Abstract
Disgust is a prototypical type of negative affect. In animal models of excessive disgust, only a few brain sites are known in which localized dysfunction (lesions or neural inactivations) can induce intense ‘disgust reactions’ (e.g., gapes) to a normally pleasant sensation such as sweetness. Here we aimed to map forebrain candidates more precisely to identify where either local neuronal damage (excitotoxin lesions) or local pharmacological inactivation (muscimol-baclofen microinjections) caused rats to emit excessive sensory disgust reactions to sucrose. Our study compared subregions of nucleus accumbens shell, ventral pallidum, lateral hypothalamus and adjacent extended amygdala. Results indicated the posterior half of ventral pallidum to be the only forebrain site where intense sensory disgust gapes to sucrose were induced by both lesions and temporary inactivations (this site was previously identified as a hedonic hotspot for enhancements of sweetness ‘liking’). By comparison, for the nucleus accumbens, temporary GABA inactivations in the caudal half of the medial shell also generated sensory disgust but lesions never did at any site. Further, even inactivations failed to induce disgust in the rostral half of accumbens shell (which also contains a hedonic hotspot). In other structures, neither lesions nor inactivations induced disgust as long as the posterior ventral pallidum remained spared. We conclude that the posterior ventral pallidum is an especially crucial hotspot for producing excessive sensory disgust by local pharmacological/lesion dysfunction. By comparison, the nucleus accumbens appears to segregate sites for pharmacological disgust induction and hedonic enhancement into separate posterior versus rostral halves of medial shell.
Keywords: Limbic, Ventral Forebrain, Brain reward system, Food intake, Hedonic
Introduction
Positive hedonic impact of reward is crucial to normal daily function. In absence of positive hedonic impact, anhedonia (loss of positive affect) or dysphoria (excessive negative affect) can characterize clinical affective disorders in humans.
A prototypical form of negative affect is disgust. Excessive disgust can occur in anxiety disorders, phobias, anorexia nervosa and obsessive-compulsive disorder (OCD) (Sprengelmeyer et al., 1997; Cisler et al., 2009; Olatunji et al., 2010; Weygandt et al., 2012). Sensory disgust has been suggested to be the prototypical and original form of this negative affect, but disgust is also a potentially complex negative emotion, which can occur as higher disgust, such as moral or aesthetic disgust. Both levels have been suggested to share the same evolutionary roots, and to have overlapping neural substrates that arose originally to mediate the sensory disgust of unpleasant tastes and smells (Rozin, 2000; Calder et al., 2001; Zald et al., 2002; Chapman & Anderson, 2012; Rozin & Haidt, 2013; Tybur et al., 2013).
Affective neuroscience experiments using animals have helped identify particular brain sites that can causally enhance the positive hedonic impact of sensory rewards, such as sweetness (Baldo & Kelley, 2007; Smith et al., 2010; Richard et al., 2013a). In particular, two interactive ‘hedonic hotspots’ have been identified as cubic-millimeter subregions: one in nucleus accumbens (NAc), in the rostrodorsal quadrant of NAc medial shell), and another in ventral pallidum (VP), in the posterior half. In those NAc or VP hotspots, opioid or related neurochemical stimulations cause increases in positive hedonic impact sweetness, measured as increased positive ‘liking’ reactions to sucrose (Peciña & Berridge, 2005; Smith & Berridge, 2005; Ho & Berridge, 2013; Castro & Berridge, 2014). ‘Liking’ here refers to objective orofacial expressions (e.g., lip licking) that are typically elicited by sweet tastes, which in rats are homologous to positive affective facial expressions elicited by sweetness in human infants and other primates (Steiner, 1973; Pfaffmann et al., 1977; Grill & Norgren, 1978a; Berridge, 2000; Steiner et al., 2001). Conversely negative sensory ‘disgust’ reactions are elicited by bitter tastes (e.g., gapes, headshakes, and chin rubs).
Do hedonic hotspots also play a special role in generating excessive ‘disgust’ that occurs under neuropathological conditions? Conceivably, neural dysfunction in hedonic hotspots might facilitate ‘disgust’, by impairing the capacity for opposing positive hedonic impact. Alternatively, disgust could arise from pathological dysfunction in separate brain sites, independent of hedonic-enhancing hotspots.
So far, hedonic hotspots of NAc and VP have been defined primarily by their capacity to enhance sensory ‘liking’ reactions, or to produce a gain of function for positive hedonic impact. Here we conversely examined the roles of these hotspots and nearby sites in producing a loss of positive hedonic function after brain lesion or inactivation, and replace ‘liking’ with excessive negative ‘sensory disgust, and compared their roles to those of other nearby subregions or structures, such as lateral hypothalamus and extended amygdala (Teitelbaum & Epstein, 1962; Cromwell & Berridge, 1993; Swanson, 2005; Zahm, 2006; Inui et al., 2007; Heimer et al., 2008; Thompson & Swanson, 2010; Zahm et al., 2013).
Materials and Methods
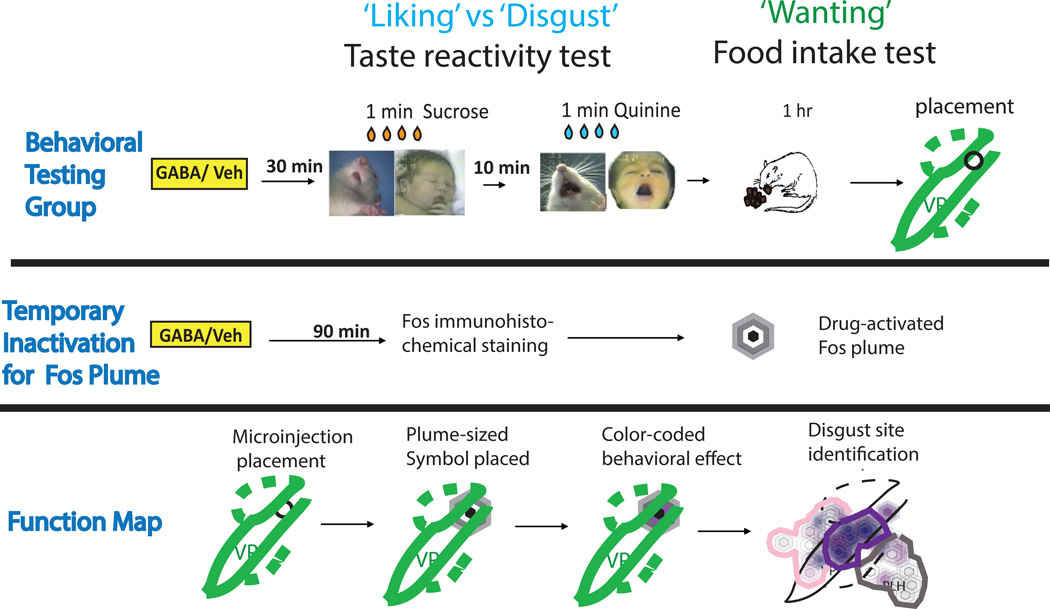
Specifically, we focused on mapping the replacement of positive ‘liking’ reactions to sweetness with intense negative ‘disgust’ reactions (e.g., gapes, headshakes, etc.) produced either by local excitotoxin lesions or by temporary pharmacological GABA inactivations of subregional sites in those structures. The affective taste reactivity test was used to assess affective ‘liking’ and ‘disgust’ orofacial reactions (Figure 1), and to identify which sites of damage or inactivation in forebrain regions caused positive hedonic reactions that are normally elicited by sweet tastes to be replaced by negative reactions such as gapes or headshakes.
Figure 1. Summary of experimental design.
Excitotoxin lesions and temporary GABAergic inactivation effects for NAc and VP were tested in separate groups of rats (VP groups shown in figure). Further, additional groups were used to assess maximal Fos plume size anatomically induced by GABAergic inactivation microinjections. Measurements of Fos plume radius and excitotoxin lesion radius were used to assign respective symbol sizes in maps. Histologically identified sites where disgust was produced by either lesions or inactivations were assessed based on brains from behaviorally-tested rats, and those sites were used to construct the respective maps of excessive disgust to sucrose taste.
Either permanent excitotoxin lesions or temporary inactivations by GABA agonist microinjections were made bilaterally in rats, targeted at sites in either: 1) ventral pallidum, 2) medial shell of nucleus accumbens, 3) lateral hypothalamus, or 4) extended amygdala (e.g., bed nucleus of stria terminalis). Sites were scattered across rats so as to fill targeted brain structures sufficiently for functional mapping, but kept as identical as possible within each rat’s bilateral placements, so that each rat individually-contributed symmetrical dysfunction at a particular bilateral site. Subregional sites were mapped histologically and specifically compared for behavioral effects on affective taste reactivity and on food intake. All experimental procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan and were carried out in accordance with the guidelines on animal care and use of the National Institutes of Health of the United States.
Surgery
Rats (n = 113 total; VP, LH and extended amygdala sites, n=94; NAc, n= 27) were anesthetized either with ketamine for microinjection cannula implantation or with methoxyflurane for excitotoxin lesions (80 mg/kg ketamine HCL, 10 mg/kg xylazine, and 0.5 mg/kg atropine sulfate; halothane induction in a chamber, followed by methoxyflurane during surgery inhaled through a ventilator system). Anesthetized rats were placed in a stereotaxic device with the mouth bar set either to flat for VP, LH or extended amygdala rats or to +5.0 mm above intra-aural zero for NAc rats. VP group coordinates were: anteroposterior (AP) from 0 to −1.2 mm relative to Bregma; mediolateral (ML), ± 3 mm; dorsoventral (DV), −5.5 mm. NAc group coordinates were AP from +2.4 to +3.4 mm ahead of bregma, ML +/− 1.0 mm, and DV from 5.7 to 6.0 mm below skull. Lateral hypothalamus and extended amygdala (BNST) group coordinates were AP from −1.2 to −1.8 behind bregma, ML +/− 2.0 mm, and DV −8.0 to 9.1 mm. Left and right bilateral coordinates were always identical to preserve symmetry within the same rat. Individual placements were staggered across rats so that these placements covered the structure or region of a structure (e.g., entire medial shell). Bilateral sites for excitotoxin infusions were always placed directly in the brain target, whereas sites for drug guide cannulae were always positioned 2.5 mm above the target (23 gauge; for subsequent inactivations).
Lesions
To induce permanent neuronal lesions while sparing fibers of passage, excitotoxin microinfusions were made during surgery of either ibotenic acid (15 ug in 1 ul 0.1M PB; in half the lesion-group; randomly assigned) or quinolinic acid (10 ug in 1 ul 0.1M PB; remaining half of group) or phosphate buffer vehicle alone as intact control condition (1 ul PB in control rats). Excitotoxin type was randomly assigned to rats, but functional effects turned out not to differ between ibotenic acid and quinolinic acid lesions (described below), which allowed eventual combination of behavioral data. The excitotoxin solution was microinjected through a stainless-steel injector cannula (29 gauge) connected to PE-20 tubing and a syringe pump at a rate of 1 ul during a period of 3 min. Microinjectors were left in place for an additional 5 min. After excitotoxin infusion, rats were maintained under halothane anesthesia for an additional 25 min. Diazepam (8mp/kg, i.p.) was given 5 min and 35 min after excitotoxin infusion to prevent seizures. In order to assess early effects of lesions, and so make assessments as comparable as possible to pharmacological inactivations, behavioral tests began 24–48 hours after lesions.
Microinjection cannulae
For rats in the pharmacological GABAergic inactivation groups, bilateral microinjection guide cannulae (23 ga, stainless steel) were embedded in an acrylic headcap anchored by skull screws. Stainless steel stylets (28 ga) were always kept inserted except during drug microinjections. Behavioral testing began 1 week after microinjection cannula implantation.
Oral cannulae
In the same surgery, all rats were additionally implanted with intraoral cannulae (PE-100 tubing) for subsequent delivery of sucrose or quinine solutions into the mouth for taste reactivity tests (Grill & Norgren, 1978a). Oral cannulae bilaterally entered the mouth lateral to the first maxillary molar, traveled beneath the zygomatic arch, and exited the dorsal head near the skull screws, where they were anchored to the headcap with dental cement. Oral cannulae did not disrupt normal eating or behavior.
After surgery, rats were given at least 7 days to recover from surgery before drug microinjection testing, and always had free access to moist cereal mash (Gerber’s baby cereal mixed with water), food pellets, and a water bottle in their home cages. During recovery after surgery, food intake was monitored by the measuring approximate amount of mash eaten each day, and by measuring body weights each day.
Aphagia monitoring and intubation feeding: Aphagia after lesions was defined as failure to eat more than 1 g chow pellets or cereal mash per day. Hypophagia was defined as failure to eat more than 5 g per day (but more than 1 g). Rats displaying aphagia or hypophagia, which lost >10 g body weight after VP or LH lesions were nourished and hydrated with supplementary intra-gastric intubations of a liquid diet, in amounts calibrated to body weight. The diet consisted of 6 to 12 ml sweetened condensed milk mixed with equal volume water in 3 daily meals. Intubation volume was gradually incremented to avoid excessive gastric distension. The first two meals consisted of 6 ml volume, the next two meals of 8 ml, the next two of 10 ml, and finally 12 ml from then on. Each aphagic/hypophagic rate received 1, 2 or 3 intubations per day, with the number of meals individually adjusted daily to prevent weight loss.
Induction of temporary inactivations
Approximately 30 min prior to a behavioral test, temporary inactivations were induced by bilateral microinjections of a mixture of GABAA and GABAB agonists (0.1ug/0.2 ul muscimol and 0.1ug/0.2 ul baclofen combined into 0.2 ul ACSF, per side), or of vehicle only as a within-subject control (0.2 ul ACSF alone), immediately before behavioral tests (Figure 1). Microinjections were made through a stainless-steel injector cannula (29 gauge) connected to PE-20 tubing and a syringe pump at a rate of 0.2 µl /min, while the rat was gently hand-held. Microinjector tips were kept in place for an additional 1 minute after end of infusion to avoid backflow. An additional group of control rats received only ACSF microinjections on all days, and never inactivations. For a particular rat designated for inactivation, the order of microinjections always was vehicle (ACSF) on the first day, and muscimol/baclofen on the second test day (VP and surrounding regions, n=35; NAc, n=15). This vehicle/drug order was chosen because our initial pilot observations indicated that GABA microinjections in VP often caused robust disgust reactions to tastes to persist for several days, and we did not wish to contaminate control vehicle tests with persistent aversion induced by previous GABAergic inactivations (persistence may have been either due to long-lasting neurobiological effects, or partly associative, if the chamber paired with intense GABAergic disgust induced on a recent day contributed as a CS toward a Pavlovian conditioned aversive response). However, our use of the separate group of vehicle/vehicle control rats that received vehicle on successive days allowed us to control for potential order effects (repeated vehicle microinjections never appeared to induce changes in taste reactivity or food intake from one test day to the next). Two minutes after microinjections were completed, stylets were inserted back to the cranial cannulae, and rats were placed into the transparent taste reactivity chamber with intraoral fluid delivery tubing attached (described in behavioral taste reactivity testing).
Behavioral taste reactivity testing
Prior to microinjections, rats were habituated in the taste reactivity chamber for 4 consecutive days for 30 minutes each, and received a mock injection of vehicle on the final habituation day.
For taste reactivity testing, on each test day tubing (PE-50 connected to a PE-10 delivery nozzle) was attached to an intraoral cannula to deliver solutions to the mouth. The other end of the tubing was attached to a syringe pump, which infused solutions at a rate of 1 ml/min during the one-minute test. Rats received sucrose solution (1%, 1 ml in 1 minute duration). Orofacial positive hedonic and negative aversive taste-reactivity responses were video recorded via an angled mirror placed underneath the transparent floor. Most rats also received a quinine infusion (3×10-4M, 1 minute duration) approximately 10 minutes after their sucrose infusion (VP, n=29 rats). As the primary goal of this study was to assess the positive hedonic impact of sucrose and its replacement by disgust, sucrose was always infused before quinine because hedonic ‘liking’ responses to sucrose are generally more vulnerable to contamination from an immediate earlier experience, whereas aversive ‘disliking’ responses to quinine are more robust.
Video scoring
Video files of neutral and aversive response patterns elicited by tastes were stored offline for subsequent slow-motion analysis (frame-by-frame to 1/10th speed using Observer software (Noldus, Netherlands). The scorer was always blind to the drug/vehicle contents, lesion status, and site placement of the rat during scoring (Berridge, 2000). Positive hedonic responses were rhythmic tongue protrusions, lateral tongue protrusions, and paw licks. Aversive or disgust responses were gapes, headshakes, face washes, forelimb flails, and chin rubs. Neutral responses (which are less consistently linked to hedonic/aversive taste valuation) included grooming, passive dripping of solution out of the mouth, and rhythmic mouth movement. A time-bin scoring procedure was used to ensure that various taste reactivity components contributed comparably to the final affective hedonic/aversive totals, even when components had different relative frequencies (Berridge, 2000). For example, rhythmic mouth movements, passive dripping of solution, paw licking, and grooming typically occur in long bouts and were thus scored in 5 s time bins (1 to 5 s equals one bout occurrence). Tongue protrusions, which occur in shorter bouts, were scored in 2 s time bins (0.2 – 2 s equals one bout score). Other taste reactivity components (lateral tongue protrusions, gapes, forelimb flails, head shakes, and chin rubs) typically occur as discrete events and were thus scored as single occurrences each time they occurred (e.g., one gape equals one score). Finally, individual totals were calculated for hedonic versus aversive categories by adding all response scores within an affective category for each rat. This total score was the sum of scores all positive hedonic reactions: lateral tongue protrusions, rhythmic tongue protrusions, and paw licks. Similarly, an aversive disgust score was the total sum of occurrences of gapes, headshakes, face washes, forelimb flails, and chin rubs.
Food intake test
A 1-hr food intake test was performed after each taste reactivity test in order to quantify voluntary food consumption (Figure 1). Rats were placed in a clean cage with bedding, chow pellets, palatable cereal mash, and a water bottle all freely available for 1 hour. Afterwards remaining amounts of water, cereal mash and chow pellets were re-weighed and subtracted from initial weights to compute amount consumed. During the test, rats were also videotaped for subsequent offline scoring of eating, locomotion, and other behaviors that occurred spontaneously during the 1-hr period.
Histology & lesion/inactivation mapping
To measure excitotoxin lesions, neuron loss was quantified and mapped using a technique for lesion mapping that counts neurons at specified locations surrounding the lesion center (Cromwell & Berridge, 1993; 1994; 1996), modified from fractionator techniques (Gundersen et al., 1988; Gundersen, 2002). To measure temporary neuronal inactivation induced by combined GABAA and GABAB agonist microinjections, suppression of Fos protein expression surrounding the microinjector tip was quantified and mapped by a related Fos plume technique that counts neurons expressing Fos at specified sites surrounding the microinjection center (Peciña & Berridge, 2005; Smith & Berridge, 2005; Richard & Berridge, 2011).
All rats received an overdose of sodium pentobarbital (0.2g/kg) prior to being transcardially perfused. Brains from rats that had been behaviorally tested were extracted and post-fixed with 10% formalin in 0.1 M phosphate buffer (PB) and then transferred to 30% sucrose in 0.1M PB, sectioned with a freezing microtome into 60 µm sections, and stained with cresyl violet. The locations of microinjection cannula tips were identified microscopically, and mapped onto a brain atlas for use in subsequent function mapping (Paxinos and Watson, 2008).
Mapping excitotoxin lesions via neuron loss
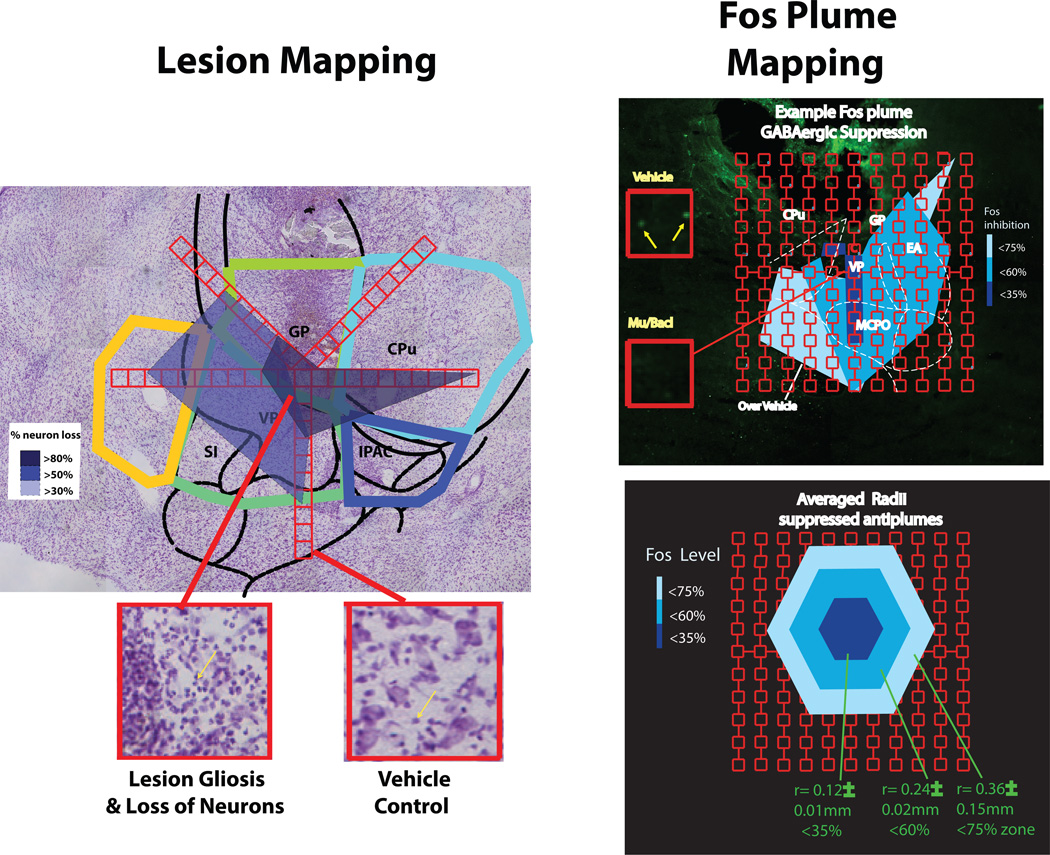
Baseline control values for neuronal density were first mapped in VP and NAc regions in normal rat brains at 6 levels along the AP axis (AP 0.3~0 mm; AP 0~-0.3 mm; AP −0.3~ −0.65 mm; AP-0.65~-1; AP −1~ −1.6 mm; AP after – 1.6 mm relative to bregma). Each AP slice section was divided into 5–7 targeted regions that included the ventral pallidum, and adjacent subareas of lateral hypothalamus, extended amygdala and globus pallidus (Cromwell & Berridge, 1993; 1994). Neuronal density was counted in contiguous sampling boxes (125 × 125 µm) at points on 5 axes emanating away from the center of excitotoxin injection (or of vehicle injection in control brains; Figure 2). For lesions, the percentage neuron death at each point was calculated relative to the baseline count at the same point in control brains.. Excitotoxin lesion blocks were deemed to have ‘intense neuron loss’ (>80% death) if fewer than 20% of the normal number of neurons remained in that block (compared to baseline norms for the same site obtained in control brains). ‘Moderate neuron loss’ was considered to be 20% to 50% of neurons survived, and ‘mild neuron loss’ was considered if more than 50% of normal neurons survived.
Figure 2. Lesion & Fos plume measurement.
Examples of how lesions or Fos plumes were measured, and sizes integrated with site data from behaviorally tested rats to create function maps for disgust induction. Excitotoxin lesion map (left). Neurons were counted in 125 × 125 µm blocks on 5 sampling arms (red) extending from the excitotoxin injection center, and compared to same location baseline counts measured control tissue that received only vehicle injection during surgery (Cromwell & Berridge, 1993; 1994). Example photo at bottom left shows sampling box of lesion, showing gliosis (yellow arrow) and loss of larger neurons, and photo of box from healthy control brain containing neurons (40X magnification). Colored areas in coronal slice above indicate zones of severe 80% neuron loss (dark-blue), 50% loss (sky-blue), and mild 30% loss (light-blue) neuron loss compared to healthy tissue at same locations. Right side shows at top an example Fos plume surrounding the tip of a microinjection of GABA agonist mixture (0.1µg muscimol, 0.1µg baclofen). Sampling was done in 50 × 50 µm blocks to count Fos-expressing neurons. Vehicle inset (Veh) shows baseline example of moderate Fos expression in a block after ACSF microinjection (10X magnification; green dots are Fos neurons). Mu/bacl inset shows example of suppressed Fos (i.e., antiplume) in equivalent block after GABAergic microinjection of muscimol and baclofen. Bottom shows the average radius of Fos antiplumes produced by muscimol-baclofen microinjections, with 3 zones for inhibition intensity, which used to assign the size of symbols in disgust maps.
Fos plume mapping
A separate group of rats received only one microinjection of GABA agonists or of vehicle 90 min prior to perfusion, and were processed for Fos plume mapping as previously described (Peciña & Berridge, 2005; Smith & Berridge, 2005; Richard & Berridge, 2011). Use of a separate group for Fos plume mapping avoids distortion by plume shrinkage that is gradually induced over a series of microinjections, and gives a more accurate measure of maximal spread of local drug impact (Richard & Berridge, 2011; Castro & Berridge, 2014). Brain slices were processed for Fos-like immunoreactivity using normal donkey serum, goat anti-c-fos (Santa Cruz Biotechnology), and donkey anti-goat Alexa Fluor 488 (InvitrogenTo visualize and quantify drug-induced Fos activities, Fos-labeled cells on tissue surface were visualized with 5x–40x magnification under the microscope followed by image processing (Figure 2). Sampling blocks (50 × 50 µm) were placed along seven radial arms emanating from the center of the microinjection site (45, 90, 135, 180, 225, 270, 315°) at 50 µm intervals (Richard & Berridge, 2011).
Statistical analyses
All behavioral analyses were two-tailed and α was always set at p<0.05. Paired samples t-tests or one-way ANOVAS with Bonferroni post-hoc test were used to test microinjection drug/vehicle effects, and between-subjects ANOVAs were used to compare lesion effects and anatomical site effects. For significant differences in behavioral taste reactivity scores, Cohen’s d statistic was used to calculate effect sizes.
Results
Overview
Our results suggest that the posterior half of VP, which contains its previously-identified hedonic hotspot, is also uniquely necessary to normal sucrose ‘liking’. We found that either localized lesions or temporary inactivations in the posterior VP produced excessive ‘disgust’ reactions to sweetness. By contrast, for the rostral half of NAc shell, although similarly containing a hedonic hotspot for opioid and related enhancements of positive ‘liking’ reactions, neither lesions nor inactivations produced disgust. Instead, only NAc sites in the posterior half of medial shell produced intense disgust (not the anterior half), and only after temporary inactivations (but not after lesions). Finally neither lesions nor inactivation sites anywhere in basal forebrain sites such as lateral hypothalamus or extended amygdala induced excessive ‘disgust’ reactions to sucrose taste, unless the posterior ventral pallidum was also impacted.
Disgust after excitotoxin lesions
Excitotoxin lesion maps
Excitotoxin microinjections produced roughly concentric spheres (1.6 mm total diameter) of neuronal death and elevated gliosis: an inner sphere of severe neuronal loss and gliosis that was 0.43 ± 0.04 mm in radius, surrounded by a middle sphere of 0.733 ± 0.041 mm radius of moderate neuron loss, surrounded by an outer sphere of 0.82 ± 0.03 mm radius of mild neuronal loss (n= 57; Figure 2). The inner severe zone was defined as containing only 20% the normal number of neurons counted at equivalent locations in normal control brains (i.e., 80% cell death). The zone of moderate neuron loss contained between 30% to 50% of the normal-control density of neurons (i.e., 50–70% cell death). The zone of mild neuron loss contained between 50% to 90% of the normal number of neurons (i.e., 10% to 50% neuron death compared to equivalent sites in control brains (Figure 2). Ibotenic acid lesions and quinolinic acid lesions did not differ detectably from each other in either radius or intensity of neuronal loss in NAc or VP, nor did the two brain structures vary systematically in their lesion sizes. As described below, evidence indicated that a damage threshold of approximately 65% to 80% neuronal loss was required to release excessive ‘disgust’ reactions at caudal VP sites, the only sites at which any lesion ever did.
NAc hotspot lesions: no release of ‘sensory disgust’
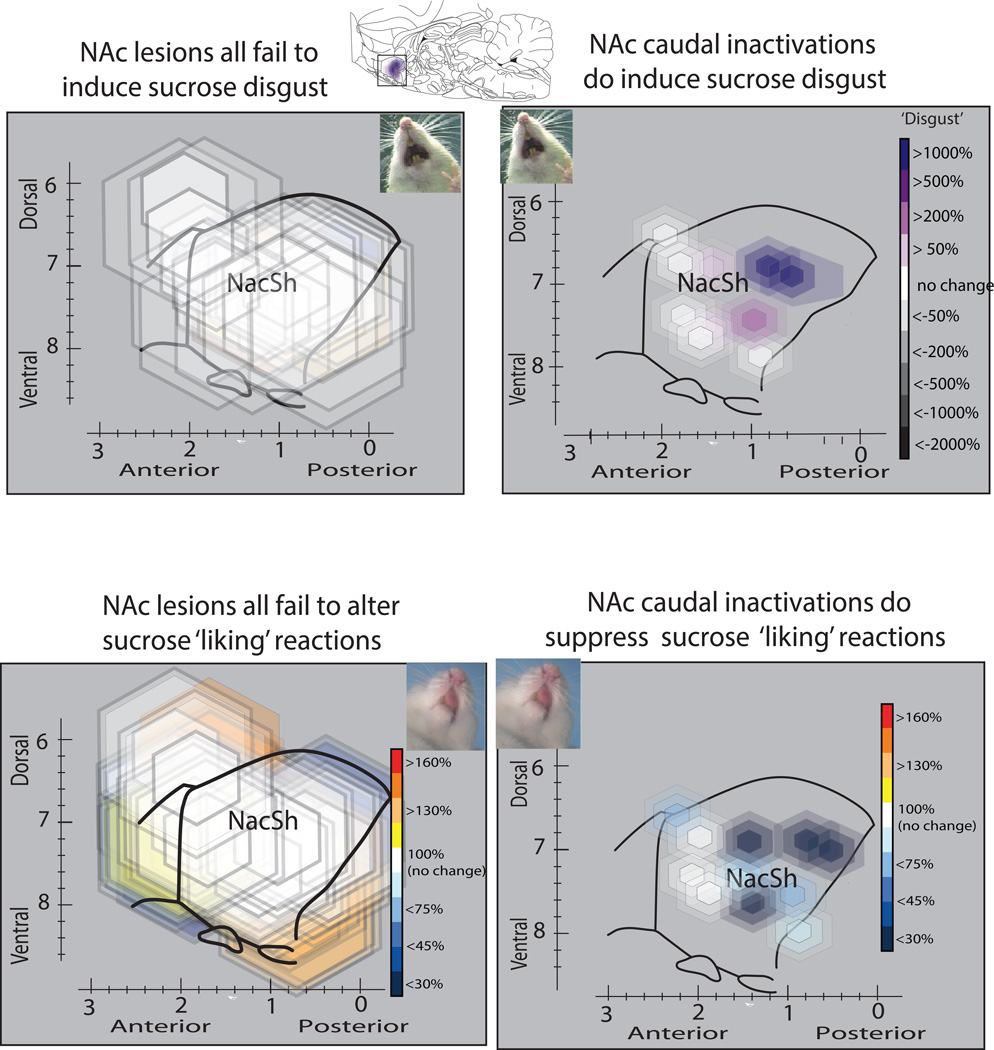
Excitotoxin lesions in the nucleus accumbens, whether in rostral or caudal halves of medial shell, completely failed to cause detectable elevation of ‘disgust’ gapes, headshakes, forelimb flails or chin rubs in response to the taste of oral sucrose infusions (Figure 3), or even to impair the number of positive hedonic reactions elicited by sucrose (p=0.78). Not even the most intense rostral NAc shell lesions that destroyed 80% neurons in the rostrodorsal quadrant of medial shell, which contains the previously identified NAc hedonic hotspot, suppressed positive hedonic reactions to sweetness or produced gapes (Figure 3). Quinine taste remained able to elicit gapes and other disgust reactions, but only at levels that remained comparable to those of vehicle control rats (p=0.36). Similarly, food and water intake were not altered by NAc lesions.
Figure 3.
Lesions of nucleus accumbens fail to alter hedonic impact, though caudal inactivations in medial shell induce disgust. NAc excitotoxin lesions had no effect on positive ‘liking’ reactions to sucrose (rhythmic tongue protrusions, lateral tongue protrusions, paw licks), and did not induce disgust reactions (gapes, headshakes, etc.), at any location in medial shell. However, temporary inactivations by GABAergic microinjections in the caudal half of medial shell did suppress ‘liking’ reactions and induce disgust reactions to sucrose taste. Maps show sagittal view to display entire NAc medial shell. Map symbols are color-coded to show changes in positive versus negative affective reactions induced at a site.
VP hotspot lesions induce excessive disgust reactions
The posterior half of the ventral pallidum (VP) proved to be chief site where excitotoxin lesions could produce intense disgust (Figure 4). Here 80% neuron loss lesions in the posterior ventral pallidum zone essentially eliminated all positive hedonic reactions normally elicited by sucrose taste, which were replaced with negative ‘disgust’ gapes, etc., in numbers >400% greater than in normal control rats (F1, 60=4.29, p<0.05; d = 0.72). These effects were visible within 24 – 48 hours after excitotoxin surgery, and persisted for several days to weeks. The intensity threshold of neural death in the posterior ventral pallidum needed to produce excessive ‘disgust’ appeared to be approximately 65% to 80% (severe neuron death: <30% neurons remaining; Figure 4). Rats with 65–70% neuron loss in posterior VP emitted a few ‘disgust’ reactions to sucrose taste, and the number of ‘disgust’ reactions appeared to rise further for lesions that produced neuronal loss reaching 80% (Figure 4). As a group, rats that emitted ‘disgust’ reactions to sucrose had an average of 74 ± 1 % neuron loss in their lesion centers (i.e., < 26% of neurons remained). The threshold for ‘disgust’ induction appeared similar to an earlier >70% threshold found by a previous study of ventral pallidal lesions (Cromwell & Berridge, 1993). By contrast to sucrose reactions, no lesions (even in posterior VP) altered ‘disgust’ reactions to bitter quinine taste, which were already at high levels in control rats (gapes, head shacks, forelimb flails, chin rubs). This may indicate that the high-levels of quinine ‘disgust’ were already near a response ceiling even for control rats, and could not be increased further by the lesions used here. All rats that showed ‘disgust’ gapes, chin rubs, headshakes and forelimb flails to sucrose were also completely aphagic and adipsic at the same time (Figure 5), neither eating nor drinking. Aphagia continued for at least 6 days in all rats with most intense lesions in VP or in LH, and for over 10 days in several rats, before food intake gradually began to recover. Aphagic rats were therefore were maintained during this period by 3 intra-gastric intubations of liquid diet each day, beginning at 6 ml of milk diet and ascending gradually to 12 ml. The intra-gastric intubations by themselves did not induce ‘disgust’ in aphagic rats, as indicated for example in failure to produce ‘disgust’ in aphagic LH lesion rats, anterior VP lesion rats or extended amygdala lesion rats that lacked VP damage. However, this does not rule out the possibility that pathological ‘disgust’ might be capable of modulation by hunger/satiety states in future studies. For example, starvation has been reported to reduce lesion induced ‘disgust’ (Fluharty & Grill, 1981), and intubation to augment LH-stimulation induced ‘disgust’ in previous studies (Hoebel & Thompson, 1969), but we did not manipulate appetite states here.
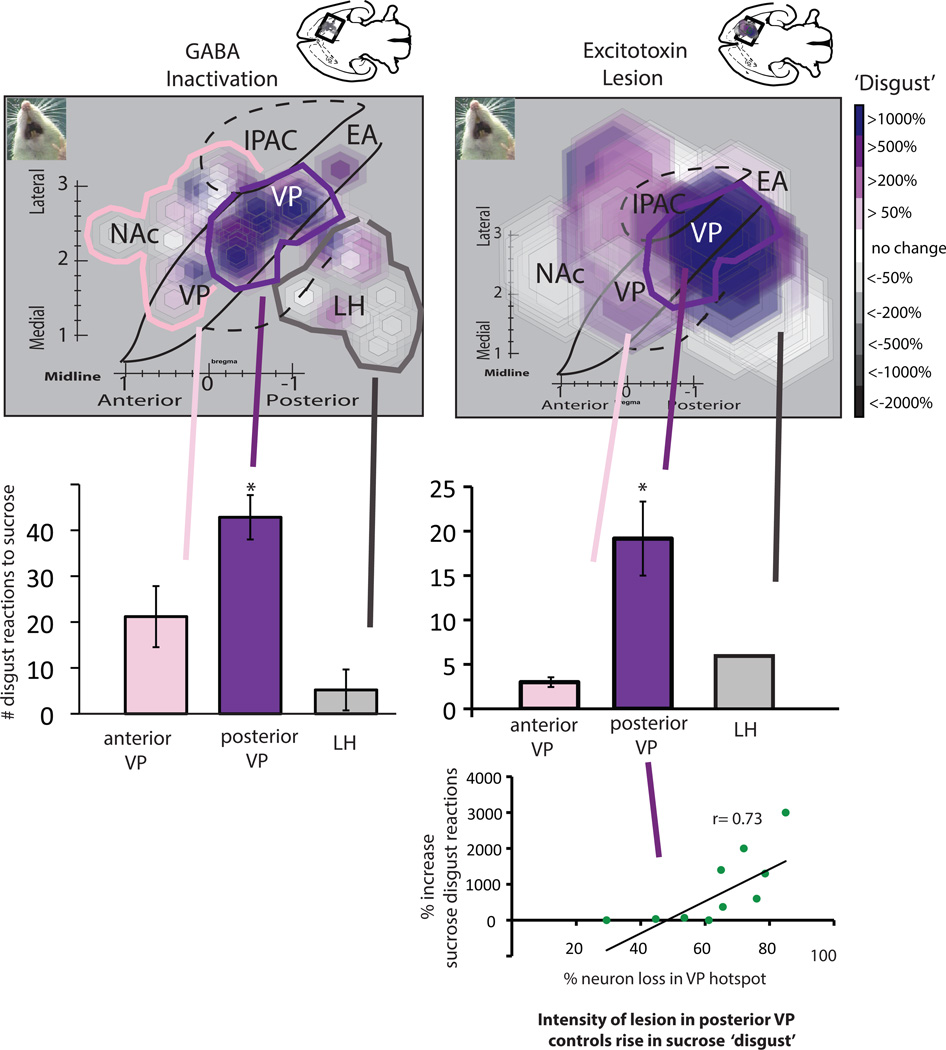
Figure 4. Induction of disgust in ventral pallidum.
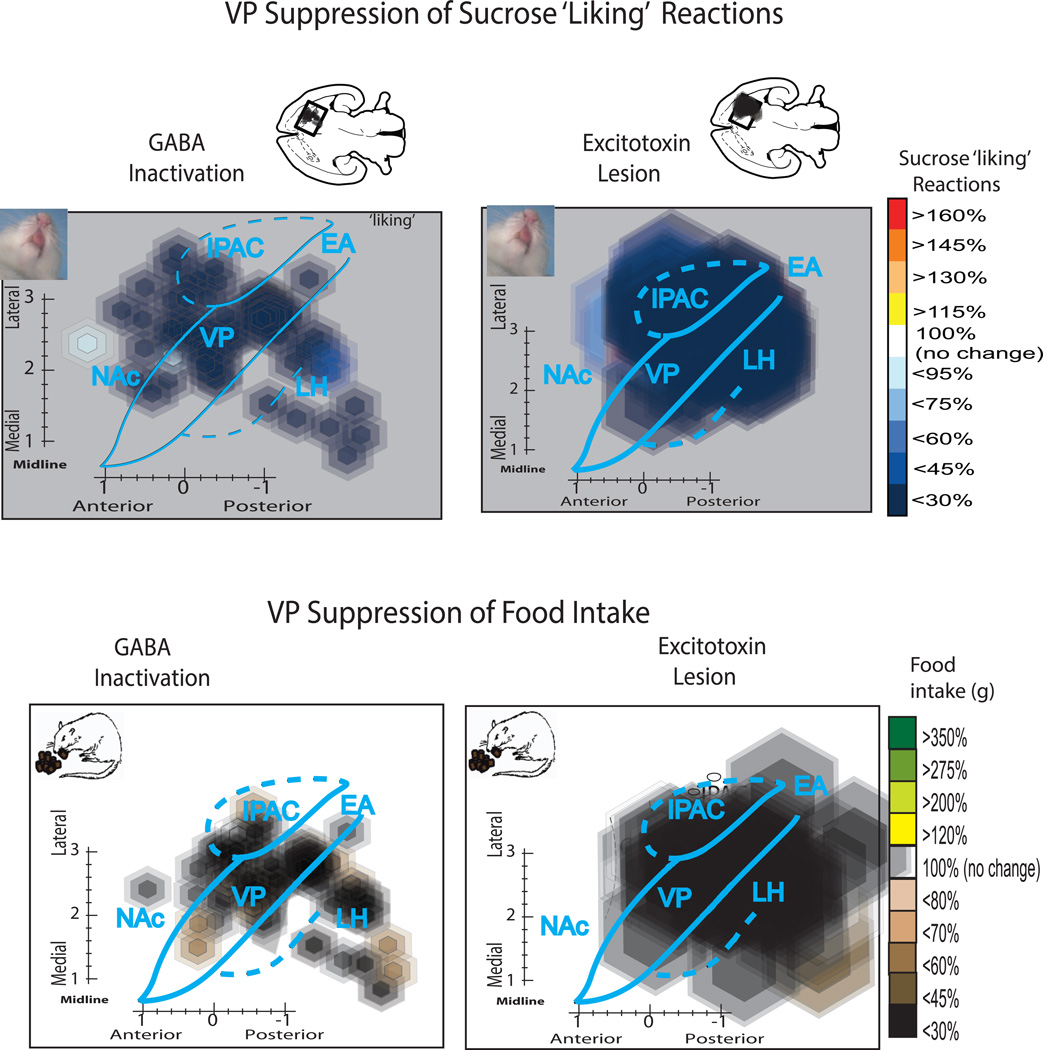
Maps of sucrose disgust reactions after either temporary inactivations by GABAergic microinjections (left) or by excitotoxin lesions (right) in VP or nearby structures. Maps show a horizontal view (−8.4 mm below skull surface) to display the entire VP. The small disgust-induction site in posterior VP is seen most clearly in the map of GABAergic inactivations due to the smaller radius of microinjection symbols. Larger lesion symbols create a more diffuse zone of disgust induction, especially because some lesions centered in LH or in extended amygdala still impinged on caudal VP. Bar graphs show the absolute number of ‘disgust’ reactions to sucrose taste induced by inactivation/lesion sites centered in posterior VP (disgust site), LH, or anterior VP compared to baseline levels measured in control rats (for lesions) or in the same rats after control vehicle microinjections (for inactivations). Scatterplot and regression analysis at bottom shows the correlation between lesion intensity (% neurons destroyed) and ‘disgust’ produced by excitotoxin lesions in the posterior VP. Abbreviations: VP= ventral pallidum, LH = lateral hypothalamus, NAc = nucleus accumbens shell, EA = extended amygdala, IPAC = interstitial nucleus of posterior limb of anterior commissure.
Figure 5. Suppression of positive ‘liking’ reactions and of food intake in ventral pallidum.
Top maps show changes in positive hedonic reactions to sucrose taste induced by temporary inactivations or lesions in VP and in nearby structures. Suppression of ‘liking’ reactions is distributed more widely across sites in VP, LH, and SI than disgust induction (especially visible for inactivations, which had smaller diameters than lesions) though still often most intense at sites in posterior VP. Bottom maps show reduction in food intake, which was also induced widely at sites in VP, LH and SI.
By contrast, several rats that had less intense lesion damage below the 65% neuron loss threshold in the same posterior hotspot region of ventral pallidum (averaging neuron loss of 53%, or >47% of neurons remaining) never emitted ‘disgust’ reactions to sucrose taste (Figure 4; although several of these rats were similarly aphagic and adipsic, and received similar gastric intubations to maintain body weight). Therefore, in constructing lesion maps for causation of disgust to sucrose, we specifically mapped zones containing 70–80% neuronal loss from rats that showed ‘disgust’ reactions to sucrose taste. This was done based on the premise that a shared zone of 70% – 80% neuron loss, unanimous among all rats showing excessive ‘disgust’ reactions, would best capture the critical brain region where neuronal loss induces abnormal ‘sensory disgust’ to sucrose.
Disgust-release site in posterior ventral pallidum
The posterior VP ‘disgust’ site was assessed by functional mapping to have a radius of about 0.6 mm, centered at approximately AP −1.3 mm posterior to bregma, L +2.4 mm lateral from the midline, and DV −8.2 mm ventral to skull surface (Figures 4 & 6). In terms of anatomical boundaries, the ‘disgust’ lesion zone extended posteriorly to the caudal border of posterior ventral pallidum, at an AP level adjacent to the sublenticular extended amygdala (SLEA)(AP −1.4 ~ −1.8 mm). Anteriorly, the disgust-induction site extended approximately to the middle of ventral pallidum (AP +0.3~ 0.0 mm), but did not appear to include the anterior half of ventral pallidum (i.e., did not extend rostral to +0.3 bregma).
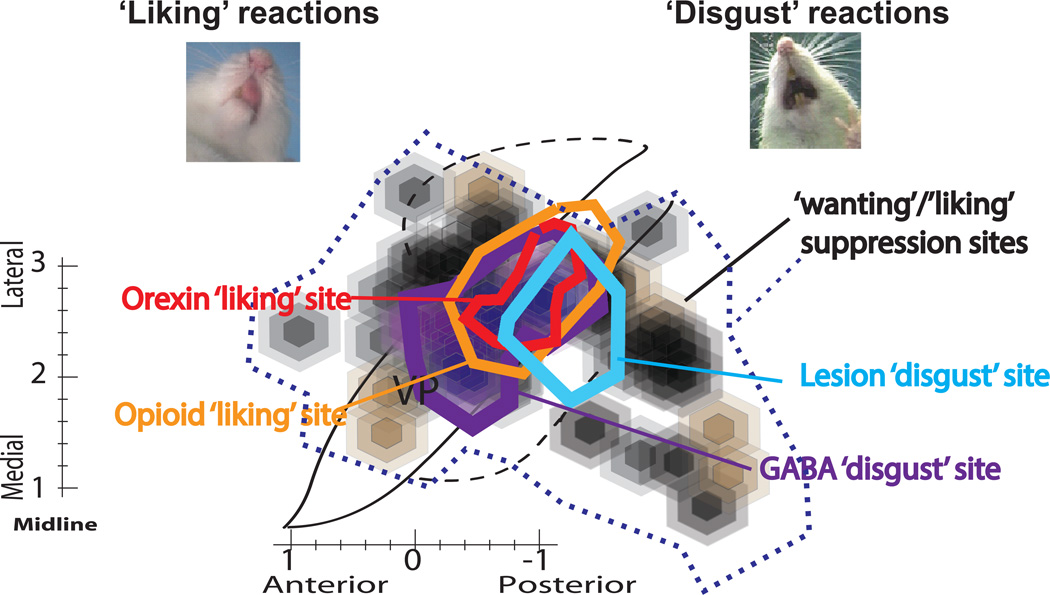
Figure 6. Summary comparison of VP disgust site to earlier opioid/orexin hedonic hotspot.
Horizontal map shows maximal disgust release sites found here for temporary GABA inactivations (purple) and for excitotoxin lesions (blue), in comparison to previous maps reported for hedonic hotspot in posterior ventral pallidum for mu opioid enhancement of sucrose ‘liking’ reactions (orange) (Smith & Berridge, 2005), and for orexin enhancement of sucrose ‘liking’ reactions (red) (Ho & Berridge, 2013). Additional black and brown symbols show motivation suppression of food intake here that extended well beyond posterior VP hotspot for pharmacological ‘liking’ enhancement or dysfunction-induced disgust.
Rats with 80% neuron loss in this posterior VP ‘disgust’ lesion zone emitted far more disgust reactions to sucrose than rats that lesions sited at other locations outside the posterior ventral pallidum, even when those other locations had comparably intense 80% neuron loss (F1, 60=4.16, p<0.05, d=-0.623). Those outside lesion sites included severe lesions placed either in anterior ventral pallidum, or lateral hypothalamus or extended amygdala sites near the VP (as well as in the nucleus accumbens shell). None of those other non-VP lesions produced intense ‘disgust’ to sucrose, even though the damaged sites contained only 20% of the normal neuronal density for those locations.
Suppression of positive ‘liking’ reactions to sucrose (without necessarily being accompanied by negative ‘disgust’ induction) was produced by a somewhat wider range of sites in and outside of VP (Figure 5). ‘Liking’ suppression was produced by lesions throughout VP, and by lesions in other adjacent structures, including LH, sublenticular extended amygdala or substantia innominata, and extending anteriorly to caudal NAc medial shell and dorsally to ventral neostriatum. Lesions centered in all of those structures appeared to induce suppression of the number of positive ‘liking’ reactions elicited by sucrose to roughly 1/10th to 1/3rd normal levels (F1, 106 = 44.37, p<0.01, d=1.12; Figure 5). However, as caveat, a number of lesions centered at those sites appeared to have wider penumbras of mild neuron loss that also penetrated near or into posterior ventral pallidum. Thus it is possible that mild VP hotspot damage participated in hedonic suppression, even if the damage was not intense enough loss to induce ‘disgust’.
Contrast of disgust to aphagia
Aphagia and adipsia (without excessive ‘disgust’) was induced by many basal forebrain lesions in a considerably larger region than posterior ventral pallidum, extending at least 3 to 5 mm3 in volume (Figure 5). Aphagia and adipsia followed lesions in the rostral VP as well as caudal VP, and also followed all lesions in the lateral hypothalamus where 70–80% cell loss was produced: food intake was near-zero, and far below that of intact control rats: F2,177=22.91, p<0.01, d=1.07). Aphagia and adipsia typically lasted from several days to a week. By contrast, nucleus accumbens lesions, either in rostral or caudal halves of medial shell, never produced aphagia or adipsia (or ‘disgust’). Even for 80% lesions centered in the NAc hotspot of rostrodorsal quadrant of medial shell, no elevation of ‘disgust’ reactions or decrease of positive hedonic reactions were produced, nor any detectable impairments of intake (n=19, n.s.).
Temporary inactivations by muscimol/baclofen microinjections
Fos inhibitory plume maps surrounding GABAergic microinjection tips
To map the brain sites responsible for behavioral effects of pharmacological temporary inhibitions, local changes in Fos protein expression (Fos plumes) were measured surrounding microinjections of GABA agonists. Inhibitory first microinjections of the muscimol/baclofen mixture in the Fos group of rats produced suppression of local Fos in neurons surrounding the microinjection site, detectable as inhibitory Fos ‘antiplumes’ compared to levels at similar VP sites in control rats that received no microinjection. Inhibitory Fos antiplumes were approximately 0.35 mm in total radius, similar to a previous muscimol microinjection study (Faure et al., 2010), containing an inner sphere of heaviest >65% Fos suppression (i.e., only <35% of normal levels of Fos in VP) of 0.12 ± 0.01 mm radius, surrounded by a middle zone of moderate 40% – 65% suppression (i.e., 35% to 60% of normal levels) of 0.24 ± 0.02 mm radius, surrounded by an outer sphere of mild 25% −40% suppression (i.e., 60% to 75% normal levels) of 0.35 ± 0.15 mm radius (Figure 2). The size of symbols in microinjection causation maps for excessive ‘disgust’ was therefore assigned to be 0.35 mm in radius to represent maximal observed spread (equal to the outer inhibitory plume). All data in plume maps other than plume radius reflect functional consequences of muscimol/baclofen microinjections measured in behaviorally tested rats, and assigned to the microinjection site to show behavioral causation (location, symbol color, bar graphs).
Nucleus accumbens inactivations: posterior aversion, but not rostral hotspot
In NAc, only sites located in the caudal two-thirds of medial shell produced excessive ‘disgust’ reactions to sucrose after GABAergic microinjections compared to vehicle control microinjections in the same rats (F1, 8= 9.232, p=0.016, d=-1.28; Figure 3). By contrast, microinjections of muscimol-baclofen into the rostral third of medial shell (including the rostrodorsal quadrant of medial shell that contains the previously identified hedonic hotspot) failed either to release ‘disgust’ reactions to sweetness, nor to significantly suppress the number of positive hedonic reactions to sucrose taste (Figure 3). This anatomical pattern followed a similar rostrocaudal gradient for ‘disgust’ induction previously reported for pure muscimol microinjections in medial shell (Reynolds & Berridge, 2001; Reynolds & Berridge, 2002; Faure et al., 2010; Richard et al., 2013b). The posterior zone of nucleus accumbens shell where temporary GABA hyperpolarization caused excessive ‘disgust’ to sucrose taste stretched approximately from A-P levels +1.2mm to +0.6mm to bregma. Food consumption was similarly blocked after NAc posterior GABAergic microinjections in the caudal half of medial shell at sites that induced ‘disgust’, (F1, 8= 9.595, p=0.01, d=-1.08)(Reynolds & Berridge, 2002). By contrast, anterior NAc microinjections of muscimol/baclofen produced if anything a trend toward increased food intake, though not statistically significant in the current study. Many previous studies have reported robust increases in food intake after muscimol microinjections in the anterior half of NAc medial shell, including in our lab (Stratford & Kelley, 1997; Basso & Kelley, 1999; Stratford & Kelley, 1999; Reynolds & Berridge, 2002; Stratford, 2005; Faure et al., 2010; Wirtshafter & Stratford, 2010; Stratford & Wirtshafter, 2012; Richard et al., 2013b). Possibly our failure to observe significant increases in feeding here induced in the anterior half of medial shell due to the temporal interpolation of taste reactivity testing between microinjection and intake test, complications arising from the addition of baclofen to muscimol (while most previous studies used one alone, usually muscimol), or to a relatively low number of microinjection sites located in rostral shell.
Inactivation of posterior ventral pallidum hotspot releases disgust
In ventral pallidum, microinjections of GABA agonists in the posterior region of VP caused subsequent sucrose taste to elicit intense ‘disgust’ reactions that reached >20X above control levels, compared to normal baselines assessed in the same rats after vehicle microinjection microinjections (F1, 62=32.141, p=0.001, d=−2.56; Figure 4). The anatomical boundaries of the ‘disgust’ site in posterior VP extended anteriorly to middle VP (AP −0.1 to −0.6 mm bregma), and posteriorly to the caudal edge of VP bordering the sublenticular extended amygdala (AP −1.5 mm bregma) (Figures 4 & 6), and the total volume of the site was estimated to be about 1.0 mm3 (which constitutes about 1/3 of the total volume of entire VP).
Sites within the posterior VP produced more intense elevations of ‘disgust’ reactions after muscimol/baclofen microinjections than sites in anterior VP, or than sites in nearby outside structures such as lateral hypothalamus or extended amygdala (SLEA) (F2,30= 9.97, p<0.05, d= 1.0). However, sites in the lateral hypothalamus that were within 1 mm of VP boundary did produce at least intermediate levels of ‘disgust’ reactions to sucrose, but no ‘disgust’ reactions were produced by further sites in LH, sublenticular extended amygdala, or ventral neostriatum that were more than 1 mm away from posterior VP (suggesting that some proximal LH ‘disgust’ impact may have been due to distant muscimol/baclofen diffusion reaching VP a bit beyond our symbol radius that was based on measured suppression of Fos). Further, many sites for GABAergic microinjections did suppress positive ‘liking’ reactions to sucrose even at further distant locations in LH or SLEA, which were more than 1 mm from VP, though still failing to generate any ‘disgust’ reactions to sucrose (Figure 2). ‘Disgust’ reactions to quinine, which were already at high levels even after vehicle microinjections, were not further elevated by muscimol-baclofen microinjections at any site (even in the posterior VP) ( F1, 72= 1.038, p>0.05, d=−0.2). This lack of effect on quinine-evoked reactions was similar to lesions above, and again perhaps indicating a response ceiling for already-aversive bitterness. Finally, muscimol/baclofen at most sites in LH and VP also suppressed or abolished food consumption in the free intake test that followed taste reactivity testing, as did most sites in VP.
Comparison of inactivation site vs lesion site in VP
Explicitly comparing excitotoxin lesions versus temporary pharmacological inactivations as ways of defining the posterior VP site for ‘disgust’ release, GABAergic microinjections produced twice the number of gapes and other ‘disgust’ reactions to sucrose than excitotoxin lesions of the same site (df= 24, p=0.015; d= −1.429; Figure 4). However, GABAergic microinjections produced smaller radius impacts, and therefore tighter maps for the site of ‘disgust’ induction. Overall, the anatomical site centered similarly on the posterior VP for both lesions and inactivations, with the lesion site map appearing larger than the GABA-inactivation site map. The reason for the size difference between mapped ‘disgust’ sites might be because any lesion intense enough to deplete at least 80% of neurons in its center also tended to be large enough to produce substantial lesser damage at more distant sites (even if the damage to those distant sites did not substantially contribute to disgust). Given that the inactivation map gives the slightly smaller site, we conclude it to be the most precise depiction, and suggest the GABAergic map most accurately reflects the true ‘disgust-induction site’ in posterior VP. Finally, it is of interest to compare the VP disgust-induction site identified here to the previously identified VP hedonic hotspot where opioid/orexin microinjections in normal rats cause enhancements of positive ‘liking’ reactions to sucrose taste (Smith & Berridge, 2005; 2007; Ho & Berridge, 2013). As expected, when laid on top of each other, a map of the anatomical center and boundaries of the VP ‘disgust-site’ for lesions or inactivations appears nearly identical to the map of the VP hedonic hotspot reported in those earlier ‘liking’ enhancement studies (Figure 6).
Discussion
Our results indicate that for excitotoxin lesions, only the posterior ventral pallidum (VP) is able to produce intense ‘disgust’ reactions to sucrose after loss of 65% – 80% of neurons within the VP hotspot. High levels of ‘disgust’ reactions to sucrose were also produced by temporary inactivation of the posterior VP hotspot caused by microinjections of GABA agonists, which presumably hyperpolarized nearby neurons via GABA opening of membrane Cl- ion gates. By comparison, for the nucleus accumbens (NAc) hedonic hotspot in the rostral medial shell, neither lesions nor inactivations caused ‘disgust’ reactions to sucrose. Instead, excessive ‘disgust’ was produced only from the posterior two-thirds of NAc medial shell, and only after temporary inactivations by muscimol/baclofen microinjections (never after excitotoxin lesions even in posterior NAc shell). Finally, for other sites in structures such as the lateral hypothalamus (LH) or extended amygdala, neither lesions nor inactivations ever caused ‘disgust’, even when impairing food and water intake, as long as the posterior VP hotspot was spared (remaining outside the LH or extended amygdala excitotoxin lesion or GABAergic microinjection spread, as indicated by neuron count or Fos plumes).
This pattern might be summarized by saying that the VP hotspot is uniquely needed for normal ‘liking’ levels to be generated in forebrain circuitry, leaving ‘disgust’ in its absence (as well as being a site able to neurochemically enhance ‘liking’ to higher levels than normal)(Smith & Berridge, 2005; 2007; Ho & Berridge, 2013). By comparison, the NAc shell segregates hedonic enhancement mechanisms into the rostral half of medial shell, whereas excessive ‘disgust’ is predominately produced more posteriorly by manipulations in the caudal half of medial shell. Within posterior NAc shell, the relatively phasic impairment of neuronal function induced by temporary GABAergic inactivation (i.e., minutes to hours after microinjection) was potent at releasing ‘disgust’,
However, posterior NAc sites did not produce not the more enduring chronic impairment induced by excitotoxin destruction of NAc neurons. The difference between GABAergic microinjections and lesions for posterior NAc might reflect compensatory adjustments occurring in remaining affective brain circuitry occurring over the first 24 – 48 hours after a NAc lesion, which are not able to occur in the shorter period of a few minutes to an hour between the GABAergic microinjection and a taste reactivity test. The difference may also reflect an intensity difference in the impact of the two manipulations. That is, GABAergic microinjections more powerfully impact function than lesions even at a single site: for example, GABAergic microinjections in posterior VP produced roughly twice the number of ‘disgust reactions’ to sucrose as did excitotoxin lesions in the same VP site. Another reason why NAc lesions failed to produce disgust, but posterior VP lesions succeeded, might be the opposite neuronal polarization mechanisms posited for NAc and VP in reward functions, which arise from reciprocal GABAergic interconnections between the two structures (Kelley et al., 2005; Baldo & Kelley, 2007; Carlezon & Thomas, 2009). That is, NAc hyperpolarization or inhibition has been proposed to be a chief neuronal mechanism of reward (Roitman et al., 2005; Carlezon & Thomas, 2009; Krause et al., 2010; Roitman et al., 2010), whereas VP neuronal depolarization or excitation appears more directly linked to reward functions (Tindell et al., 2004; Tindell et al., 2006; Carlezon & Thomas, 2009; Root et al., 2010; Smith et al., 2011). In that framework, NAc may thus play a relatively permissive role in reward and incentive motivation, via disinhibiting targets including VP. By contrast, VP excitation may play a more direct role in generating reward functions. Loss of VP neurons might therefore more dramatically tilt affective balance towards negative affect, including disgust. However, we note as counterpoint to this explanation that NAc excitations (perhaps of different neuronal subpopulations) also may participate in reward (Ambroggi et al., 2011), and clearly NAc pharmacological inhibitions in caudal shell can induce ‘disgust’, as seen here, suggesting NAc inhibitions are not purely linked to reward.
Another potential basis, at least for taste ‘disgust’, is that VP has more extensive reciprocal projections than NAc with brainstem gustatory relays in rodents such as the parabrachial nucleus of the pons (Norgren & Leonard, 1973; Wu et al., 2012). It may also be relevant that VP neurons more directly synapse onto dopamine neurons in VTA, which might especially mediate appetitive motivation, whereas NAc projections synapse only on non-dopaminergic neurons (Xia et al., 2011; Hjelmstad et al., 2013). However, as a caveat to that explanation, the GABAergic inhibitory nature of both projections does present a puzzle for how VP excitations could drive dopamine firing, unless additional other synapses or co-neurotransmitters were involved (Sesack & Grace, 2010).
Whatever the neuronal mechanism for ‘disgust’ production, it seems intriguing that reciprocal projections may connect the posterior half of NAc medial shell (where ‘disgust’ is produced at least by GABAergic microinjections) to the posterior half of VP (Groenewegen et al., 1993) (where ‘disgust’ is produced by both GABAergic microinjections and lesions), raising the possibility that the two disgust-inducing sites in NAc and VP may be anatomically interconnected. NAc core also projects to posterior VP, whereas by comparison, rostral medial shell of NAc may connect more predominately with anterior VP (Groenewegen et al., 1993; Thompson & Swanson, 2010).
Ventral pallidum hotspot: unique site for normal pleasure and ‘liking’ enhancement
The ‘disgust-releasing site’ in posterior ventral pallidum appeared essentially identical in anatomical boundaries to the VP hedonic hotspot previously identified for the enhancements of sensory pleasure via microinjections that deliver opioid or orexin neurochemical stimulation (Smith & Berridge, 2005; 2007; Ho & Berridge, 2013)(Figure 6). That same VP hotspot also contains neurons in which firing increases code hedonic palatability of sweet, salty and bitter tastes, and their modulation of palatability via changes in natural appetite/satiety states or brain manipulations (Tindell et al., 2004; Tindell et al., 2005; Tindell et al., 2006; Smith et al., 2011).
Our results also confirm previous reports that VP lesions or muscimol microinjections produce excessive ‘disgust’ reactions to sweet tastes (Cromwell & Berridge, 1993; Shimura et al., 2006), whereas the same manipulations of lateral hypothalamus or extended amygdala do not (if VP remains spared) (Cromwell & Berridge, 1993). Our results additionally reveal that the VP subregion most responsible for ‘disgust’ appears limited to the posterior half of ventral pallidum. By contrast, only mere passive aphagia or hypophagia (without active disgust to sucrose, though often with suppression of positive hedonic ‘liking’ reactions) was produced by damage in a larger surrounding 5-mm3 area, including anterior VP, lateral hypothalamus and sublenticular extended amygdala (Schallert & Whishaw, 1978). The large region for induction of aphagia and adipsia is also consistent with previous reports of diverse lateral hypothalamus or globus pallidus lesions (Morgane, 1961; Teitelbaum & Epstein, 1962; Schallert & Whishaw, 1978; Cromwell & Berridge, 1993; 1994).
Regarding differences in function between anterior VP versus posterior VP, further support is provided by reports that temporary inactivation of the posterior VP in rats reduces reinstatement of cocaine reward pursuit primed by either cocaine administration or by aversive stress, whereas inactivation of the anterior VP more effectively reduces priming of cocaine pursuit by learned drug-predicting cues (McFarland & Kalivas, 2001; McFarland et al., 2004; Mahler et al., 2014). Further, opioid microinjections in the posterior VP increase unconditioned consumption of food as well as enhancing ‘liking’ orofacial reactions to sucrose hedonic impact, and also increase instrumental responses to earn electrode self-stimulation in lateral hypothalamus (in addition to increasing ‘liking’ reactions to sucrose taste), whereas the same opioid microinjections in anterior VP suppress eating and self-stimulation (in addition to suppressing sucrose ‘liking’) (Johnson et al., 1993; Smith & Berridge, 2005). Conversely, opioid antagonist microinjection in posterior VP blocks hedonic alliesthesia or palatability enhancement normally produced by hunger or by NAc opioid stimulation (Smith & Berridge, 2007; Wassum et al., 2009). Thus VP appears to have strong localization of function differences between rostral and caudal subregions.
The neurobiological basis of why the posterior VP hotspot is more important than the anterior half of VP to ‘disgust’ induction remains unknown, but there are at least several special neurobiological features of the posterior VP that might be important to localization of hedonic/disgust function. For example, the posterior part of VP is reported to have higher levels of enkephalin neurotransmitter expression than anterior VP (accompanied by a lower density of pre-synaptic μ-opioid receptors), and also a higher ratio of non-cholinergic neurons to cholinergic neurons (Maidment et al., 1989; Bengtson & Osborne, 2000). There are also important connectivity differences between posterior VP and anterior VP involving projections with nucleus accumbens, medial prefrontal cortex, insular, and somatosensory cortex (Zahm & Heimer, 1990; Groenewegen et al., 1993; Zahm et al., 1996; Zahm et al., 2013).
Nucleus accumbens: segregation of rostral pleasure enhancement vs caudal disgust induction
By contrast to VP hotspot, our results indicate for NAc that the rostrodorsal quadrant of medial shell is not a site for ‘disgust’ induction, despite containing another hotspot for hedonic enhancement by opioid or endocannabinoid stimulation (Peciña & Berridge, 2005; Mahler et al., 2007; Smith & Berridge, 2007; Smith et al., 2011; Castro & Berridge, 2014). Neither permanent excitotoxin lesions nor temporary GABAergic inactivations in the anterior half of NAc medial shell were able to elevate ‘disgust’ reactions to sucrose taste, or even to suppress positive ‘liking’ reactions to sucrose.
For NAc, ‘disgust’ was induced only by GABAergic microinjections, and only at sites in the posterior two-thirds of medial shell. That NAc caudal bias for temporary inactivation ‘disgust’ may also fit within a larger rostrocaudal affective keyboard pattern of anatomical organization of valence function previously found for NAc shell. For example, GABAergic muscimol microinjections in posterior medial shell also produce actively-coping ‘fear’ (e.g., defensive anti-predator reactions such as directional treading/burying) and establish conditioned place avoidance, whereas rostral NAc shell sites produce appetitive increases in eating and food-seeking behavior and sometimes even ‘liking’ enhancements (Stratford & Kelley, 1997; Reynolds & Berridge, 2002; Faure et al., 2010; Wirtshafter & Stratford, 2010; Richard et al., 2013b). Localization of function across different rostrocaudal subregions of NAc medial shell might be viewed as simply extending the principle of anatomical heterogeneity for reward motivation that is already well recognized for the larger shell versus core components of NAc (Meredith et al., 2008; Besson et al., 2010; Rocha & Kalivas, 2010; Ambroggi et al., 2011; Cacciapaglia et al., 2012; Resendez et al., 2013).
Disgust release in historical context
Over a century ago, the neurologist John Hughlings Jackson proposed that active behavioral symptoms caused by brain damage might be understood as ‘release phenomena’ (Hughlings Jackson, 1958). Jackson wrote: “We must never speak of destructive lesions causing positive symptoms. It is erroneous, I submit to say that any sort of disease causes elaborate positive mental symptoms illusions, hallucinations, etc.” Instead “it causes a negative mental condition, the elaborate positive mental symptoms are permitted” (italics original, p. 192, 1958; originally published 1879). By ‘negative’ mental condition Jackson meant a loss of some particular function (e.g., loss of pleasure or ‘liking’ reactions here) and by ‘positive’ symptom he meant any intense new and pathologically intense process (e.g., ‘disgust’). That is, applied here, Jackson’s term ‘positive mental symptom’ would not refer to positive-valence ‘liking’ but rather to intense negative-valence ‘disgust’.
By this Jacksonian view a VP lesion causes a release phenomenon: sudden removal of inhibitory influences that are normally exerted over other circuitry, producing disinhibition and apparent excitation of the dramatic new symptoms. Arguably, this release phenomenon framework applies to the induction of intense ‘sensory disgust’ by our posterior VP lesions/inactivations or by posterior NAc shell inactivations. That would mean that lesions of the ventral pallidum did not quite ‘cause’ the excessive ‘disgust’. Instead, VP lesions removed either antagonistic opposition by ‘liking’ (via impairing a positive hedonic mechanism) and/or removed descending inhibitory control (via impairing hierarchical modulation that normally suppresses disgust). In either case, the removal freed remaining negative-affect circuitry elsewhere to generate excessive ‘disgust’. A release interpretation is also consistent with observations that the excessive ‘disgust’ to sweetness released by lesions gradually fades over days to weeks, suggesting that hyper-reactivity of remaining disgust circuitry eventually declines and restores affective balance (Teitelbaum & Epstein, 1962; Cromwell & Berridge, 1993). The identity of that remaining negative-valenced circuitry which generates disgust in absence of posterior VP has yet to be identified, but plausibly involves anterior ventral pallidum and regions of the hypothalamus, as well as striatum, amygdala and insula cortex (Calder et al., 2001; Calder et al., 2007; Hayes et al., 2007) (Heining et al., 2003; Murphy et al., 2003; Sambataro et al., 2006; Johnson et al., 2007; Mataix-Cols et al., 2008; Chapman & Anderson, 2012; Klucken et al., 2012).
Historically, only a few subcortical forebrain ablations or lesions have produced intense negative emotional reactions in the forms of excessive disgust, fear or rage. In transection studies, disgust reactions to sweetness as well as irritable aggression to handling were reported by Grill and Norgren in so-called ‘thalamic rats’, which possessed a thalamus and brainstem but had suction ablation of all telencephalic structures: basal ganglia (including neostriatum, nucleus accumbens and probably globus pallidus and ventral pallidum), septum, hippocampus and neocortex (Grill & Norgren, 1978a). Similarly, earlier transection studies of cats by Bard and Cannon observed intense negative affective reactions in the form of ‘thalamic rage’ after ablations of telencephalic structures above either the thalamus, or above the hypothalamus (and including the thalamus) (Cannon, 1927; Bard, 1934). Conceivably, all of those ablations may have eliminated the posterior ventral pallidum if they succeeded in destroying all structures rostral to the anterior edge of the hypothalamus and thalamus. Compared to the intense negative affect produced by such thalamic/hypothalamic ablations, neither ‘rage’ nor ‘disgust’ is produced when transections are made more anterior to the thalamic level, such as removing neocortex alone but leaving intact striato-pallidal circuitry and other telencephalic subcortical structures (Sorenson & Ellison, 1970; Warren et al., 1972; Vanderwolf et al., 1978; Wirsig & Grill, 1982). Similarly, no excessive ‘rage’ or ‘disgust’ is caused by transections more posterior to hypothalamus, such as mesencephalic decerebration in rats or cats, effectively removes the entire diencephalon, including hypothalamus as well as rostral structures, and rebalances the ratio of taste-elicited positive and negative affective reactions remaining as reflexes (Sherrington, 1906; Miller & Sherrington, 1915; Grill & Norgren, 1978b; Grill, 2006).
A few early specific lesion studies in 1920s-1040s similarly observed ‘chronic rage’ in cats and dogs after localized lesions in rostral regions of the basal forebrain (which it is tempting to speculate might also have damaged ventral pallidum) (Fulton & Ingraham, 1929; Spiegel et al., 1940). By the 1960s, the rough outline of a specific site for ‘disgust’ induction began to emerge in the results from large electrolytic lesions of the ventral forebrain region containing lateral hypothalamus (LH) and globus pallidus (which also probably damaged the ventral pallidum) (Morgane, 1961; Teitelbaum & Epstein, 1962). As Teitelbaum and Epstein wrote, after those large LH lesions (which probably intruded into VP) a rat “actively resists having milk placed in its mouth by a medicine dropper, and it does not swallow the milk once it is there…”, but instead “does engage in the same paw-waving and wiping, chin-rubbing, poor grooming and rejection (as) when very bitter quinine (1% weight/volume is put in its mouth… This suggests that mouth contact with food and water is highly aversive to a rat with lateral (hypothalamic) lesions during this stage” (pp. 75–76) (Teitelbaum & Epstein, 1962). However, subsequent studies using smaller and more precise lesions suggested further that only damage to the anterior part of the greater lateral hypothalamus region induced ‘disgust’ (i.e., the portion of lateral hypothalamus that borders upon the posterior half of ventral pallidum identified here), which again possibly intruded into VP (Schallert & Whishaw, 1978; Stellar et al., 1979). The same rats with anterior lesions that showed ‘disgust’ to food also showed antisocial behavior towards other rats, “actively rejected the normal (other rat) by pushing it away or by kicking it away in a stereotyped manner” (p.733), rather than approaching and positively engaging as control rats did. By contrast, damage to the posterior portion of LH failed to produce ‘disgust’ (producing only aphagia without aversion to tastes, similar to LH lesions here) or anti-social behavior (Schallert & Whishaw, 1978).
The ‘disgust lesion site’ was further moved entirely out of lateral hypothalamus proper, and further anterolaterally toward ventral pallidum, based on the results of an earlier study in our laboratory by Cromwell (Cromwell & Berridge, 1993). Cromwell mapped excitotoxin lesions that released negative ‘disgust’ reactions to sucrose, and was first to report that the disgust release site was actually not in lateral hypothalamus at all, but rather more anteriorly and laterally in the ventral pallidum or substantia innominata (Cromwell and Berridge, 1993). The ventral pallidum was further highlighted for disgust pharmacologically by Shimura and colleagues, who reported that GABA agonist microinjections in unspecified regions of VP similarly produced excessive gape reactions to sucrose (Shimura et al., 2006). Finally, our present results suggest the ‘disgust release site’ for both pharmacological microinjections and excitotoxin lesions can be narrowed further to the posterior half of VP, and is essentially identical to the VP hedonic hotspot for ‘liking’ enhancements. In retrospect, it seems reasonable to entertain the explanatory hypothesis that all the earlier ablation, lesion, and pharmacological studies described above that induced intense ‘disgust’ or ‘rage’ reactions may have released negative affect by impinging on the same site: the hotspot in the posterior half of VP.
By contrast, lesions in the nucleus accumbens typically never have been reported to induce either eating deficits or a major shift towards negative affect (Koob et al., 1978; Kelley & Stinus, 1985; Ramaswamy et al., 1998). Instead, only a mild alteration in eating patterns or sucrose preference has been found or none at all (Koob et al., 1978; Kelley & Stinus, 1985; Ramaswamy et al., 1998). Although NAc lesions may effectively impair rewarding impact of some drugs of abuse (Koob et al., 1978; Zito et al., 1985; Bardo, 1998), NAc damage has remarkably little impact on the capacity for natural sensory pleasures, such as food, or on motivation to consume natural reward. Our results confirm reports that NAc lesions do not induce intense negative affect or even suppress the positive hedonic impact of sucrose taste. We show this conclusion applies even to lesions that specifically target the hedonic hotspot in the rostrodorsal quadrant of medial shell, which is capable of generating opioid/endocannabinoid hedonic enhancements.
Relation to theories of positive versus negative affect: one or two dimensions?
Two major views exist in psychology over whether affect is best described as a single dimension (e.g. a line from positive pole to negative pole), or instead as two separate dimensions (one dimension for positive affect and an independent dimension for negative affect) (Norris et al., 2010). The 1-dimension view posits every affective reaction to be a point along a single line: intense pleasant reactions at one end, intense unpleasant reactions at the opposite end, and neutrality in the middle (Wundt, 1904; Young, 1918; Cacioppo et al., 1997; Feldman Barrett & Russell, 1999; Russell & Carroll, 1999; Kuppens et al., 2013). An implication for the brain of the 1-dimension view, is that a single affective brain mechanism or circuit could in principle generate all pleasant or unpleasant reactions via particular neural activation intensities or states. The 2-dimension view suggests instead that the positive affect dimension is separate and orthogonal to the negative affect dimension, so that the two could be depicted as positive x-axis versus negative y-axis forming a plane of intersection, in which all affective reactions are placed as 2-dimensional points (Gray, 1982; Berridge & Grill, 1984; Lang, 1995; Gray & McNaughton, 1996; Cacioppo et al., 1997; Larsen et al., 2001; Norris et al., 2010). A brain-related implication of the 2-dimension view is that neural mechanisms for positive affect are different from those for negative affect.
How do our current results on sensory disgust relate to these views? Our finding that particular VP hotspot lesions, VP inhibitions, and NAc inhibitions producing intense ‘disgust’ reactions also simultaneously reduced positive ‘liking’ reactions to sucrose fits best on its face with the 1-dimension view. By the 1-dimensional view, any increase in negative affect is necessarily also a decrease in positive affect. On the other hand, reciprocal inhibition between positive and negative mechanisms also has been posited at least by several advocates of the 2-dimension view (Gray, 1982; Berridge & Grill, 1984; Lang, 1995; Gray & McNaughton, 1996; Cacioppo et al., 1997; Larsen et al., 2001; Norris et al., 2010). If reciprocal inhibition is allowed between the two dimensions, then our reciprocal change results become more ambiguous. They could fit with the 2-dimension view, presuming that excessive ‘disgust’ activation is intense enough activation of the negative dimension to produce reciprocal inhibition of the positive dimension’s expression.
By the 2-dimensional view, negative affect can change independently of positive affect, and both positive and negative affect could exist at the same time. Our results did find a relatively univalent nonreciprocal or 2-dimension change in affect after damage to a larger region of ventral pallidum outside the hotspot, lateral hypothalamus and/or extended amygdala. Those lesions reduced the number of positive ‘liking’ reactions elicited by sucrose (often producing aphagia too), but did not produce detectable ‘disgust’ reactions. However, a 1-dimension view could counter that those lesions produced a mild change along a universal dimension, sufficient to reduce positive hedonic impact but not to cross the neutrality midpoint into negative territory. In either case, it remains unclear mechanistically whether that selective positive suppression was truly caused by damage to the outside-VP anatomical zones or perhaps to spread of milder damage into the VP hotspot (i.e., less than 65%). Finally, a 2-dimensional view probably best fits the Jacksonian-style interpretation described above, in which posterior VP damage is conceived to disrupt a mechanism of positive affect, so releasing a separate negative ‘disgust’ mechanism from reciprocal inhibition or hierarchical suppression.
In the end, our current results are not conclusive for the 1-dimension vs. 2-dimension debate. Future identification of a separate forebrain generator for negative disgust would support a 2-dimension or 2-mechanism view, whereas a potential future finding that posterior VP generates both ‘liking’ and ‘disgust’ via different modes would better support a 1-dimension or single-mechanism view.
Relation to other forms of disgust
Human disgust spans several levels of psychological complexity, from simple sensory disgust for a nasty taste or smell as studied here, to contamination disgust for the sight of excrement, bloody bodily wounds or particular sexual acts, to moral disgust at horrific unfairness or atrocity (Rozin & Fallon, 1987; Schnall et al., 2008; Chapman & Anderson, 2012). Sensory versus higher levels of disgust are distinguishable psychologically and perhaps even in facial expression (Rozin et al., 1994). However, the facial expressions do overlap between sensory and higher disgusts.
Most strikingly, both sensory and higher levels of disgust activate overlapping patterns of brain circuitry in fMRI studies (Small et al., 1999; Zald & Pardo, 2000; Zald et al., 2002; Calder, 2003; Zald, 2003; Calder et al., 2007; Mataix-Cols et al., 2008; von dem Hagen et al., 2009). Neuroimaging and electrophysiological studies have implicated particular roles in disgust for the subcortical ventral pallidum and globus pallidus, the neostriatum and nucleus accumbens, as well as the anterior insula cortex and cingulate cortex (Calder et al., 2001; Heining et al., 2003; Calder et al., 2007; Mataix-Cols et al., 2008; Shabel et al., 2011; Borg et al., 2012; Chapman & Anderson, 2012; Klucken et al., 2012; Borg et al., 2014). Within the ventral pallidum in particular, the rostral half has been reported to activate to disgusting images of food, whereas the caudal half to pleasant food images, a difference that seems compatible with the notion that caudal VP dysfunction might impair positive hedonic reactions to release negative disgust generated elsewhere (and perhaps involving rostral VP)(Beaver et al., 2006; Calder et al., 2007).
Sensory disgust to taste has been suggested to be the prototypical form and evolutionary origin of disgust, emerging first as a preadaptation to ovoid oral pathogens, which later became co-opted into more complex levels by biological and cultural evolution into higher forms of disgust (Rozin, 2000; Rozin et al., 2008; Chapman et al., 2009; Tybur et al., 2013). Cross-talk between levels also may still occur psychologically, as when experience of a bad taste potentiates or primes subsequent judgments about moral disgust (Eskine et al., 2011; Herz, 2014).
Although speculative, an application of our results to implications for human psychopathology would suggest special roles for posterior shell of nucleus accumbens and for the posterior regions ventral pallidum as sites where localized neural dysfunctions might induce excessive sensory disgust, and even conceivably higher levels such as contamination disgust or moral disgust. To the degree that neural circuitry overlaps for generating different levels of discuss, our findings at least pose the hypothesis that these subregions of NAc and VP might deserve special scrutiny when more complex forms of excessive disgust occur in humans.
Some clinical evidence from human studies supports the idea that VP may be needed for positive hedonic ‘liking’ in humans, and that normal pleasure is replaced by anhedonia or by negative affect after VP damage. Although it is rare for a human clinical brain lesion to destroy ventral pallidum on both left and right sides of the brain without also destroying so much other ventral forebrain that the patient becomes unable to communicate affective status, still a few cases of relatively specific VP lesions do exist. For example, one patient with VP damage on both sides due to hypoxia was reported to become dominated by depression, hopelessness, guilt, and anhedonia (Miller et al., 2006). Drinking alcohol, which was previously abused, lost its pleasure for him, and he no longer craved other drugs of abuse that he had previously avidly consumed. However, this lesion probably did not fully destroy his ventral pallidum, and perhaps this is why he was not as strongly seized by disgust as our rats seemed here. Instead, the patient still continued to eat and drink normally after his lesion, and even gained weight. But affectively, he was reported to become dominated by feelings of depression, hopelessness, guilt, and anhedonia (Miller et al., 2006). In another patient with globus pallidus lesions that were described as possibly extending into ventral pallidum, the patient reported an “inability to feel emotions”, and was described by analysts as having flat affect and “a profound lack of motivation” (Vijayaraghavan et al., 2008). Further, the patient was less motivated and less aroused by rewards such as pleasant food images, though still motivated to avoid unpleasant stimuli (Vijayaraghavan et al., 2008). While more evidence is needed, these clinical case studies seem at least consistent with the idea that damage to VP impairs positive affect as well as motivation for rewards in humans, and possibly releases excessive negative affect.
Conclusion
Overall, our data suggest that NAc segregates medial shell into separate zones for generating positive hedonic enhancement (rostral half of medial shell) versus release of excessive negative disgust or fear induced by temporary pharmacological GABA inactivations (caudal half of medial shell). By contrast, the ventral pallidum seems to combine excessive ‘disgust’ release and positive enhancement of pleasure into the same caudal hotspot in posterior VP. Further, the VP posterior site appears unique as the only brain lesion site known so far where excitotoxin-induced destruction of neurons also releases intense ‘disgust’, as well as GABAergic inactivations, beyond being a hotspot for hedonic enhancement. These results help advance understanding of how excessive negative affect, in the form of abnormally intense ‘sensory disgust’ reaction, is produced by localized brain dysfunction.
Acknowledgements
This research was supported by MH63649 and DA015188 grants from the NIH.
Abbreviations
- NAc
nucleus accumbens
- VP
ventral pallidum
- LH
lateral hypothalamus
- SLEA
sublenticular extended amygdala
References
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31:6820–6830. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo B, Kelley A. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Bard P. On emotional expression after decortication, with some remarks on certain theoretical views. Psychol Rev. 1934;41:309–329. [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: Beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Beaver J, Lawrence A, van Ditzhuijzen J, Davis M, Woods A, Calder A. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Osborne PB. Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. J Neurophysiol. 2000;83:2649–2660. doi: 10.1152/jn.2000.83.5.2649. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ. Isohedonic tastes support a two-dimensional hypothesis of palatability. Appetite. 1984;5:221–231. doi: 10.1016/s0195-6663(84)80017-3. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg C, de Jong PJ, Georgiadis JR. Subcortical BOLD responses during visual sexual stimulation vary as a function of implicit porn associations in women. Soc Cogn Affect Neurosci. 2014;9:158–166. doi: 10.1093/scan/nss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg C, de Jong PJ, Renken RJ, Georgiadis JR. Disgust trait modulates frontal-posterior coupling as a function of disgust domain. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology. 2012;62:2050–2056. doi: 10.1016/j.neuropharm.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. Beyond bipolar conceptualizations and measures: the case of attitudes and evaluative space. Personality and Social Psychology Review. 1997;1:3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Calder A, Beaver J, Davis M, van Ditzhuijzen J, Keane J, Lawrence A. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ. Disgust discussed. Ann Neurol. 2003;53:427–428. doi: 10.1002/ana.10565. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Reviews Neuroscience. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The James-Lange theory of emotion: A critical examination and an alternative theory. Am. J. Psychol. 1927;39:10–124. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and dappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Kim D, Susskind J, Anderson A. In bad taste: evidence for the oral origins of moral disgust. Science. 2009;323:1222–1226. doi: 10.1126/science.1165565. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Anderson AK. Understanding disgust. Ann N Y Acad Sci. 2012;1251:62–76. doi: 10.1111/j.1749-6632.2011.06369.x. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Lohr JM. Disgust, fear, and the anxiety disorders: a critical review. Clin Psychol Rev. 2009;29:34–46. doi: 10.1016/j.cpr.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Mapping of globus pallidus and ventral pallidum lesions that produce hyperkinetic treading. Brain Res. 1994;668:16–29. doi: 10.1016/0006-8993(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: A lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskine KJ, Kacinik NA, Prinz JJ. A bad taste in the mouth: gustatory disgust influences moral judgment. Psychol Sci. 2011;22:295–299. doi: 10.1177/0956797611398497. [DOI] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman Barrett L, Russell JA. Structure of current affect. Curr. Dir. Psychol. 1999;8:10–14. [Google Scholar]
- Fluharty SJ, Grill HJ. Taste reactivity of lateral hypothalamic lesioned rats: effects of deprivation and tube feeding. Neuroscience Abstracts. 1981;7:29. [Google Scholar]
- Fulton JF, Ingraham FD. Emotional disturbances following experimental lesions of the base of the brain (Pre-chiasmal) Am J Physiol. 1929;90:353. [Google Scholar]
- Gray JA. The Neuropsychology of Anxiety - an Inquiry into the Functions of the Septo-Hippocampal System. Behav Brain Sci. 1982;5:469–484. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebraska Symposium on Motivation. 1996;43:61–134. [PubMed] [Google Scholar]
- Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978b;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. The smooth fractionator. J Microsc. 2002;207:191–210. doi: 10.1046/j.1365-2818.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M. Disgust and Huntington's disease. Neuropsychologia. 2007;45:1135–1151. doi: 10.1016/j.neuropsychologia.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, Zahm DS. Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and its Implications for Neuropsychiatric Illness. Amsterdam: Elsevier: Academic Press; 2008. [Google Scholar]
- Heining M, Young AW, Ioannou G, Andrew CM, Brammer MJ, Gray JA, Phillips ML. Disgusting smells activate human anterior insula and ventral striatum. Ann N Y Acad Sci. 2003;1000:380–384. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Herz RS. Verbal priming and taste sensitivity make moral transgressions gross. Behav Neurosci. 2014;128:20–28. doi: 10.1037/a0035468. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid Modulation of Ventral Pallidal Afferents to Ventral Tegmental Area Neurons. J Neurosci. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Thompson RD. Aversion to lateral hypothalamic stimulation caused by intragastric feeding or obesity. J Comp Physiol Psychol. 1969;68:536–543. doi: 10.1037/h0027651. [DOI] [PubMed] [Google Scholar]
- Jackson Hughlings J, editor. Selected writings of John Hughlings Jackson. London: Staples Press; 1958. [Google Scholar]
- Inui T, Shimura T, Yamamoto T. The role of the ventral pallidum GABAergic system in conditioned taste aversion: effects of microinjections of a GABAA receptor antagonist on taste palatability of a conditioned stimulus. Brain Res. 2007;1164:117–124. doi: 10.1016/j.brainres.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32:1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, Ross CA, Nance M, Kayson E, Julian-Baros E, Hayden MR, Kieburtz K, Guttman M, Oakes D, Shoulson I, Beglinger L, Duff K, Penziner E, Paulsen JS. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain : a journal of neurology. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L. Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behav. Neurosci. 1985;99:531–545. doi: 10.1037//0735-7044.99.3.531. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, Merz CJ, Kagerer S, Walter B, Sammer G, Vaitl D, Stark R. Neural correlates of disgust- and fear-conditioned responses. Neuroscience. 2012;201:209–218. doi: 10.1016/j.neuroscience.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. Journal of comparative and physiological psychology. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A Pause in Nucleus Accumbens Neuron Firing Is Required to Initiate and Maintain Feeding. J. Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P, Tuerlinckx F, Russell JA, Barrett LF. The relation between valence and arousal in subjective experience. Psychol Bull. 2013;139:917–940. doi: 10.1037/a0030811. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The Emotion Probe - Studies of Motivation and Attention. Am Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? J Pers Soc Psychol. 2001;81:684–696. [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neuroscience. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giampietro V, Brammer MJ, Phillips ML. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. The European journal of neuroscience. 2008;27:3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and Motor Circuitry Underlying Footshock-Induced Reinstatement of Cocaine-Seeking Behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Sherrington CS. Some observations on the bucco-pharyngeal stage of reflex deglutition in the cat. Q J Exp Physiol. 1915;9:147–186. [Google Scholar]
- Miller J, Vorel S, Tranguch A, Kenny E, Mazzoni P, van Gorp W, Kleber H. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- Morgane PJ. Alterations in feeding and drinking behavior of rats with lesions of the globus pallidi. Am J Physiol. 1961;201:420–428. doi: 10.1152/ajplegacy.1961.201.3.420. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Norgren R, Leonard CM. Ascending central gustatory pathways. J. Comp. Neurol. 1973;150:217–237. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biol Psychol. 2010;84:422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Cisler J, McKay D, Phillips ML. Is disgust associated with psychopathology? Emerging research in the anxiety disorders. Psychiatry Res. 2010;175:1–10. doi: 10.1016/j.psychres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffmann C, Norgren R, Grill HJ. Sensory affect and motivation. Ann N Y Acad Sci. 1977;290:18–34. doi: 10.1111/j.1749-6632.1977.tb39713.x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy C, Ghosh B, Vasudev R. Regulatory role of nucleus accumbens in the ingestion of sucrose and saccharine. Indian J Exp Biol. 1998;36:820–823. [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nev√°rez N, Hamid AA, Aragona BJ. Mu-Opioid Receptors within Subregions of the Striatum Mediate Pair Bond Formation through Parallel Yet Distinct Reward Mechanisms. J Neurosci. 2013;33:9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus Accumbens Dopamine/Glutamate Interaction Switches Modes to Generate Desire versus Dread: D1 Alone for Appetitive Eating But D1 and D2 Together for Fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Castro D, DiFeliceantonio AG, Robinson MJF, Berridge KC. Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neuroscience and Biobehavioral Review. 2013a doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Plawecki AM, Berridge KC. Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine. Eur J Neurosci. 2013b;37:1789–1802. doi: 10.1111/ejn.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus Accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn. Mem. 2010;17:539–546. doi: 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Ma S, Barker DJ, West MO. Rapid phasic activity of ventral pallidal neurons during cocaine self-administration. Synapse. 2010;64:704–713. doi: 10.1002/syn.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P. Disgust. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. New York: Guilford; 2000. pp. 637–653. [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychol Rev. 1987;94:23–41. [PubMed] [Google Scholar]
- Rozin P, Haidt J. The domains of disgust and their origins: contrasting biological and cultural evolutionary accounts. Trends Cog Sci. 2013;17:367–368. doi: 10.1016/j.tics.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis M, Haviland J, editors. Handbook of Emotions. Gilford; 2008. pp. 757–776. [Google Scholar]
- Rozin P, Lowery L, Ebert R. Varieties of disgust faces and the structure of disgust. Journal of Personality & Social Psychology. 1994;66:870–881. doi: 10.1037//0022-3514.66.5.870. [DOI] [PubMed] [Google Scholar]
- Russell JA, Carroll JM. On the bipolarity of positive and negative affect. Psychological Bulletin. 1999;125:3–30. doi: 10.1037/0033-2909.125.1.3. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Dimalta S, Di Giorgio A, Taurisano P, Blasi G, Scarabino T, Giannatempo G, Nardini M, Bertolino A. Preferential responses in amygdala and insula during presentation of facial contempt and disgust. The European journal of neuroscience. 2006;24:2355–2362. doi: 10.1111/j.1460-9568.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Two types of aphagia and two types of sensorimotor impairment after lateral hypothalamic lesions: observations in normal weight, dieted, and fattened rats. Journal of Comparative and Physiological Psychology. 1978;92:720–741. doi: 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- Schnall S, Haidt J, Clore GL, Jordan AH. Disgust as embodied moral judgment. Pers Soc Psychol Bull. 2008;34:1096–1109. doi: 10.1177/0146167208317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Schairer W, Donahue RJ, Powell V, Janak PH. Similar neural activity during fear and disgust in the rat basolateral amygdala. PloS one. 2011;6:e27797. doi: 10.1371/journal.pone.0027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. New York: C. Scribner's sons; 1906. [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic Hotspots: Generating Sensory Pleasure in the Brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford, U.K: Oxford University Press; 2010. pp. 27–49. [Google Scholar]
- Sorenson CA, Ellison GD. Striatal organization of feeding behavior in the decorticate rat. Exp Neurol. 1970;29:162–174. doi: 10.1016/0014-4886(70)90047-6. [DOI] [PubMed] [Google Scholar]
- Spiegel EA, Miller HR, Oppenheimer MJ. Forebrain and rage reactions. J. Neurophysiol. 1940;3:538–548. [Google Scholar]
- Sprengelmeyer R, Young AW, Pundt I, Sprengelmeyer A, Calder AJ, Berrios G, Winkel R, Vollmoeller W, Kuhn W, Sartory G, Przuntek H. Disgust implicated in obsessive-compulsive disorder. Proceedings. Biological sciences / The Royal Society. 1997;264:1767–1773. doi: 10.1098/rspb.1997.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973:254–278. [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Brooks FH, Mills LE. Approach and withdrawal analysis of the effects of hypothalamic stimulation and lesions in rats. Journal of Comparative and Physiological Psychology. 1979;93:446–466. doi: 10.1037/h0077590. [DOI] [PubMed] [Google Scholar]
- Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res. 2012;226:548–554. doi: 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Tybur JM, Lieberman D, Kurzban R, DeScioli P. Disgust: Evolved function and structure. Psychol Rev. 2013;120:65–84. doi: 10.1037/a0030778. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Kolb B, Cooley RK. Behavior of the rat after removal of the neocortex and hippocampal formation. J Comp Physiol Psychol. 1978;92:156–175. doi: 10.1037/h0077447. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan L, Vaidya J, Humphreys C, Beglinger L, Paradiso S. Emotional and motivational changes after bilateral lesions of the globus pallidus. Neuropsychology. 2008;22:412–418. doi: 10.1037/0894-4105.22.3.412. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Beaver JD, Ewbank MP, Keane J, Passamonti L, Lawrence AD, Calder AJ. Leaving a bad taste in your mouth but not in my insula. Social cognitive and affective neuroscience. 2009;4:379–386. doi: 10.1093/scan/nsp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, Warren HB, Akert K. The behavior of chronic cats with lesions in the frontal association cortex. Acta Neurobiol Exp (Wars) 1972;32:361–392. [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Nat Acad Sci. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Blecker CR, Schafer A, Hackmack K, Haynes JD, Vaitl D, Stark R, Schienle A. fMRI pattern recognition in obsessive-compulsive disorder. NeuroImage. 2012;60:1186–1193. doi: 10.1016/j.neuroimage.2012.01.064. [DOI] [PubMed] [Google Scholar]
- Wirsig CR, Grill HJ. Contribution of the rat's neocortex to ingestive control: I. Latent learning for the taste of sodium chloride. Journal of Comparative and Physiological Psychology. 1982;96:615–627. doi: 10.1037/h0077911. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR. Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol Biochem Behav. 2010;96:342–346. doi: 10.1016/j.pbb.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundt W. Principles of Physiological Psychology. New York: MacMillen; 1904. [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus Accumbens Medium Spiny Neurons Target Non-Dopaminergic Neurons in the Ventral Tegmental Area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PT. An experimental study of mixed feelings. Am. J. Psychol. 1918;29:237–271. [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional--anatomical 'macrosystems'. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J. Comp. Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic ‘hotspot’ of Peciña and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J. Comp. Neurol. 2013;521:50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J. Comp. Neurol. 1996;364:340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. J. Neurophysiol. 2002;87:1068–1075. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chem. Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- Zito KA, Vickers G, Roberts DC. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacology, Biochemistry & Behavior. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]