Abstract
Resuscitation of patients after hemorrhage often results in pulmonary inflammation and places them at risk for the development of acute respiratory distress syndrome. Our previous data indicate that macrophage-derived chemokine (MDC/CCL22) is elevated after resuscitation, but its direct role in this inflammatory response is unknown. MDC signaling through the C-C chemokine receptor type 4 (CCR4) is implicated in other pulmonary proinflammatory conditions, leading us to hypothesize that MDC may also play a role in the pathogenesis of lung inflammation following hemorrhage and resuscitation. To test this, C57BL/6 mice underwent pressure-controlled hemorrhage followed by resuscitation with lactated Ringer’s solution. Pulmonary inflammation and inflammatory cell recruitment were analyzed with histological staining, and serum- and tissue-level cytokines were measured by ELISA. Pulmonary inflammation and cell recruitment following hemorrhage and resuscitation were associated with systemic MDC levels. Inhibition of MDC via injection of a specific neutralizing antibody prior to hemorrhage and resuscitation significantly reduced pulmonary levels of the chemotactic cytokines KC, MIP-2, and MIP-1α as well as inflammatory cell recruitment to the lungs. Intravenous administration of recombinant MDC prior to resuscitation augmented pulmonary inflammation and cell recruitment. Histological evaluation revealed the expression of CCR4 within the bronchial epithelium, and in vitro treatment of activated bronchial epithelial cells with MDC resulted in production and secretion of neutrophil chemokines. The present study identifies MDC as a novel mediator of lung inflammation after hemorrhage and resuscitation. MDC neutralization may provide a therapeutic strategy to mitigate this inflammatory response.
Keywords: hemorrhagic shock, pulmonary neutrophil recruitment, CCR4, bronchial epithelium
INTRODUCTION
Hemorrhage followed by resuscitation initiates a cascade of systemic inflammatory events that often leads to multi-organ impairment including acute respiratory distress syndrome (ARDS) and delayed mortality (1). Models of hemorrhage have demonstrated that acute lung injury after resuscitation manifests as hypoxia, pulmonary edema, and polymorphonuclear neutrophil (PMN) infiltration into the pulmonary interstitium and alveolar space (2-4). The pathogenesis of acute lung inflammation following hemorrhage and resuscitation is the result of an inflammatory response mediated by proinflammatory cytokines, chemokines, and adhesion molecules (5, 6). For example, PMN recruitment to the lungs is regulated by the CXC class of chemokines—in particular, interleukin (IL)-8 or its functional murine homologues keratinocyte-derived chemokine (KC/CXCL1) and macrophage inflammatory protein (MIP)-2 (CXCL2) (7-9), which, in turn, are mediated by IL-1, tumor necrosis factor-alpha (TNF-α), and IL-6 (5, 10, 11). Using an established murine model of pressure-controlled hemorrhage, our laboratory recently demonstrated upregulation of several of these known proinflammatory mediators following resuscitation in addition to the previously unexamined cytokine macrophage-derived chemokine (MDC; CCL22) (4).
MDC binds to and signals through the C-C chemokine receptor type 4 (CCR4) and is known to function as a potent chemoattractant for CCR4-expressing Th2 lymphocytes, monocytes, monocyte-derived dendritic cells, and natural killer cells (12-14). The role of MDC in the type-2 inflammatory response is well established, especially in asthmatic patients in which antigen exposure leads to an up-regulation of the CCR4 ligands MDC and thymus-and activation-regulated chemokine (TARC/CCL17) as well as an accumulation of Th2 cells in the lungs (15). Previous reports also suggest that MDC may play an active role in proinflammatory responses, as increased levels of MDC have been detected in such Th1-mediated events as atherosclerosis (16), Crohn’s disease (17), cigarette smoke-induced pulmonary inflammation (18), endotoxemia (19, 20) and sepsis (21).
The role of MDC in the development of the lung inflammatory response to hemorrhage and resuscitation is unknown. Therefore, the goal of the present work was to investigate the relationship between MDC and pulmonary inflammation using a murine model of hemorrhage and resuscitation in combination with MDC neutralization and augmentation with recombinant MDC. Our findings suggest that MDC produced during hemorrhage and resuscitation participates in the regulation of pulmonary inflammation via mediation of inflammatory cell chemotaxis. Furthermore, evidence is provided for the involvement of CCR4+ bronchial epithelial cells in the production of proinflammatory cytokines after exposure to MDC.
MATERIALS AND METHODS
Hemorrhage and resuscitation
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati Medical Center. Male C57BL/6 mice were anesthetized with intraperitoneal pentobarbital (0.1 mg/gram body weight) and hemorrhaged as previously described (4). Briefly, mice underwent femoral artery cannulation followed by a 10 min equilibration period, a 60 min period of hemorrhage, and 20 min period of fluid resuscitation. During hemorrhage, blood was withdrawn in a controlled manner via the femoral artery to achieve a mean arterial blood pressure of 25 mmHg. Mice were maintained at a pressure of 25 mmHg ± 5 mmHg for 60 min followed by resuscitation with lactated Ringer’s solution (LR) to a mean arterial blood pressure of 80 mmHg ± 5 mmHg for 20 min. Sham animals underwent identical femoral artery cannulation and monitoring for 90 min, but were neither hemorrhaged nor resuscitated. Mice were then decannulated, monitored and sacrificed at specified time intervals. Each experimental and control group consisted of four animals per treatment and time point.
MDC neutralization and recombinant MDC treatment experiments
For neutralization studies, a polyclonal goat anti-mouse MDC antibody (10 μg, R&D Systems, Wiesbaden, Germany) was administered by intraperitoneal injection 2 hours prior to the start of hemorrhage to ensure adequate systemic and pulmonary availability of the antibody at the onset of hemorrhage. Control mice were treated with a polyclonal goat IgG molecule (10 μg, R&D Systems). Subsequently, mice were hemorrhaged and resuscitated as described. In separate studies, mice were hemorrhaged and injected intravenously with 1 μg of recombinant mouse MDC (R&D Systems) prior to resuscitation.
Cytokine quantification in serum and lung homogenates
Murine blood was collected via cardiac puncture at intervals after hemorrhage and resuscitation. Serum was separated from cellular components by centrifugation and analyzed via enzyme-linked immunosorbent assay (ELISA) for levels of MDC (R&D Systems) according to manufacturer’s instructions. Lungs were snap frozen in liquid nitrogen at the time of collection, then protein was extracted with tissue extraction buffer supplemented with Soybean Trypsin Inhibitor (0.1 mg/mL, Sigma), PMSF (2 mM, Sigma), Complete Tabs (Roche), and a protease inhibitor cocktail of leupeptin, aprotinin, and pepstatin (all 1 mg/mL). Cytokine levels were measured by ELISA (Quansys Biosciences, Logan, Utah).
Lung histology and immunostaining
To assess lung inflammation, the left lung was infused via the mainstem bronchus with neutral buffered formalin at 4 h after resuscitation and harvested. Following fixation, tissue specimens were embedded in paraffin, sectioned and stained. After hematoxylin and eosin staining, lungs were evaluated with light microscopy. Inflammatory cells were identified using a specific monoclonal rat anti-mouse Ly-6B.2 alloantigen antibody (1:500, AbD Serotec, Raleigh NC) following antigen retrieval. For detection, horseradish peroxidase (HRP; BD Pharmingen, San Diego, CA) and Ultravision Detection System-DAB Plus Substrate System (Fremont, CA) were used. Inflammatory cells were quantified by blindly counting positively stained cells within ten high-powered fields per mouse.
In order to determine CCR4 expression in pulmonary tissue, sections were stained with a polyclonal antibody against mouse CCR4 (1:1000, AbCam, Cambridge, MA) and DAB staining. CCR4 expression was identified on cultured human bronchial epithelial cells with an anti-human CCR4 monoclonal antibody (1:100, R&D Biosystems) and an AlexaFluor®-555 secondary antibody (1:1000, Life Technologies, Grand Island, NY). For CCR4 immunostaining, IgG controls were used to confirm antibody specificity.
In vitro experiments
Normal human bronchial epithelial cells (hBEC) were purchased and cultured according to manufacturer recommendations (Clonetics™; Lonza, Walkersville, MD). Cells were maintained in Clonetics™ Bronchial Epithelial Growth Medium (BEGM™, Lonza). Prior to treatment, cells were seeded at 10,000 cells/cm2 and grown to confluence on tissue culture plates. Confluent cells were treated with 100 ng/mL recombinant human MDC (R&D Systems) with and without 20 ng/mL recombinant human TNF-α (R&D Systems). After 24 h of treatment, culture media was collected and cytokine levels analyzed by multiplex ELISA (Quansys Biosciences).
Statistical analyses
Data were assessed for normality and equal variances and analyzed using a Student’s t-test, analysis of variance (ANOVA) with Student-Newman-Keuls post-hoc tests or Kruskal-Wallis test. Normally distributed data are reported as mean + standard deviation (SD). Statistical analyses were performed using SigmaPlot 11 software (Systat Software, Chicago, IL).
RESULTS
Hemorrhage and resuscitation leads to increased serum MDC levels, pulmonary inflammation, and inflammatory cell infiltration
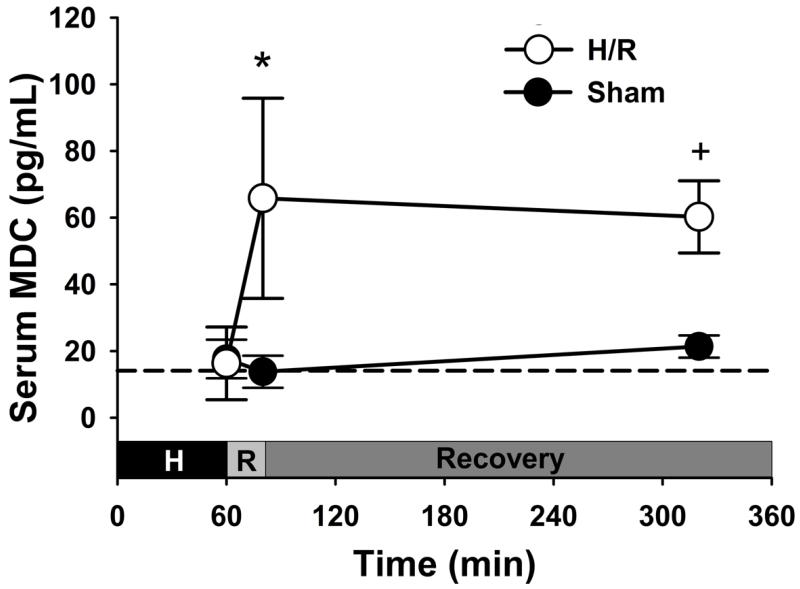
Systemic levels of MDC following hemorrhage and resuscitation were significantly increased over baseline and sham mice and remained elevated throughout the 4h post-resuscitation period (Figure 1). Consistent with previous studies (2-4), pulmonary inflammation was increased following hemorrhage and resuscitation as demonstrated by thickening of the alveolar walls and the presence of inflammatory cells. Histological evidence of increased inflammatory cell recruitment in resuscitated mice was confirmed by examination with a Ly-6B.2 stain as well as quantification of positively-stained cells (see Supplemental Figure 1, Supplemental Digital Content 1, at http://links.lww.com/SHK/A228; Hemorrhage and resuscitation (H/R) results in lung inflammation and inflammatory cell recruitment. (A-D) Representative micrographs of lung tissue 4h post-resuscitation stained with hematoxylin and eosin (A-B) and anti-mouse Ly-6B.2 (C-D) for inflammatory cell identification. Mice hemorrhaged and resuscitated (B,D) demonstrated increased inflammation and inflammatory cell infiltration as compared with sham mice (A,C). Scale bars represent 200μm. (E) Quantification of Ly-6B.2 stained cells confirmed increased pulmonary recruitment following H/R. *p<0.001 versus Sham.). Together, these data indicate that hemorrhage and resuscitation results in elevated serum MDC levels, inflammatory cell recruitment to the lungs, and acute lung inflammation.
Figure 1. Serum MDC levels are significantly elevated following hemorrhage and resuscitation (H/R).
Compared to Sham animals, elevated MDC levels were detected in the serum of mice immediately following resuscitation with lactated Ringer’s solution and for 4h post-resuscitation. Dashed line represents the baseline level of serum MDC in naive (untreated) mice. *p<0.05 and +p<0.01 versus corresponding Sham time point.
Lung inflammation following hemorrhage and resuscitation is attenuated by neutralization of endogenous MDC and exacerbated by treatment with exogenous MDC
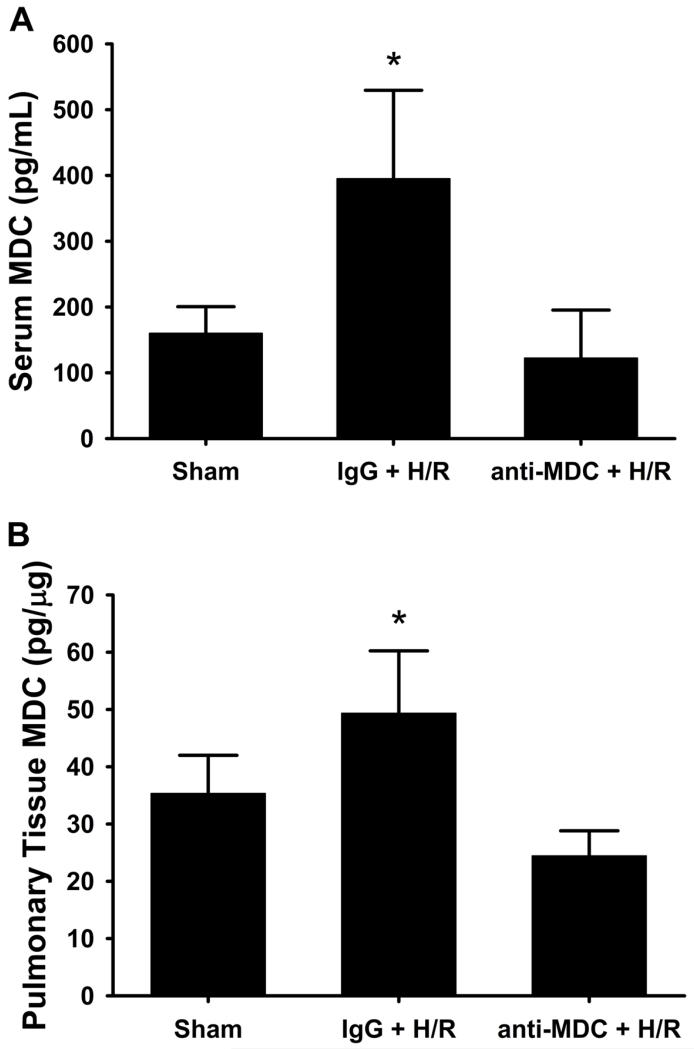
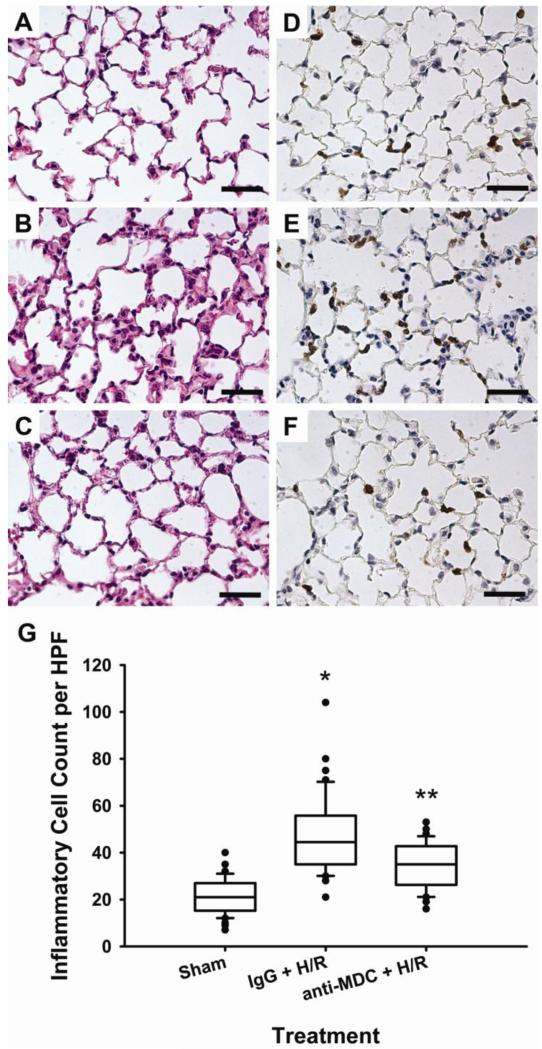
To more directly investigate the relationship between elevated levels of MDC after hemorrhage and resuscitation and pulmonary inflammation, mice were treated with a control (IgG) or MDC-neutralizing antibody prior to hemorrhage. Neutralization of MDC prior to hemorrhage led to decreased serum (Figure 2A) and pulmonary (Figure 2B) MDC levels, attenuated pulmonary inflammation (Figure 3A-C), and decreased inflammatory cell recruitment (Figure 3D-G).
Figure 2. Neutralization of MDC reduces (A) systemic and (B) pulmonary levels of MDC following hemorrhage and resuscitation (H/R).
Mice treated with an IgG control antibody prior to hemorrhage and resuscitation (IgG+H/R) demonstrated elevated levels of MDC 4h after resuscitation. Pre-treatment with a specific MDC neutralizing antibody (anti-MDC+H/R) resulted in post-resuscitation MDC levels equivalent to Sham levels. Data are presented as amount of MDC per mL of serum (A) and amount of MDC per microgram of tissue protein (B). *p<0.05 versus all groups.
Figure 3. Neutralization of MDC reduces pulmonary inflammation and inflammatory cell recruitment following hemorrhage and resuscitation (H/R).
Representative micrographs of lung tissue stained with hematoxylin and eosin (A-C) and anti-mouse Ly-6B.2 (D-F) for inflammatory cell identification. Sham mice (A, D) and mice injected with an anti-MDC neutralizing antibody prior to hemorrhage (C, F) demonstrated less pulmonary edema and cell infiltration than mice receiving the IgG antibody control (B, E). (G) Quantification of positively-stained cells confirmed decreased pulmonary inflammatory cell recruitment following neutralization of MDC. **p<0.05 versus all groups and *p<0.05 versus Sham.
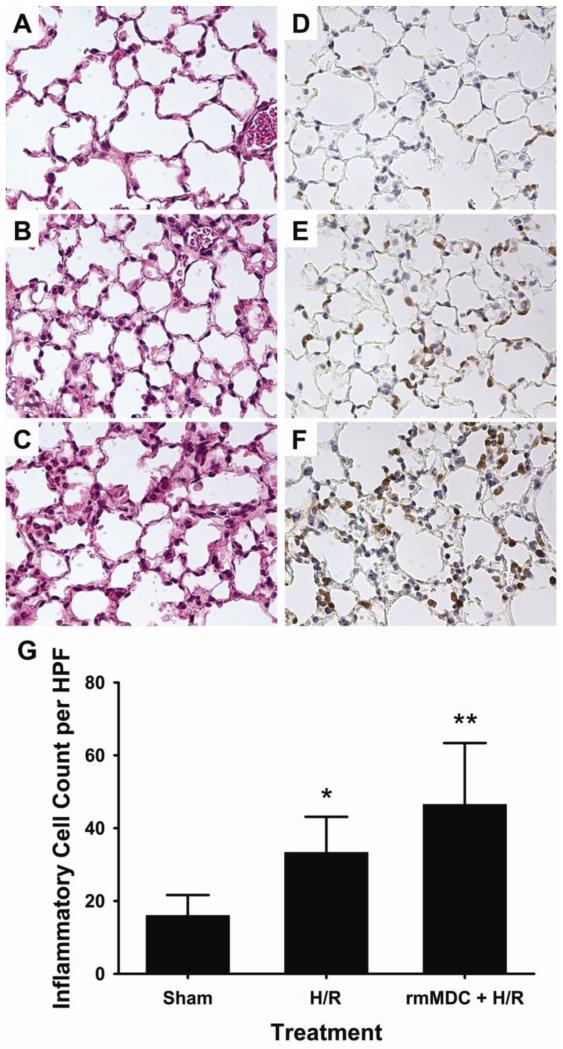
In order to evaluate whether augmenting MDC levels would exacerbate lung inflammation after hemorrhage and resuscitation, recombinant murine MDC was administered intravenously after hemorrhage and prior to resuscitation. This experiment revealed that additional MDC further increased pulmonary inflammation as compared to hemorrhage and resuscitation alone (Figure 4). MDC administered to normal non-hemorrhaged mice did not lead to pulmonary recruitment of inflammatory cells or pulmonary inflammation (see Supplemental Figure 2, Supplemental Digital Content 1, at http://links.lww.com/SHK/A228, Intravenous injection of MDC does not lead to increased pulmonary cell recruitment in normal (i.e. non-hemorrhaged) mice. *p<0.05 versus PBS.). Together, these findings suggest MDC plays an important role in the pathogenesis of acute lung inflammation in the setting of hemorrhage and resuscitation.
Figure 4. Recombinant mouse MDC (rmMDC) exacerbates lung inflammation following hemorrhage and resuscitation (H/R).
Representative micrographs of lung tissue stained with hematoxylin and eosin (A-C) and anti-mouse Ly-6B.2 (D-F) for inflammatory cell identification. The lungs from mice injected with rmMDC prior to resuscitation (C,F) had substantial interstitial tissue edema and cell infiltration as compared to sham mice (A,D) and mice resuscitated with lactated Ringer’s solution only (B,E). (G) Quantification of positively-stained cells confirmed the effect of rmMDC on increased inflammatory cell recruitment. **p<0.001 versus all groups and *p<0.001 versus Sham
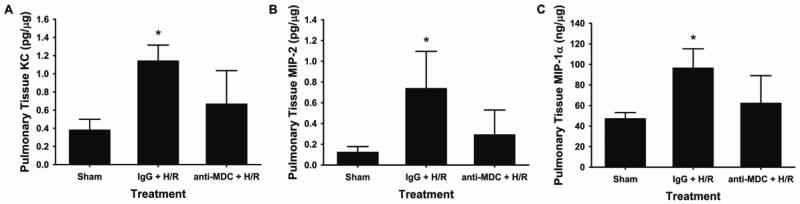
MDC is a known chemoattractant for CCR4+ monocytes; however, there is no known role for the direct involvement of MDC in mediating neutrophil chemotaxis (12). In order to determine if MDC may be acting upstream from known neutrophil chemoattractants to mediate pulmonary infiltration of neutrophils, the effect of MDC neutralization on pulmonary expression of the neutrophil chemokines KC, MIP-2, and MIP-1 was determined. Mice underwent hemorrhage and resuscitation with addition of either IgG control or anti-MDC antibody. Lung tissue was harvested and tissue levels of KC, MIP-2, and MIP-1 were determined by ELISA. Each of these mediators of neutrophil chemotaxis was increased after hemorrhage and resuscitation but decreased after pre-treatment with a neutralizing antibody to MDC (Figure 5). These data suggest that MDC may act to increase neutrophil tissue infiltration after hemorrhage and resuscitation as a secondary effect by altering levels of these chemokines.
Figure 5. Neutralization of MDC results in decreased pulmonary levels of the chemoattractant cytokines (A) keratinocyte-derived chemokine (KC), (B) macrophage inflammatory protein 2 (MIP-2), and (C) MIP-1α 2h after hemorrhage and resuscitation (H/R).
Mice pre-treated with MDC neutralizing antibody (anti-MDC+H/R) prior to resuscitation had attenuated levels of chemokines compared to mice receiving the IgG antibody control (IgG+H/R). Data are presented as amount of cytokine per microgram of tissue protein. *p<0.05 versus all groups.
Bronchial epithelial cells express the MDC receptor CCR4 and produce IL-8 under proinflammatory conditions
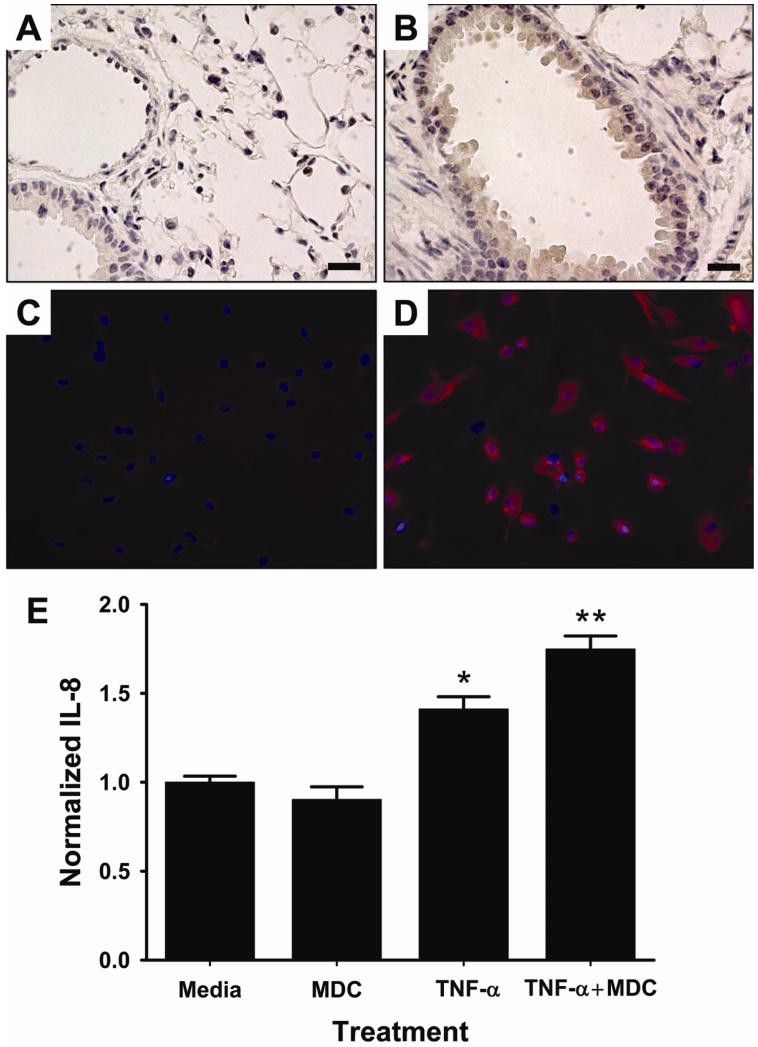
Taken together, our in vivo data suggest that hemorrhage and resuscitation results in increased circulating MDC levels, leading to increased pulmonary chemokine levels with resultant chemotaxis of inflammatory cells into the pulmonary parenchyma. In order to identify potential target cells for MDC in the lung that may in turn mediate inflammatory cell infiltration, we evaluated lung expression of CCR4 with a monoclonal antibody specific for this receptor (Figure 6). Positive CCR4 staining was observed most strongly within the bronchial epithelium (Figure 6B), suggesting that these cells may participate in MDC-mediated acute lung inflammation after hemorrhage and resuscitation. No differences in pulmonary CCR4 expression were observed in Sham mice versus those exposed to hemorrhage and resuscitation, suggesting that the inflammatory response to hemorrhage did not alter CCR4 expression (data not shown).
Figure 6. Bronchial epithelial cells express the MDC receptor CCR4 and produce IL-8 under proinflammatory conditions in response to MDC.
(A) Minimal background staining was observed in paraffin-embedded sections of mouse lung tissue treated with the IgG control antibody. (B) The receptor CCR4 was identified in lung tissue stained with an anti-mouse polyclonal CCR4 antibody and DAB staining. Scale bars represent 200μm. (C) No staining was observed in cultured human bronchial epithelial cells with the IgG control antibody. (D) CCR4 was identified on human bronchial epithelial cells (hBEC) using an anti-human CCR4 primary antibody and an AlexaFluor®-555 secondary antibody. (E) In culture, hBEC did not increase production of IL-8 in response to MDC treatment alone. Treatment of hBEC with the proinflammatory mediator TNF-α increased IL-8 expression levels above untreated (media) controls, and concurrent treatment with TNF-α and MDC resulted in significantly more IL-8 production as compared to TNF-α alone. *p<0.001 versus Media and MDC and **p<0.01 versus TNF-α.
Our data suggest that MDC regulates pulmonary levels of key chemokines, including KC, MIP-2, and MIP-1 (Figure 5) and that the CCR4 receptor for MDC is present on murine bronchial epithelial cells (Figure 6B). In order to examine the possibility that bronchial epithelial cells may directly respond to MDC stimulation and to verify that the signaling mechanisms exist in human cells, we cultured primary human bronchial epithelial cells (hBEC). In culture, these cells strongly express the CCR4 receptor (Figure 6D). Treatment of these cells with MDC did not result in increased production of the chemokine IL-8, the human analogue of KC and MIP-2 (Figure 6E). In order to determine the possible role of MDC in IL-8 production in hBEC under proinflammatory conditions, we treated the cells with TNF-α. TNF-α has been previously shown to regulate pulmonary cytokine production and inflammatory lung injury following hemorrhage and resuscitation (5, 11). In our model, we found that TNF-α was elevated as early as 30 minutes after hemorrhage and resuscitation (see Supplemental Figure 3 Supplemental Digital Content 1, at http://links.lww.com/SHK/A228; Serum levels of TNF- are elevated 30 min after hemorrhage and resuscitation with lactated Ringer’s (LR) solution. *p<0.05 versus Sham.). Therefore, TNF-α was used as an inflammatory stimulus to evaluate the responsiveness of hBEC to MDC cultured under proinflammatory conditions. As expected, treatment of hBEC with TNF-α was associated with increased IL-8 production (Figure 6E). Concurrent treatment of hBEC with TNF-α and MDC resulted in increased IL-8 production over treatment of TNF-α alone (Figure 6E), suggesting that under proinflammatory conditions hBEC cells may produce and secrete chemotactic cytokines in response to MDC.
DISCUSSION
In the present study, we have described a novel role for MDC in mediating the proinflammatory response leading to pulmonary inflammation following hemorrhage and resuscitation. Our data demonstrate that systemic and pulmonary levels of MDC after resuscitation correlate with pulmonary infiltration of inflammatory cells and lung inflammation, a response that is attenuated by neutralization of MDC and exacerbated by the addition of recombinant MDC. Investigation into the mechanism of MDC’s role in this proinflammatory response revealed that alterations in MDC levels directly correlate with pulmonary levels of the chemoattractant cytokines KC, MIP-2 and MIP-1α. Histological evaluation revealed that CCR4 is expressed within the bronchial epithelium, and in vitro studies demonstrated that cultured human bronchial epithelial cells express the CCR4 receptor and produce IL-8 in response to MDC treatment under proinflammatory conditions. Together, this work demonstrates a previously unidentified role for MDC in regulating lung inflammation following hemorrhage and resuscitation.
MDC and its only known receptor, CCR4, are best characterized for their roles in the recruitment of Th2 cells to sites of allergic inflammation (15, 22, 23) and have thus been previously regarded as mediators of chronic inflammation. A few previous reports suggest that MDC may also play a role in more acute inflammatory processes (16-19, 21). For instance, MDC released by alveolar macrophages is implicated for its involvement in cigarette-smoke induced pulmonary inflammation via signaling of the CCR4+ bronchial epithelium to promote pulmonary recruitment of Th1 cells (18). In sepsis, endogenous MDC within the peritoneum is associated with increased recruitment of peritoneal macrophages resulting in enhanced bacterial clearance and survival (21). High levels of MDC mRNA are expressed in some human arteries with advanced atherosclerotic lesions (16) and in the inflamed intestinal mucosa of Crohn’s disease patients (17). MDC-CCR4 signaling has also been implicated in regulation of the inflammatory response observed during endotoxemia (19, 20). Together, these reports highlight the potential role of MDC in mediating the acute inflammatory response in a variety of diseases/disorders and support the concept that MDC may differentially contribute to Th1- or Th2-mediated events depending on the physiologic setting. Our current data implicate MDC in mediating a pulmonary inflammatory response that is observed in the setting of hemorrhage and resuscitation but absent under normal (i.e. non-hemorrhaged) conditions.
Our current and prior work indicate that hemorrhage and resuscitation results in increased systemic levels of the chemokine MDC (4); however, the source of MDC in these studies is unknown. The relatively high serum, as opposed to pulmonary, MDC concentrations suggest that the lung is not the primary source of MDC production under these conditions. MDC is constitutively produced by macrophages and monocyte-derived dendritic cells (12). In addition, natural killer cells, monocytes, and CD4 lymphocytes are capable of producing MDC after stimulation (23). It is plausible that these peripheral blood cells are responsible for the increased systemic levels of MDC observed in our studies following hemorrhage and resuscitation. In addition, potential pulmonary sources of MDC include alveolar macrophages and smooth muscle cells (18, 22), and these cell types may also contribute to the inflammatory reaction in the lung following hemorrhage (24).
Our data demonstrate that the MDC produced during hemorrhage and resuscitation is associated with an influx of inflammatory cells into the lung, but the exact identity of these cells is unknown at present. The antibody that we utilized in our experiments (Ly-6B.2, clone 7/4) has been shown to be specific for neutrophils (25), but a recent study has indicated that it also stains for inflammatory monocytes and some activated macrophages (26). Thus, in the setting of hemorrhage and resuscitation, MDC-mediated inflammatory cell chemotaxis may represent the direct pulmonary recruitment of CCR4+ monocytes and macrophages and/or the extravasation of neutrophils as an indirect response to MDC-mediated production of chemotactic cytokines by pulmonary epithelial cells (as implied by the data presented in Figures 5 and 6). MDC/CCR4 signaling in systemic inflammation has been previously established by a previous study (19), but the current work does not rule out the potential for intrapulmonary MDC/CCR4 signaling that may also contribute to inflammatory cell recruitment and lung inflammation.
Our data indicate that cultured human bronchial epithelial cells produce the chemokine IL-8 following stimulation with MDC and TNF-α an established regulator of pulmonary cytokine production and inflammatory lung injury following hemorrhage and resuscitation (11). These data suggest that hBEC are responsive to MDC in the proinflammatory conditions generated by TNF-α and that the cellular mechanisms responsible for our in vivo murine observations are also present in human cells. Data from other laboratories have also demonstrated the role of pulmonary epithelial cells in inflammatory cell recruitment during the development of lung inflammatory injury (2, 27-29). In a rat model of hemorrhagic shock, Hierholzer et al. (2) demonstrated the involvement of bronchoepithelial cells in the development of pulmonary injury via the production of granulocyte colony-stimulating factor (G-CSF), a potent cytokine involved in neutrophil chemotaxis and activation. Human bronchial epithelial cells have also been shown to produce inflammatory mediators in response to particulate matter exposure such as that present in ambient air pollution (27, 28). In addition, alveolar type II epithelial cells were shown to produce the chemokines KC and MIP-2 in response to alveolar macrophage-produced TNF-α (29). Our data confirm the presence of CCR4 within the bronchial epithelium (Figure 6B) and support the notion that MDC may target pulmonary epithelial cells and mediate neutrophil chemotaxis via the production of chemokines like KC, MIP-1, and MIP-2 (Figure 6E). Our data, however, do not exclude the involvement of other cell types, including type I or type II pneumocytes, in mediating pulmonary cytokine production and/or lung inflammatory injury following hemorrhage and resuscitation, and future work is needed to evaluate these cell types in terms of CCR4 expression and responsiveness to MDC. Furthermore, there is some evidence to suggest that MDC may serve as a ligand for receptors other than CCR4 since MDC modified to block its interaction with CCR4 still showed appreciable chemotactic activity for monocytes (30, 31). Ultimately, however, the identities of alternative receptors for MDC have not been defined, and further research is needed to consider whether CCR4-independent signaling mechanisms are involved in MDC’s mediation of pulmonary inflammation following hemorrhage and resuscitation.
Previously, our group and others have demonstrated an integral relationship between resuscitation strategy and clinical outcome following hemorrhagic shock (4, 32-34). These works provide evidence that resuscitative fluids may differentially modulate the systemic inflammatory response syndrome that ensues following hemorrhage and subsequent resuscitation. The resuscitative approach for the current work involved the use of LR solution based on current Advanced Trauma Life Support (ATLS) guidelines and common practice among trauma/surgical centers worldwide. The outcomes of this work highlight MDC as a mediator of pulmonary inflammation, but these conclusions are limited to the physiologic setting that is present following hemorrhage and subsequent resuscitation with LR. Given the significance of the findings and potential for therapeutic intervention, additional investigations are warranted to determine the role of MDC in modulating inflammation following the use of other resuscitative approaches, such as those utilizing colloidal solutions or blood-based products.
In summary, our data provide evidence for a novel role of MDC in regulating the proinflammatory response in lung following hemorrhage and resuscitation. We demonstrate for the first time that MDC is upregulated in response to fluid resuscitation and is capable of mediating inflammatory cell trafficking and inflammation, at least in part through regulation of chemoattractant cytokines. Although the pathogenesis of lung inflammation is complex, our data suggest that interventions to neutralize MDC are a potential method to attenuate lung inflammation after hemorrhage and resuscitation.
Supplementary Material
Supplemental Figure 1. Hemorrhage and resuscitation (H/R) results in lung inflammation and inflammatory cell recruitment. (A-D) Representative micrographs of lung tissue 4h post-resuscitation stained with hematoxylin and eosin (A-B) and anti-mouse Ly-6B.2 (C-D) for inflammatory cell identification. Mice hemorrhaged and resuscitated (B,D) demonstrated increased inflammation and inflammatory cell infiltration as compared with sham mice (A,C). Scale bars represent 200μm. (E) Quantification of Ly-6B.2 stained cells confirmed increased pulmonary recruitment following H/R. *p<0.001 versus Sham.
Supplemental Figure 2. Intravenous injection of MDC does not lead to increased pulmonary cell recruitment in normal (i.e. non-hemorrhaged) mice. *p<0.05 versus PBS.
Supplemental Figure 3. Serum levels of TNF- are elevated 30 min after hemorrhage and resuscitation with lactated Ringer’s (LR) solution. *p<0.05 versus Sham.
Acknowledgments
Source of Funding
This research was supported by the following grants: NIH K08GM088589 and NIH T32GM08478.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34(6):397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 2.Hierholzer C, Kelly E, Tsukada K, Loeffert E, Watkins S, Billiar TR, Tweardy DJ. Hemorrhagic shock induces G-CSF expression in bronchial epithelium. Am J Physiol. 1997;273(5 Pt 1):L1058–64. doi: 10.1152/ajplung.1997.273.5.L1058. [DOI] [PubMed] [Google Scholar]
- 3.Makley AT, Goodman MD, Belizaire RM, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Damage control resuscitation decreases systemic inflammation after hemorrhage. J Surg Res. 2012;175(2):e75–82. doi: 10.1016/j.jss.2011.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makley AT, Goodman MD, Friend LA, Deters JS, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. 2010;68(2):305–11. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 42(4):640–51. [PubMed] [Google Scholar]
- 6.van Merus M, Wulfert FM, Knol AJ, De Haes A, Houwertjes M, Aarts LP, Molema G. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock. 2008;29(2):291–9. doi: 10.1097/SHK.0b013e318145a7c1. [DOI] [PubMed] [Google Scholar]
- 7.Lee BH, Lee TJ, Jung JW, Oh DJ, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. The Role of Keratinocyte-derived Chemokine in Hemorrhage-induced Acute Lung Injury in Mice. Journal of Korean Medical Science. 2009;24(5):775–781. doi: 10.3346/jkms.2009.24.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1137–45. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 9.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003;19(4):358–65. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hierholzer C, Kalff JC, Omert L, Tsukada K, Loeffert JE, Watkins SC, Billiar TR, Tweardy DJ. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol. 1998;275(3 Pt 1):L611–21. doi: 10.1152/ajplung.1998.275.3.L611. [DOI] [PubMed] [Google Scholar]
- 11.Abraham E, Jesmok G, Tuder R, Allbee J, Chang YH. Contribution of tumor necrosis factor-alpha to pulmonary cytokine expression and lung injury after hemorrhage and resuscitation. Crit Care Med. 1995;23(8):1319–26. doi: 10.1097/00003246-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185(9):1595–604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273(3):1764–8. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) J Leukoc Biol. 2000;68(3):400–4. [PubMed] [Google Scholar]
- 15.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107(11):1357–64. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaves DR, Hakkinen T, Lucas AD, Liddiard K, Jones E, Quinn CM, Senaratne J, Green FR, Tyson K, Boyle J, Shanahan C, Weissberg PL, Gordon S, Yla-Hertualla S. Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus and activation-regulated chemokine, are expressed in human atherosclerotic lesions. Arteriosclerosis Thrombosis and Vascular Biology. 2001;21(6):923–929. doi: 10.1161/01.atv.21.6.923. [DOI] [PubMed] [Google Scholar]
- 17.Judge F, Alizadeh M, Boissier C, Chantry D, Siproudhis L, Corbinals S, Quelvennec E, Dyard F, Campion J-P, Gosselin M, Bretagne J-F, Semana G, Heresbach D. Quantitation of chemokines (MDC, TARC) expression in mucosa from Crohn’s disease and ulcerative colitis. Euro Cyto Network. 2001;12(3):468–77. [PubMed] [Google Scholar]
- 18.Ritter M, Goggel R, Chaudhary N, Wiedenmann A, Jung B, Weith A, Seither P. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem Biophys Res Commun. 2005;334(1):254–62. doi: 10.1016/j.bbrc.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 19.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191(10):1755–64. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnier DI, Bailey SR, Schuster RM, Gangidine MM, Lentsch AB, Pritts TA. Proinflammatory chemokines in the intestinal lumen contribute to intestinal dysfunction during endotoxemia. Shock. 2012;37(1):63–9. doi: 10.1097/SHK.0b013e31823cbff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J Immunol. 2000;164(10):5362–8. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AEI, Coyle AJ, Gearing D, Gutierrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. Journal of Immunology. 1999;163(1):403–411. [PubMed] [Google Scholar]
- 23.Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG., 3rd STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161(9):5027–38. [PubMed] [Google Scholar]
- 24.Niesler U, Palmer A, Radermacher P, Huber-Lang MS. Role of Alveolar Macrophages in the Inflammatory Response After Trauma. Shock. 2014 doi: 10.1097/SHK.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18(3):229–39. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 26.Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leukoc Biol. 2010;88(1):169–80. doi: 10.1189/jlb.0809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol. 2002;27(1):34–41. doi: 10.1165/ajrcmb.27.1.4787. [DOI] [PubMed] [Google Scholar]
- 28.Ishii H, Fujii T, Hogg JC, Hayashi S, Mukae H, Vincent R, van Eeden SF. Contribution of IL-1 beta and TNF-alpha to the initiation of the peripheral lung response to atmospheric particulates (PM10) Am J Physiol Lung Cell Mol Physiol. 2004;287(1):L176–83. doi: 10.1152/ajplung.00290.2003. [DOI] [PubMed] [Google Scholar]
- 29.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L105–13. doi: 10.1152/ajplung.00470.2006. [DOI] [PubMed] [Google Scholar]
- 30.Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, De Clercq E, Schols D, Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161(6):2672–5. [PubMed] [Google Scholar]
- 31.Proost P, Struyf S, Schols D, Opdenakker G, Sozzani S, Allavena P, Mantovani A, Augustyns K, Bal G, Haemers A, Lambeir AM, Scharpe S, Van Damme J, De Meester I. Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Biol Chem. 274(7):3988–93. doi: 10.1074/jbc.274.7.3988. [DOI] [PubMed] [Google Scholar]
- 32.Gunter OL, Jr., Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–34. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 34.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Hemorrhage and resuscitation (H/R) results in lung inflammation and inflammatory cell recruitment. (A-D) Representative micrographs of lung tissue 4h post-resuscitation stained with hematoxylin and eosin (A-B) and anti-mouse Ly-6B.2 (C-D) for inflammatory cell identification. Mice hemorrhaged and resuscitated (B,D) demonstrated increased inflammation and inflammatory cell infiltration as compared with sham mice (A,C). Scale bars represent 200μm. (E) Quantification of Ly-6B.2 stained cells confirmed increased pulmonary recruitment following H/R. *p<0.001 versus Sham.
Supplemental Figure 2. Intravenous injection of MDC does not lead to increased pulmonary cell recruitment in normal (i.e. non-hemorrhaged) mice. *p<0.05 versus PBS.
Supplemental Figure 3. Serum levels of TNF- are elevated 30 min after hemorrhage and resuscitation with lactated Ringer’s (LR) solution. *p<0.05 versus Sham.