loss trial. The percent of participants who received a low-intensity intervention, as defined in Methods, differed across studies, (e.g., 18% in SHARE and 51% in Think Health). Several of the other variables shown in Table 2 differed by study as well. Studies were similar in that most participants were females, approximately 47 years of age, with a BMI of approximately 37 kg/m2, and most were employed. Nearly a third were severely obese (BMI ≥ 40), and most had a positive history for at least one obesity-related comorbidity.
Table 2.
Baseline participant characteristics overall and by weight loss trial.
| Variable | All (n=604) |
HELP (n=128) |
SHARE (n=309) |
Think Health! (n=167) |
Chi- square or F test statistic (p-value) |
|---|---|---|---|---|---|
| N (%) unless otherwise indicated | |||||
| Low intensity intervention | 183 (30) | 42 (33) | 56 (18) | 85 (51) | 55.6 (<0.001) |
| Age, mean (sd) | 46.8 (10.0) | 45.4 (10.2) | 46.7 (9.1) | 47.9 (11.2) | 2.3 (0.104) |
| Female | 540 (89) | 116 (91) | 278 (90) | 146 (87) | 1.0 (0.608) |
| Baseline weight, mean kg (sd) | 102.4 (18.8) | 101.6 (17.0) | 103.6 (19.3) | 100.8 (19.0) | 1.3 (0.269) |
| BMI, mean (sd) | 37.3 (6.0) | 36.5 (5.2) | 37.9 (6.2) | 37.0 (6.1) | 2.7 (0.068) |
| BMI category | 3.4 (0.488) | ||||
| <35 | 250 (41) | 60 (47) | 119 (39) | 71 (43) | |
| 35–40 | 169 (28) | 35 (27) | 87 (28) | 47 (28) | |
| >=40 | 18 (31) | 33 (26) | 103 (33) | 49 (29) | |
| Employed | 494 (82) | 121 (95) | 258 (84) | 115 (69) | 33.3 (<0.001) |
| Number in household, mean (sd) | 3.2 (1.6) | 3.9 (1.6) | 3.0 (1.4) | 3.0 (1.8) | 19.0 (<0.001) |
| Married | 278 (46) | 64 (50) | 152 (49) | 62 (37) | 7.5 (0.023) |
| Current smoker | 85 (14) | 18 (14) | 30 (10) | 37 (22) | 13.8 (0.001) |
| Current drinker | 165 (27) | 29 (23) | 85 (28) | 51 (31) | 2.3 (0.320) |
| Physical activity | 45.1 (<0.001) | ||||
| High | 162 (27) | 37 (29) | 87 (29) | 38 (23) | |
| Moderate | 252 (42) | 27 (21) | 155 (51) | 70 (42) | |
| Low | 184 (31) | 62 (49) | 63 (21) | 59 (35) | |
| Excellent self rated health | 137 (23) | 28 (22) | 85 (28) | 24 (14) | 11.2 (0.004) |
| Positive medical history | 400 (66) | 88 (69) | 210 (68) | 102 (61) | 2.8 (0.252) |
| Heart disease | 52 (9) | 21 (17) | 20 (6) | 11 (7) | 12.8 (0.002) |
| Lung disease | 107 (18) | 17 (13) | 47 (15) | 43 (26) | 10.3 (0.006) |
| Mobility limitations | 202 (33) | 46 (36) | 135 (44) | 21 (13) | 47.6 (<0.001) |
| High blood pressure | 260 (43) | 51 (40) | 135 (44) | 74 (44) | 0.5 (0.781) |
| Diabetes | 79 (13) | 16 (13) | 38 (12) | 25 (15) | 0.7 (0.703) |
| Cohen Stress Score, mean (sd) | 5.2 (3.1) | 5.4 (2.9) | 5.3 (3.0) | 4.9 (3.2) | 1.5 (0.228) |
| Any prior weight loss programs | 333 (56) | 81 (63) | 179 (59) | 73 (44) | 13.8 (0.001) |
| Number prior weight loss programs | 41.4 (<0.001) | ||||
| 0 | 268 (45) | 45 (36) | 129 (42) | 94 (56) | |
| 1–2 | 271 (45) | 51 (40) | 153 (50) | 67 (40) | |
| 3+ | 62 (10) | 30 (24) | 26 (8) | 6 (4) | |
| SisterTalk Food Habits Score, mean (sd)a | 2.6 (0.6) | 2.9 (0.3) | 2.0 (0.3) | 3.0 (0.4) | 299.2 (<0.001) |
| Desired weight at end of study, mean lbs (sd) | 150.8 (39.9) | 157.3 (28.6) | 140.3 (45.7) | 165.3 (28.8) | 25.2 (<0.001) |
| BMI based on desired weight, mean (sd) | 24.9 (6.1) | 25.6 (3.7) | 23.3 (7.4) | 27.4 (3.7) | 28.0 (<0.001) |
n=431, not measured on 10 HELP and 163 SHARE participants.
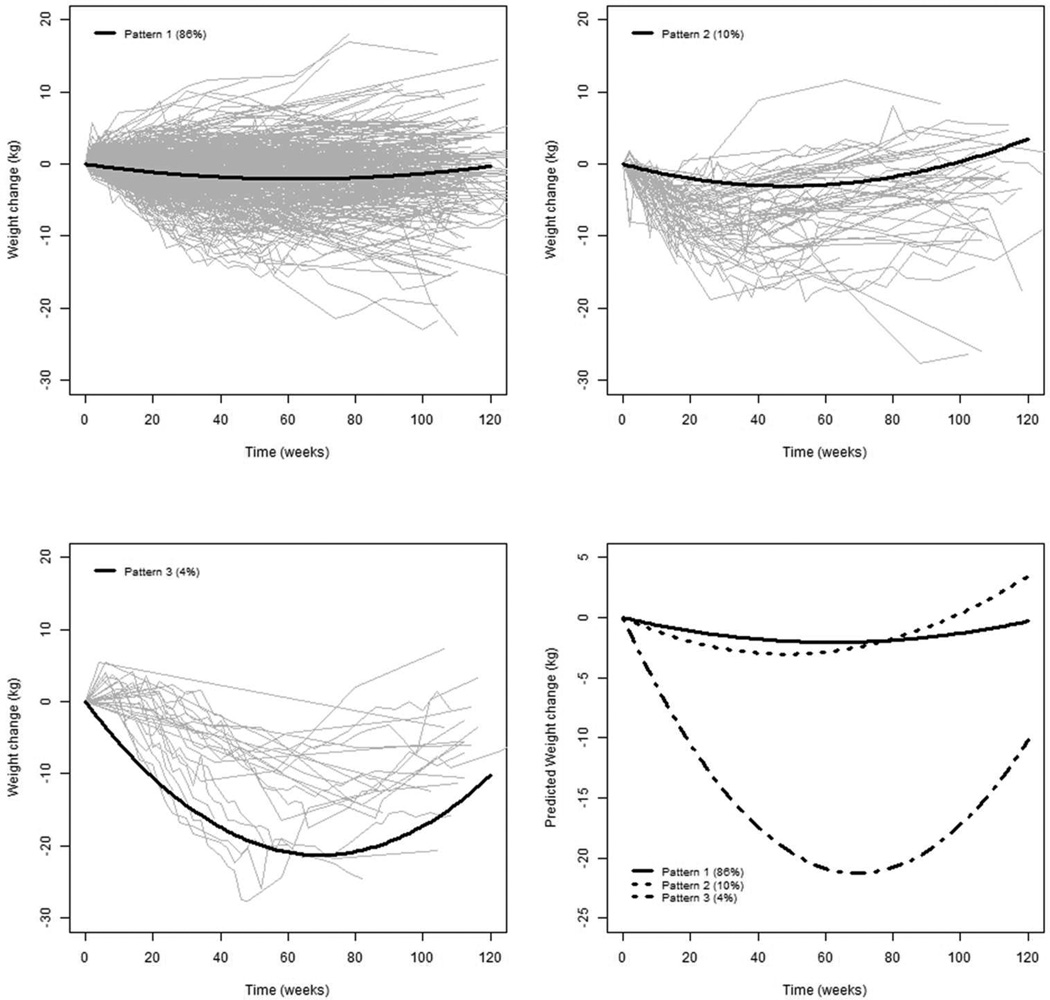
The 604 participants contributed a total of 5349 weight measurements that were included in the growth mixture models. The model containing a quadratic term for time yielded an improved fit of the model compared to a linear term (Likelihood ratio Chi-square (df=4)=2614.66, p<0.001), so we based our results on the quadratic trend in time which was assumed for each class. In comparing the number of classes, the 4-class model yielded the best fit. However the class structure did not differ, and the more parsimonious 3-class model was chosen. Figure 1 graphically represents the mean trajectory of weight loss for each class along with the raw data for individuals with the highest probability of class assignment. The class 1 pattern, “Pattern 1,” is relatively linear with modest weight loss of about 2 kg below baseline (86% of the sample). Participants with Pattern 1 had a mean baseline weight of 101.4 kg with a statistically significant decline in weight at 12 months (linear time =−2.63, 95% CI [−3.44, −1.83], quadratic time=0.84, 95% CI [0.57, 1.12]). The second class, “Pattern 2”, demonstrates a gradual curve of weight loss to about 3 kg at 12 months (10% of the sample). Participants in Pattern 2 had a mean baseline weight of 104.1 kg, with a statistically significant decline in weight (linear time = −4.99, 95% CI [−8.54, −1.45]) and a significant rebound (quadratic time = 2.05, 95% CI [0.78, 3.32]). The third class, “Pattern 3”, demonstrates a gradual curve of substantial weight loss of about 20 kg at 12 months, followed by a regain of about 4 kg (4% of sample). Participants in Pattern 3 had a mean baseline weight of 120.0 kg with a statistically significant decline in weight (linear time = −24.5, 95% CI [−30.2, −18.7]) and a significant rebound (quadratic time = 7.02, 95% CI [4.93, 9.11]).
Figure 1.
Three patterns reflect growth classes derived from a growth mixture model that produced three classes of participants. The gray lines reflect observed weight loss patterns, while the solid lines reflect the average patterns from the three classes.
Table 3 contains the results of the preliminary examination of predictors of class membership. Pattern 1 was predominant and pattern 2 secondary in all three studies. However, all except one person with Pattern 3 were from the SHARE study. The two approaches to analysis of covariates yielded consistent results. Study membership, low intensity intervention, marital status, prior experience with weight loss programs, food habit score, excellent self-rated health, and baseline BMI were significant predictors of class membership at the p<0.2 level and were included in the final model.
Table 3.
Bivariate associations between participant characteristics and class membership using model based assignments of individuals to their most likely class and growth mixture models with covariates.
| Variable | All (n=604) |
Pattern 1 (n=519) |
Pattern 2 (n=61) |
Pattern 3 (n=24) |
GMM Wald Chi-square test statistic (df) p-value |
Fisher’s Exact or F test statistic (p-value) |
|---|---|---|---|---|---|---|
| N(%) unless otherwise indicated | ||||||
| Tria | ne | ne <0.1 (<0.001) | ||||
| HELP | 128 (21) | 118 (23) | 10 (16) | 0 (0) | ||
| SHARE | 309 (51) | 247 (48) | 39 (64) | 23 (96) | ||
| Think Health! | 167 (28) | 154 (30) | 12 (20) | 1 (4) | ||
| Low intensity intervention | 183 (30) | 168 (32) | 12 (20) | 3 (13) | 6.5 (2) 0.040 | <0.1 (0.018) |
| Age, mean (sd) | 46.8 (10.0) | 46.8 (10.2) | 46.2 (9.1) | 47.5 (8.6) | 0.1 (2) 0.968 | 0.1 (0.870) |
| Female | 540 (89) | 466 (90) | 52 (85) | 22 (92) | 0.4 (2) 0.834 | <0.1 (0.491) |
| Baseline weight, mean (sd) | 102.4 (18.8) | 101.4 (18.3) | 104.1 (19.7) | 120.0 (18.2) | 10.2 (2) 0.006 | 11.9 (<0.001) |
| BMI, mean (sd) | 37.3 (6.0) | 37.0 (5.8) | 37.9 (6.3) | 43.5 (5.8) | 17.9 (2) <0.001 | 14.5 (<0.001) |
| BMI group | 5.0 (4) 0.289 | <0.1 (<0.001) | ||||
| <35 | 250 (41) | 225 (43) | 23 (38) | 2 (8) | ||
| 35–40 | 169 (28) | 145 (28) | 19 (31) | 5 (21) | ||
| >=40 | 185 (31) | 149 (29) | 19 (31) | 17 (71) | ||
| Employed | 494 (82) | 421 (81) | 53 (87) | 20 (83) | 3.5 (2) 0.175 | <0.1 (0.601) |
| Number in household, mean (sd) | 3.2 (1.6) | 3.2 (1.6) | 3.3 (1.5) | 3.0 (2.0) | 0.6 (2) 0.735 | 0.4 (0.642) |
| Married | 278 (46) | 228 (44) | 36 (59) | 14 (61) | 2.5 (2) 0.286 | <0.1 (0.029) |
| Current smoker | 85 (14) | 75 (15) | 8 (13) | 2 (8) | 0.2 (2) 0.911 | <0.1 (0.804) |
| Current drinker | 165 (27) | 148 (29) | 14 (23) | 3 (13) | 3.7 (2) 0.160 | <0.1 (0.171) |
| Physical activity | 5.0 (4) 0.286 | <0.1 (0.501) | ||||
| High | 162 (27) | 136 (26) | 17 (28) | 9 (38) | ||
| Moderate | 252 (42) | 215 (42) | 29 (48) | 8 (33) | ||
| Low | 184 (31) | 163 (32) | 14 (23) | 7 (29) | ||
| Excellent self rated health | 137 (23) | 112 (22) | 15 (25) | 10 (42) | 4.9 (2) 0.088 | <0.1 (0.075) |
| Positive medical history | 400 (66) | 343 (66) | 43 (70) | 14 (58) | 1.0 (2) 0.595 | <0.1 (0.540) |
| Heart disease | 52 (9) | 47 (9) | 3 (5) | 2 (8) | 0.8 (2) 0.673 | <0.1 (0.600) |
| Lung disease | 107 (18) | 95 (18) | 11 (18) | 1 (4) | 2.7 (2) 0.258 | <0.1 (0.220) |
| Mobility limitations | 202 (33) | 170 (33) | 24 (39) | 8 (33) | 0.4 (2) 0.812 | <0.1 (0.572) |
| High blood pressure | 260 (43) | 223 (43) | 26 (43) | 11 (46) | 1.5 (2) 0.479 | 0.017 (0.965) |
| Diabetes | 79 (13) | 69 (13) | 6 (10) | 4 (17) | 1.9 (2) 0.396 | 0.025 (0.623) |
| Cohen Stress Score, mean (sd) | 5.2 (3.1) | 5.2 (3.0) | 5.2 (3.5) | 4.5 (2.9) | 1.1 (2) 0.572 | 0.7 (0.484) |
| Any prior weight loss programs | 333 (56) | 279 (54) | 34 (56) | 20 (87) | 6.0 (2) 0.050 | <0.1 (0.006) |
| Number prior weight loss programs | 1.2 (4) 0.885 | <0.1 (0.058) | ||||
| 0 | 268 (45) | 237 (46) | 27 (44) | 4 (17) | ||
| 1–2 | 271 (45) | 226 (44) | 28 (46) | 17 (71) | ||
| 3+ | 62 (10) | 53 (10) | 6 (10) | 3(13) | ||
| SisterTalk Food Habits Score, mean (sd) | 2.6 (0.6) | 2.7 (0.6) | 2.5 (0.6) | 2.1 (0.5) | 149.3 (2) <0.001 | 7.9 (<0.001) |
| Desired weight, mean lbs (sd) | 150.8 (39.9) | 150.3 (40.1) | 157.1 (38.7) | 145.4 (39.1) | 2.2 (2) 0.333 | 1.0 (0.362) |
| Desired BMI, mean (sd) | 24.9 (6.1) | 24.9 (6.2) | 26.0 (5.8) | 24.0 (6.3) | 3.9 (2) 0.145 | 1.2 (0.306) |
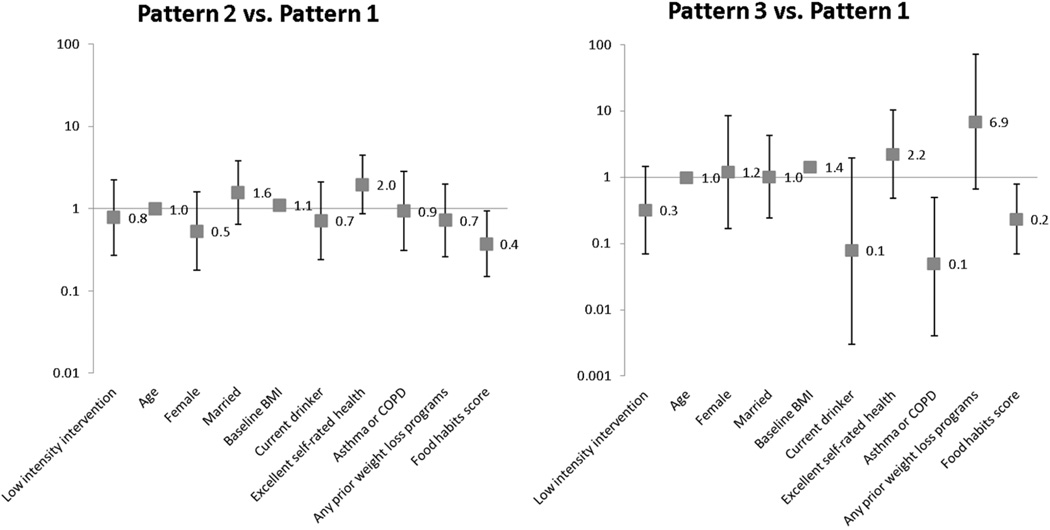
Results of the final adjusted model that incorporates the selected predictors from the preliminary analyses are shown in Figure 2. Participants reporting dietary behaviors indicative of higher fat intake were less likely to be in Pattern 2 (OR= 0.37, 95%CI [0.15, 0.94]) or Pattern 3 (OR=0.23, 95%CI [0.07, 0.79]) Participants with higher BMI were more likely to be in Pattern 2 (OR= 1.10, 95%CI [1.03, 1.17]) or Pattern 3 (OR=1.42, 95%CI [1.25, 1.63]) compared to Pattern 1. Participants who reported having asthma or lung disease were also less likely to be in Pattern 3 compared to Pattern 1 (OR=0.05, 95%CI [0.01, 0.50]). No other predictors were statistically significant.
Figure 2.
Odds ratios [95% CI] derived from growth mixture models of participant characteristics as predictors of weight loss patterns.
Discussion
We identified three weight loss patterns, of which one—gradual weight loss over 2 years to a mean of 2 kg below baseline (Pattern 1) applied to most (86%) participants. Pattern 3, indicative of the highest degree of success (large and relatively well-maintained weight loss), included very few (4%) participants. The least successful pattern (Pattern 2) was one of modest (3 kg) initial weight loss followed by regain to 1 kg above baseline and included 10% of participants. Participants with higher BMI and higher fat intake were less likely to be in Patterns 2 and 3 compared to the predominant, Pattern 1. Participants with asthma/COPD were less likely to be in Pattern 3. No other baseline characteristics examined predicted these patterns.
The predominance of Pattern 1 is consistent with findings from the three included trials when analyzed separately (16, 17, 19, 20). The 2 kg weight loss, equivalent to loss of 2% from the baseline weight of approximately 100 kg, is below the accepted 5% threshold for clinical significance (4). This level of weight loss is often associated with low-intensity interventions (30), but we also observed this pattern in 80% of the participants in the trial (SHARE) that offered weekly and then biweekly intervention during the first 6 months, which is high-intensity. The Pattern 1 weight loss is also similar to weight losses of black participants after the same length of follow up in the efficacy trials from which the HELP, SHARE, and Think Health! treatment protocols were adapted: the Trials of Hypertension Prevention (TOHP) I and II (15, 31), the Trial of Nonpharmacologic Interventions in the Elderly (TONE) (9), and the Diabetes Prevention Program (DPP) (32). This, small weight losses may be typical of what can be expected on average with black adults based on currently available lifestyle change approaches even under best case scenarios.
Taking the most positive view, this pattern of weight loss suggests that engagement in lifestyle change programs may have value for preventing the gradual weight gain of 0.5 to 1 kg per year that has been observed among black participants in cohort studies or control groups in clinical trials (11, 15, 33). Prevention of further weight gain among those already overweight has been associated with health benefits (34). However, in practice, programs designed for weight loss should have a reasonable potential of facilitating clinically significant weight loss in a substantial percentage of participants, including black participants.
Obesity treatment studies that focus on or report outcomes for black Americans are relatively few and do not form a coherent body of evidence to guide the design of effective interventions. A series of systematic reviews undertaken to address the need for progress in this area of research confirms the dearth of evidence but offers some insights about potentially relevant research directions (35). Electronic interventions delivered through the Internet or mobile devices have received limited study in black and other ethnic/minority populations but may have promise. Improving the quality of cultural adaptations is another area worth pursuing, including studies conducted in faith based and other culturally relevant settings. Cultural adaptations and cultural tailoring of weight loss programs have long been recommended to account for cultural influences on food and eating, physical activity, and body size perceptions and aspirations (4). However, there is much room for improvement in how these adaptations are designed, assessed, and linked to outcomes.
The other key area for future study relates to the influences of physical, economic, and other environmental factors on the ability of black Americans to lose weight and sustain weight loss. U.S. environments in general have become ‘obesigenic’ and tend to promote weight gain (36). Food marketing and physical activity environments of black Americans are relatively less favorable than those of whites with respect to support for healthy eating and active living (37, 38), which suggests that—all other things being equal—adherence to recommendations to reduce caloric intake and increase physical activity will be more difficult to follow. Yet few studies have evaluated the effects on behavior of changing aspects of food or physical activity environments in black communities (39). Multilevel studies to elucidate interactions of weight loss program participants with their environmental contexts for adherence would be of particular interest, e.g., using mixed-methods designs (40).
Strengths of this study include the use of pooled data from three RCTs, which increased statistical power for identifying patterns evident in studies conducted in different settings. Furthermore, the analytic approach used all available weights for any participant with at least one weight measurement after baseline and was, therefore, less affected than other approaches by missing data. The flexible models made use of actual rather than planned measurement times, and then allowed for nonlinear trajectories. It would have been desirable to examine weight loss trajectories earlier than at 1 year, but we did not have uniformly available weights during the early months of enrollment. The small number of men in the data set limited our ability to explore gender differences in weight loss patterns.The absence of a broader array of covariates was also a potential limitation. This is less important with one predominant pattern, as observed here, but would be more relevant with several more evenly represented patterns.
In conclusion, our main finding was that one pattern of non-clinically significant weight loss applied to most participants. The consistency of this finding with previously published reports indicates a need to design and evaluate new approaches and more refined versions of current approaches to facilitate meaningful weight loss in this high-risk population. Our findings relate primarily to women but obesity prevalence in black men suggests a need for research that applies to both men and women.
What is already known about this subject
Obesity prevalence is notably higher in black compared to white Americans
Behavioral weight loss programs are less effective in blacks than whites
Even programs adapted for relevance to black Americans yield small weight losses
What this study adds
Longitudinal clinical trial data from 604 black Americans revealed 3 different weight change patterns
A pattern of modest weight loss that was not clinically significant was predominant
A pattern of major weight loss was observed but included very few participants
Acknowledgements (including author contributions)
This analysis was supported by National Institute of Diabetes, Digestive, and Kidney Diseases (R21DK089422). Dr. Morales’ effort was partly supported by K01MH073903. The primary studies contributing data were supported by American Heart Association National Center (9970068N), National Heart, Lung, and Blood Institute (R01HL69400), and the Pennsylvania Department of Health Commonwealth Universal Research Enhancement Program. Drs. Morales and Kumanyika designed the study, analyzed the data, and prepared the first draft of the manuscript. Ms. Fassbender and Ms. Good had major responsibility for creating the pooled data set. Drs. Wadden and Localio provided critical feedback on analyses. All authors reviewed and provided critical feedback on the final draft.
Footnotes
Conflict of Interest Statement
Thomas Wadden serves on advisory boards for Novo Nordisk, Nutrisystem, and Orexigen Pharmaceuticals and has received grants on behalf of the University of Pennsylvania from each entity. He also has consulted with Boehringer Ingelheim. The remaining authors declared no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2012: With Special Feature on Emergency Care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- 3.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23(Suppl 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 4.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obesity research. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA : the journal of the American Medical Association. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 7.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Archives of Internal Medicine. 1997;157:657–667. [PubMed] [Google Scholar]
- 8.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10:96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- 10.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 11.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 12.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Archives of Internal Medicine. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 14.Espeland MA, Bray GA, Neiberg R, Rejeski WJ, Knowler WC, Lang W, et al. Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) research group. Annals of epidemiology. 2009;19:701–710. doi: 10.1016/j.annepidem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study. Prev Med. 2005;41:488–502. doi: 10.1016/j.ypmed.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Kumanyika SK, Wadden TA, Shults J, Fassbender JE, Brown SD, Bowman MA, et al. Trial of family and friend support for weight loss in African American adults. Archives of Internal Medicine. 2009;169:1795–1804. doi: 10.1001/archinternmed.2009.337. [DOI] [PubMed] [Google Scholar]
- 18.Kumanyika S, Fassbender J, Phipps E, Tan-Torres S, Localio R, Morales KH, et al. Design, recruitment and start up of a primary care weight loss trial targeting African American and Hispanic adults. Contemp Clin Trials. 2011;32:215–224. doi: 10.1016/j.cct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumanyika SK, Fassbender JE, Sarwer DB, Phipps E, Allison KC, Localio R, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring) 2012;20:1249–1257. doi: 10.1038/oby.2011.329. [DOI] [PubMed] [Google Scholar]
- 20.Kumanyika S. Modeling Effective Approaches to Obesity Treatment in Primary Care. Final Report to the Commonwealth of Pennsylvania. 2010 [Google Scholar]
- 21.Ware JE, Kosinski M, Dewey JE, Gandek B. A Manual for Users of the SF-8 Health Survey. Lincoln, RI: 2001. [Google Scholar]
- 22.Rand Corporation. 36-Item Short Form Survey from the RAND Medical Outcomes Study. 2013 Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. [Google Scholar]
- 23.Anderson CA, Kumanyika SK, Shults J, Kallan MJ, Gans KM, Risica PM. Assessing change in dietary-fat behaviors in a weight-loss program for African Americans: a potential short method. J Am Diet Assoc. 2007;107:838–842. doi: 10.1016/j.jada.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 25.Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM Algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 26.Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- 27.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 28.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 29.Rosner B. Fundamentals of Biostatistics, Sixth Edition. Belmont, CA: Brooks/Cole; 2006. [Google Scholar]
- 30.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Archives of Internal Medicine. 1993;153:849–858. [PubMed] [Google Scholar]
- 32.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. American journal of epidemiology. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 34.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. International journal of obesity. 2006;30:1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumanyika SK, Whitt-Glover MC, Haire-Joshu D. What works for obesity prevention and treatment in African–Americans? Research directions. Obesity Reviews. 15(Supp 4) doi: 10.1111/obr.12213. in press. [DOI] [PubMed] [Google Scholar]
- 36.Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, et al. Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science) Circulation. 2008;118:428–464. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 37.Grier SA, Kumanyika SK. The context for choice: health implications of targeted food and beverage marketing to African Americans. American journal of public health. 2008;98:1616–1629. doi: 10.2105/AJPH.2007.115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiologic reviews. 2009;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- 39.Kumanyika SK, Swank M, Stachecki J, Whitt-Glover M, Brennan L. Examining the Evidence for Policy and Environmental Strategies to Prevent Childhood Obesity in Black Communities: New Directions and Next Steps. Obesity Reviews. 15(Supp 4) doi: 10.1111/obr.12206. in press. [DOI] [PubMed] [Google Scholar]
- 40.Pluye P, Hong QN. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annual review of public health. 2014;35:29–45. doi: 10.1146/annurev-publhealth-032013-182440. [DOI] [PubMed] [Google Scholar]