Abstract
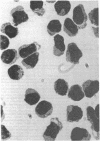
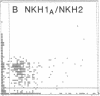
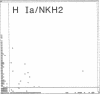
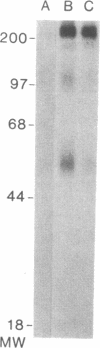
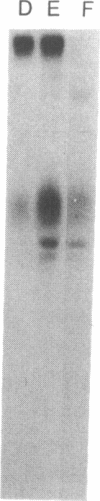
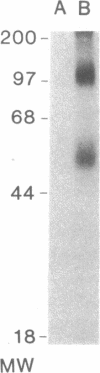
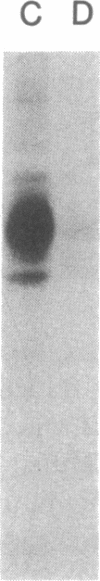
The initial characterization of two monoclonal antibodies directed at antigens selectively expressed on large granular lymphocytes (LGL) is reported in the present paper. These two reagents, anti-natural killer (NK) H1A and anti-NKH2, were obtained following immunization of mouse spleen cells with a cloned human NK cell line termed JT3. In fresh human peripheral blood, both anti-NKH1A and anti-NKH2 selectively reacted with cells that appeared morphologically as large granular lymphocytes. However, complement lysis studies and two color fluorescence analysis demonstrated that some LGL express both antigens and other cells express only NKH1A or NKH2. Functional analysis of these subsets indicated that the population of NKH1A+ cells contains the entire pool of NK active lymphocytes, whereas expression of NKH2 antigen appeared to delineate a unique subpopulation of LGL which, in a resting state, display a low degree of spontaneous cytotoxicity. Expression of NKH1A and NKH2 was also investigated using a series of nine well characterized human NK clones. All NK clones were found to be NKH1A+ and four out of nine also expressed NKH2. These results strongly supported the view that NKH1A is a "pan-NK" associated antigen, and indicated that at least a fraction of cloned NKH2 + LGL are strongly cytotoxic. Anti-NKH1A was shown to have the same specificity as the previously described N901 antibody and was found here to precipitate a 200,000-220,000-mol wt molecule in SDS-polyacrylamide gel electrophoresis (PAGE) analysis. Anti-NKH2 was specific for a structure that migrates at 60,000 mol wt in SDS-PAGE analysis under reducing conditions. Two color immunofluorescence analysis of NKH1A, NKH2, and other NK-associated antigens (Leu7 and B73.1) demonstrated variable degrees of coexpression of these antigens, which confirmed that NKH1A and NKH2 define distinct cell surface structures. Anti-NKH1A and anti-NKH2 appear to be useful reagents for characterizing LGL present in human peripheral blood and for identifying functionally relevant subsets within this heterogeneous population of cytotoxic lymphocytes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Abo T., Miller C. A., Balch C. M., Cooper M. D. Interleukin 2 receptor expression by activated HNK-1+ granular lymphocytes: a requirement for their proliferation. J Immunol. 1983 Oct;131(4):1822–1826. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., O'Brien C., Schlossman S. F. Delineation of an effector population responsible for natural killing and antibody-dependent cellular cytotoxicity in man. Clin Immunol Immunopathol. 1981 Jan;18(1):145–150. doi: 10.1016/0090-1229(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Beveridge R. P., Schlossman S. F. Isolation of myeloid progenitor cells from peripheral blood of chronic myelogenous leukemia patients. Blood. 1982 Jul;60(1):30–37. [PubMed] [Google Scholar]
- Griffin J. D., Hercend T., Beveridge R., Schlossman S. F. Characterization of an antigen expressed by human natural killer cells. J Immunol. 1983 Jun;130(6):2947–2951. [PubMed] [Google Scholar]
- Hercend T., Meuer S., Brennan A., Edson M. A., Acuto O., Reinherz E. L., Schlossman S. F., Ritz J. Identification of a clonally restricted 90 kD heterodimer on two human cloned natural killer cell lines. Its role in cytotoxic effector function. J Exp Med. 1983 Nov 1;158(5):1547–1560. doi: 10.1084/jem.158.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercend T., Meuer S., Reinherz E. L., Schlossman S. F., Ritz J. Generation of a cloned NK cell line derived from the "null cell" fraction of human peripheral blood. J Immunol. 1982 Sep;129(3):1299–1305. [PubMed] [Google Scholar]
- Hercend T., Reinherz E. L., Meuer S., Schlossman S. F., Ritz J. Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature. 1983 Jan 13;301(5896):158–160. doi: 10.1038/301158a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Lust J. A., Kumar V., Burton R. C., Bartlett S. P., Bennett M. Heterogeneity of natural killer cells in the mouse. J Exp Med. 1981 Aug 1;154(2):306–317. doi: 10.1084/jem.154.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Phillips J. H., Babcock G. F. NKP-15: a monoclonal antibody reactive against purified human natural killer cells and granulocytes. Immunol Lett. 1983 Mar;6(3):143–149. doi: 10.1016/0165-2478(83)90096-2. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Rumpold H., Kraft D., Obexer G., Böck G., Gebhart W. A monoclonal antibody against a surface antigen shared by human large granular lymphocytes and granulocytes. J Immunol. 1982 Oct;129(4):1458–1464. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Zarling J. M., Clouse K. A., Biddison W. E., Kung P. C. Phenotypes of human natural killer cell populations detected with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2575–2580. [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]