Abstract
Objective
Primary-care (PC) settings may be an opportune place to deliver obesity interventions. Scalable interventions utilizing motivational interviewing (MI), supported by internet resources, may overcome obstacles to effective obesity treatment dissemination. This study was a randomized controlled trial (RCT) testing two web-supported interventions, motivational interviewing (MIC) and nutrition psychoeducation (NPC), an attention-control intervention, to usual care (UC).
Design and Methods
89 overweight/obese patients, with and without binge eating disorder (BED), were randomly assigned to MIC, NPC, or UC for 3 months in PC. Patients were assessed independently at post-treatment and at 3-month follow-up
Results
Weight, triglyceride levels, and depression scores decreased significantly in NPC when compared to UC but not MIC; UC and MIC did not differ significantly. Weight-loss results maintained at 3-month follow-up: approximately 25% MIC and NPC patients achieved at least 5% weight-loss which did not differ by BED status. Fidelity ratings were high and treatment adherence was associated with weight loss.
Conclusions
This is the first RCT in PC testing MI for obesity to include an attention-control intervention (NPC). NPC, but not MI, showed a consistent pattern of superior benefits relative to UC. BED status was not associated but treatment adherence was associated with weight loss outcomes.
Keywords: obesity, randomized controlled trial, weight loss, primary care, motivational interviewing, binge eating
Introduction
The prevalence of overweight and obesity has risen dramatically in the United States, with estimated rates now at 69.2%.1 The life-threatening medical consequences of excess weight continue to drive efforts to develop accessible weight loss interventions. Primary care (PC) offers an advantageous setting in which to disseminate scalable weight loss treatment.2
Weight loss treatment provided within PC, however, is limited3-5 due to several implementation barriers, including limited resources, time, and training.6 Developing scalable and effective methods for providing treatment in PC is imperative. Motivational interviewing (MI), combined with web-supported resources, has potential to overcome these unique barriers. MI, an evidence-based, time limited, person-centered counseling approach for strengthening a person's motivation and commitment to behavior change,7 can be effectively implemented by general medical practitioners, without prior therapeutic experience, to treat health-related behavioral concerns.8 A review9 and meta-analysis10 support the effectiveness of MI for weight loss.
MI interventions, typically combined with behavioral weight loss techniques, with relatively modest time requirements (e.g., 8 phone calls), within PC have shown average weight losses ranging from 011−7.312 pounds when compared to usual care. Weight loss outcomes, however, may be improved further by adding web-supported resources (e.g., resources for tracking food/exercise, setting weight loss, calorie, or exercise goals).13 Existing studies examining this combined intervention MI and web resources,11,13-16 however, are limited in several ways. They tend to exclude overweight individuals (i.e., recruiting obese only), include clinicians who are not often available in PC (e.g., dieticians), omit information about MI training and fidelity, metabolic outcomes, and follow-up assessments after treatment cessation, and fail to compare the MI intervention to an attention-control group.
An important subgroup of overweight and obese individuals also has been overlooked in the aforementioned studies, those with binge eating disorder (BED),17 which is defined by recurrent binge eating without regular compensatory behaviors.18 This is despite the fact that relative to obese persons without BED, BED is associated with increased medical co-morbidity and health-care utilization19,20 and may decrease individuals' ability to lose weight.21
The current study sought to address these issues by testing the effectiveness of scalable PC weight loss programs that are easily accessible by patients and by expanding the patients recruited, outcomes examined, and assessment protocols. Two treatment conditions, one incorporating MI (MIC) and one nutrition psychoeducation (NPC) designed as an attention-control intervention, both augmented by web-supported resources, were compared to usual care (UC). Both active conditions were provided by medical assistants (MAs). We hypothesized that MIC patients would lose significantly more weight and experience more weight-related improvements compared to NPC and UC patients, with BED patients losing significantly less weight than patients without BED.
Methods and Procedures
Participants
Participants (>18 years old) were 89 overweight and obese patients (body mass index (BMI)≥25, ≤55) receiving PC services at an urban university-based medical healthcare center. They were recruited through PC provider referrals and flyers placed in waiting/patient rooms. Recruitment was intended to enhance generalizability by utilizing relatively few exclusionary criteria. Exclusion criteria included over 65 years old, severe psychiatric problems (e.g., schizophrenia), severe medical problems (e.g., cardiac disease), pregnancy/breastfeeding, or uncontrolled liver, thyroid disease, hypertension, or diabetes. The Physical Activity Readiness Questionnaire (PAR-Q)22 was used to exclude individuals with cardiovascular problems, chest pains, and unexplained/frequency dizziness23 and PC provider consent was required if patients endorsed high blood pressure, physical conditions that may prohibit physical activity, or infrequent/explainable dizziness.23 Participants were required to have regular internet and telephone access.
Measures
The following measures were collected at baseline, post-treatment (week 12), and 3-month follow-up (week 24).
The Eating Disorder Examination (EDE),24 a semi-structured interview for assessing eating disorders and diagnosing BED (edited to correspond with DSM-5), has demonstrated good inter-rater and test-retest reliability.25 The EDE-Global score provides an index of eating disorder symptomatology, with higher scores reflecting greater severity. Current Cronbach's alpha was .88.
The Beck Depression Inventory (BDI)26 assesses current depression level with higher scores reflecting increased depression; the BDI has excellent reliability and validity.27 Current Cronbach's alpha was .88.
The Autonomous Motivation (AM)28 subscale of the Treatment Self-Regulation Questionnaire measures internal/personal reasons for losing weight with satisfactory reliability. Higher scores reflect higher levels of motivation. Current Cronbach's alpha was .86.
Physical and Metabolic Measurements
Height was measured at baseline-only using a wall measure, weight was measured using a large capacity digital scale. Blood pressure and pulse were measured at baseline, post, and 3-month follow-up using automated blood pressure monitors, recorded readings were an average of two measurements obtained in a standardized manner by the clinicians. Blood work was drawn and analyzed by Quest Diagnostics at baseline and post only.
Procedures
The study had IRB approval. All participants provided written informed consent. Patients completed the self-report measures and were screened by master- or doctoral-level psychology clinicians trained in eating/weight disorders and who were blinded to the patients' treatment condition. Participants were randomly assigned, stratified by BED diagnosis, to one of three conditions. Participants were reimbursed at assessment points, receiving up to $200 total.
Interventions
Motivational Interviewing and Internet Condition (MIC)
This five-session, manualized, 12-week intervention included guidelines to help MAs flexibly apply MI with strategies to motivate patients for weight-related behavior change and allowed focus on BED as needed. The first session included an initial 60-minute in-person individual session, which consisted of 40 minutes of MI focused on motivation for weight loss and treatment adherence, ending with patients setting their chosen and specific weight-related goals. Use of MI-inconsistent strategies (e.g., confrontation) was proscribed. The final 20 minutes (of first session) was training in the use of supplemental materials: 1) a free weight loss website (Livestrong.com, which does not include MI materials); and 2) a LEARN manual (Lifestyle, Exercise, Attitudes, Relationships, Nutrition),29 a readily-available, well-researched weight loss manual. Clinicians taught patients to login to Livstrong.com, enter pertinent information (height, weight, age, activity level) and weekly weight loss goals. Livestrong.com then provided patients with daily calorie guidelines for attaining this goal. Patients were shown how to track food, weight, and exercise, and to monitor other nutrition related information (e.g., carbohydrate intake). At the patients' discretion, they received personalized feedback on food journals at subsequent sessions. Following this first appointment, patients received up to four additional 20-minute MI sessions (in-person at weeks 6, 12, by phone at weeks 3, 9). Clinicians used MI strategies (e.g., open-ended questions, change planning) in these sessions to enhance patient motivation to meet weight-related goals (e.g., decreasing calories, increasing fruit/vegetable intake).
Nutrition Psychoeducation and Internet Condition (NPC) was designed as a five-session psychoeducation only, attention-control. The sessions provided basic nutritional information (e.g., recommended fruit/vegetable intake) based on the recommendations of the American Heart Association and United States Department of Agriculture and allowed patients to ask questions to better understand the material. NPC participants received the same manual, and opportunity to sign up and use Livstrong.com to set weight loss/calorie goals as in MIC. However, any further discussion about motivation, food tracking, goal setting, or personalized feedback was proscribed.
Usual Care (UC) patients met briefly for the first appointment to receive their randomization and were asked to continue working with their PC providers for weight-related concerns and not to start any structured/commercial weight loss programs (e.g., Weight Watchers) until after the 3-month follow-up assessment. They were not informed of the weight loss website or manual.
Training, clinicians, and fidelity
The MAs did not have prior weight loss treatment or MI training. Four MIC clinicians attended two eight-hour training sessions by a member of the Motivational Interviewing Network of Trainers. Clinicians needed to demonstrate adequate MI skills, based on an a priori criterion-level of performance,30,31 with three mock and one real MI sessions, before delivering treatment. Three NPC clinicians attended one eight-hour training session. All clinicians attended separate 60- to 90-minute group supervisions once every three weeks. All sessions were recorded.
Fidelity ratings
Two independent research-clinicians were trained to rate treatment adherence. For MIC, consistent with standardized protocols,30,31 we used the Independent Tape Rater Scale to judge the degree to which the MAs performed MI (Table 1) adequately (i.e., strategy present at least 3 times per session with adequate skill) and avoided MI-inconsistent techniques. For NPC, we created two items modeled on those used to rate MIC that targeted psychoeducation about nutrition, as well as the use of MI-oriented strategies proscribed in NPC.
Table 1. Rater agreement on adequate performance in the delivery of MIC and NPC strategies.
| Number of Sessions Meeting Criterion (n=15) | Rater Agreement | ||
|---|---|---|---|
|
| |||
| Rater 1 | Rater 2 | ||
| MIC Sessions | |||
| MI-Consistent Strategies | |||
| MI spirit | 14 | 15 | 93% |
| Open questions | 15 | 15 | 100% |
| Reflections | 15 | 15 | 100% |
| Affirmations | 13 | 15 | 87% |
| Fostering collaboration | 12 | 15 | 80% |
| Motivation for change | 12 | 14 | 87% |
| Client-centered discussion & feedback | 12 | 12 | 80% |
| Exploring pros/cons/ambivalence | 0 | 0 | 100% |
| Developing discrepancies | 1 | 0 | 93% |
| Change planning discussion | 14 | 15 | 93% |
| MI-Inconsistent Strategies | |||
| Unsolicited advice | 4 | 1 | 80% |
| Direct confrontation | 0 | 0 | 100% |
| Asserting authority | 0 | 0 | 100% |
| NPC Sessions | |||
| NPC-Consistent Strategies | |||
| Psychoeducation about nutrition topic | 15 | 15 | 100% |
| Provide patient opportunity to ask questions | 14 | 14 | 100% |
| NPC-Inconsistent Strategies | |||
| Discuss patient's motivations to lose weight | 0 | 0 | 100% |
| Change planning discussion | 0 | 0 | 100% |
Note: MIC=Motivational Interviewing and Internet Condition. NPC=Nutrition Psychoeducation and Internet Condition. UC=Usual Care. Adequate performance criterion = demonstration of the strategy at least 3 times in the session with adequate skill. Fifteen sessions per condition (N=30) were randomly selected for two trained judges to independently rate.
Statistical Analyses
Missing data were minimal. Intent-to-treat data are presented (exceptions: Treatment Credibility, Satisfaction), with the last observation carried forward, and outliers beyond 3 standard deviations replaced with highest recorded value within the 3 standard deviation range. Baseline characteristics for the treatment groups were compared using chi-square and ANOVAs. Percent weight loss was calculated so negative scores indicate weight loss. 3 (MIC, NPC, UC) × 2 (baseline, post) and 3 (MIC, NPC, UC) × 3 (baseline, post, 3-month follow-up) repeated measures ANOVAs compared treatments on outcomes. Follow-up ANCOVAs were used to test for differences when repeated measures ANOVA condition-by-time interactions were significant. One-way ANOVAs, chi-square, Fisher's tests, correlations, and t-tests also were used to examine treatment-related outcomes.
Results
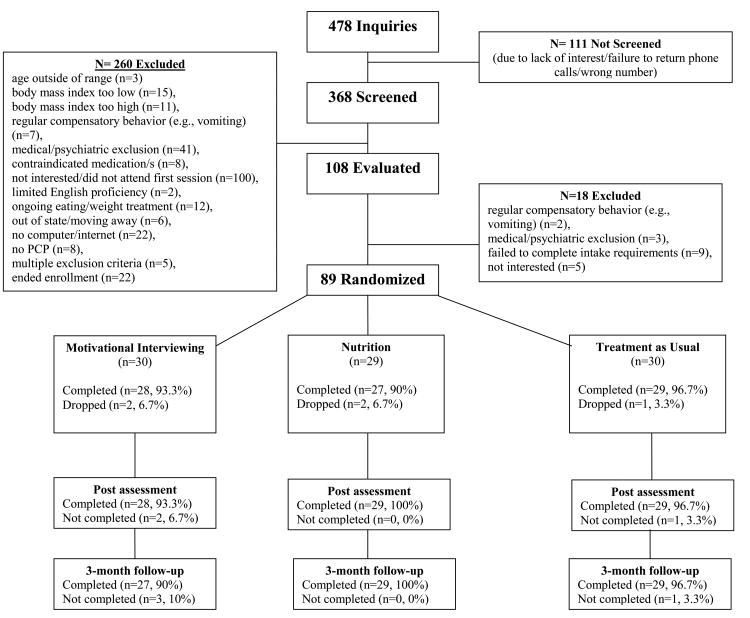
Figure 1 summarizes the flow of study participants. Patient retention was excellent, 94.4%, 96.6%, and 95.5% of patients completed treatment, post-treatment assessment, and 3-month follow-up assessment, respectively. Participants had a mean age of 47.9 years (SD=10.5, range 22-65), a mean BMI of 35.3 kg/m2 (SD=7.0), 76.4% (n=68) were female, 25.8% (n=23) met DSM-5 BED criteria, and the sample was relatively diverse, 65.2% (n=58) of participants identified as White, not Hispanic (Table 2). Treatment groups did not differ significantly at baseline (Tables 2, 3, and 4), with the exception of EDE-Global scores.
Figure 1. Treatment recruitment and retention consort.
Table 2. Demographic and clinical characteristics of 89 randomized patients across treatments.
| Variable | MIC (n=30) | NPC (n=29) | UC (n=30) | Test statistic |
|---|---|---|---|---|
| Age, Mean (SD) | 47.07 (9.97) | 48.93 (11.59) | 47.77 (10.05) | F(2,86)=0.23, p=.792 |
| Body Mass Index, Mean (SD) | 34.65 (7.06) | 35.07 (7.52) | 36.08 (6.44) | F(2,86)=0.33, p=.719 |
| Female, n (%) | 24 (80%) | 20 (69%) | 24 (80%) | x2(2)=1.32, p=.517 |
| Ethnicity, n (%) | x2(8)=6.48, p=.594 | |||
| White, not Hispanic | 19 (63%) | 20 (69%) | 19 (63%) | |
| White, Hispanic | 0 (0%) | 2 (7%) | 2 (7%) | |
| African-American | 8 (27%) | 3 (10%) | 7 (23%) | |
| Bi/multi-racial | 1 (3%) | 3 (10%) | 1 (3%) | |
| Bi/multi-racial, Hispanic | 2 (7%) | 1 (3%) | 1 (3%) | |
| Education, n (%) | x2(4)=2.13, p=.712 | |||
| ≤ High school diploma | 3 (10%) | 4 (14%) | 4 (13%) | |
| Some college | 9 (30%) | 6 (21%) | 11 (37%) | |
| ≥ College degree | 18 (60%) | 19 (66%) | 15 (50%) | |
| DSM-5 BED Diagnosis n (%) | 8 (27%) | 7 (24%) | 8 (27%) | x2(2)=0.07, p=.968 |
Note: MIC=Motivational Interviewing and Internet Condition. NPC=Nutrition Psychoeducation and Internet Condition. UC=Usual Care. Test statistic = chi-square for categorical variables and ANOVAs for continuous variables. P-values are for two-tailed tests. SD = standard deviation. n = number. BED = binge eating disorder.
Table 3. Intent-to-treat physical and metabolic measurements of 89 randomized patients across treatment assessments.
| Pre-treatmenta | Post-treatment | 3-month follow-up | Test Statistic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | NPC | UC | MIC | NPC | UC | MIC | NPC | UC | F-test/ (Partial Eta2) | |
| Variable | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | Time (Main effect) |
| Time × Randomization | ||||||||||
| Total Cholesterol1 | 90.6(38.2) | 94.6(32.7) | 94.7(28.3) | 87.6(33.3) | 85.1(34.6) | 95.8(35.1) | F(1, 86)=3.51, p=.064/ (.039) | |||
| F(2,86)=2.30, p=.106/ (.051) | ||||||||||
| HDL | 56.1(14.7) | 55.1(14.2) | 56.0(13.4) | 56.6(17.2) | 53.4(12.9) | 54.7(14.2) | F(1, 86)=1.05, p=.309/ (.012) | |||
| F(2,86)=0.64, p=.532/ (.015) | ||||||||||
| LDL | 112.1(36.4) | 116.3(29.0) | 116.1(20.6) | 106.6(32.3) | 112.0(32.0) | 116.8(26.5) | F(1, 85)=3.36, p=.070/ (.038) | |||
| F(2,85)=1.35, p=.265/ (.031) | ||||||||||
| Triglycerides | 117.8(66.3) | 116.4(40.2) | 113.1(59.6) | 118.0(69.9) | 99.0(31.9) | 121.6(63.2) | F(1, 86)=0.56, p=.458/ (.039) | |||
| F(2,86)=3.75, p=.028/ (.080) | ||||||||||
| Glucose | 97.3(15.9) | 100.4(16.2) | 95.8(14.8) | 97.4(17.8) | 96.5(14.9) | 97.2(15.7) | F(1, 85)=0.47, p=.493/ (.006) | |||
| F(2,85)=1.71, p=.186/ (.039) | ||||||||||
| HbA1c | 5.7(0.5) | 5.7(0.4) | 5.7(0.4) | 5.7(0.5) | 5.7(0.5) | 5.7(0.5) | F(1, 85)=2.21, p=.141/ (.025) | |||
| F(2,85)=0.87, p=.423/ (.020) | ||||||||||
| Systolic BP | 120.3(11.1) | 127.7(13.6) | 123.3(13.2) | 116.1(10.5) | 121.7(13.7) | 121.3(12.0) | 118.2(14.0) | 122.7(14.8) | 125.2(13.0) | F(2, 172)=5.45,p=.005/ (.060) |
| F(4, 172)=1.32, p=.265/ (.030) | ||||||||||
| Diastolic BP | 76.8(9.6) | 77.5(10.8) | 73.3(10.2) | 73.8(8.9) | 76.0(10.0) | 72.8(8.5) | 76.0(11.0) | 76.2(9.9) | 75.0(9.4) | F(2, 172)=2.34, p=.100/ (.026) |
| F(4, 172)=0.66, p=.618/ (.015) | ||||||||||
| Pulse | 72.9(11.4) | 75.0(11.6) | 74.7(12.1) | 72.0(12.5) | 72.1(12.0) | 76.0(11.8) | 73.9(12.0) | 72.8(10.6) | 73.9(11.7) | F(2, 172)=0.37, p=.692/ (.004) |
| F(4, 172)=1.21, p=.305/ (.028) | ||||||||||
Note: MIC=Motivational Interviewing and Internet Condition. NPC=Nutrition Psychoeducation and Internet Condition. UC=Usual Care. HDL=High-density lipoprotein. LDL=Low-density lipoprotein. HbA1c=Glycated hemoglobin. BP=Blood pressure.
= no significant differences at baseline.
Table 4. Intent-to-treat psychological and motivation measurements of 89 randomized patients across treatment assessments.
| Pre-treatmenta | Post-treatment | 3-month follow-up | Test Statistic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | NPC | UC | MIC | NPC | UC | MIC | NPC | UC | F-test/ (Partial Eta2) | |
| Variable | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | (M/SD) | Time (Main effect) |
| Time × Randomization | ||||||||||
| EDE-Global | 2.1(0.9) | 1.6(0.8) | 1.6(0.9) | 1.7(0.8) | 1.2(0.5) | 1.5(0.9) | 1.7(1.0) | 1.3(0.7) | 1.5(0.9) | F(2, 172)=12.18, p<.0005/ (.124) |
| F(4, 172)=2.14, p=.078/ (.047) | ||||||||||
| BDI | 7.8(6.5) | 7.9(5.7) | 6.9(5.7) | 6.3(5.7) | 5.1(3.9) | 6.9(6.2) | 7.3(7.1) | 5.4(4.7) | 7.8(8.4) | F(2, 172)=4.97, p=.008/ (.055) |
| F(4, 172)=2.52, p=.043/ (.055) | ||||||||||
| AM | 6.6(0.7) | 6.7(0.6) | 6.7(0.4) | 6.6(0.7) | 6.5(0.7) | 6.3(1.0) | 6.4(0.9) | 6.4(0.8) | 6.5(1.0) | F(2, 172)=5.69, p=.004/ (.062) |
| F(4, 172)=1.44, p=.222/ (.032) | ||||||||||
Note: MIC=Motivational Interviewing and Internet Condition. NPC=Nutrition Psychoeducation and Internet Condition. UC=Usual Care. EDE=Eating Disorder Examination. BDI=Beck Depression Inventory, AM=Autonomous Motivation subscale.
= no significant differences at baseline, with the exception of EDE-Global.
Treatment Credibility, Satisfaction, Fidelity, and Adherence
Participants completed a five-item treatment credibility measure after randomization, with higher scores indicating more credibility. One-way ANOVA was significant (F(2,75)=3.15, p=.049); LSD post-hoc analyses showed no significant specific condition differences, however, the average rating for UC (M=12.35, SD=7.49) was lower than MIC (M=16.63, SD=7.84) and NPC (M=16.54, SD=5.56). Participants completed an eight-item satisfaction measure (i.e., possible scores ranging from 8-80, with higher scores reflecting more satisfaction) at post-treatment. One-way ANOVA was significant (F(2, 81)=10.35, p<.0005); LSD post-hoc analyses showed UC (M=66.0; SD=11.1) patients were significantly less satisfied than MIC (M=73.3; SD=6.6, p=.001) and NPC (M=75.2; SD=5.3, p<.0005) patients. MIC and NPC did not differ (p=.393), with both reporting high levels of satisfaction. Both treatments were delivered as intended (Table 1).
96.4% of patients tracked on Livestrong.com at least once. MIC patients were significantly more likely than NPC patients to have tracked food in the 28 days prior to their 3-month follow-up assessment, x2(1)=4.03, p=.045 (37.0% versus 13.8%) but not in the 28 days prior to their post-treatment assessment, x2(1)=2.70, p=1.00 (66.7% versus 44.8%). Of the 12 LEARN manual chapters read, there were no significant differences between MIC (M=5.7;SD=54.9) and NPC (M=6.6;SD=4.3), t(57)=0.814, p=.419.
Weight
The one-way ANOVA examining percent weight loss from baseline to post-treatment was significant, F(2,86)=4.52, p=.014 (Figure 2). LSD post-hocs revealed NPC lost significantly more weight than UC (p=.004) but not MIC (p=.318). The difference between MIC and UC approached significance (p=.053). The one-way ANOVA examining percent weight loss from baseline to 3-month follow-up assessment was similar, F(2,86)=3.89, p=.024. LSD post-hoc analyses revealed NPC lost significantly more than UC (p=.007) but not MIC (p=.148). There were no significant differences between MIC and UC (p=.184). Percent weight change translates to the following average pounds lost by post-treatment, MIC M=−3.3 (SD=6.5), NPC M= −4.9 (SD=6.1), UC M= −0.4 (SD=6.2), and 3-month follow-up assessment, MIC M= −2.6 (SD=8.7), NPC M= −5.7 (SD= 9.0), UC M= 0.4 (SD=7.2).
Figure 2. Intent-to-treat percent weight loss.
Note. MIC= Motivational Interviewing and Internet Condition. NPC= Nutrition Psychoeducation and Internet Condition. UC=Usual Care.
Percent weight loss (MIC/NPC combined) was significantly correlated with tracking frequency and LEARN chapters read (Table 5).
Table 5. Relationship between weight loss and treatment compliance across active treatment groups.
| MIC/NPC Combined | MIC/NPC Combined | |||
|---|---|---|---|---|
| PWL Baseline to Post | PWL Baseline to 3-month | Lost 5% by Post | Lost/maintained 5% by 3-month | |
|
|
||||
| Tracking Post | r(56) = −.579,p<0005 | r(56) = −.468,p<.0005 | t(54) =−5.61,p<.0005 | t(17.85) =−4.45,p<.0005 |
| Tracking 3-month | r(56) = −.300,p=.025 | r(56) = −.207, p=.126 | t(54) = −1.71, p =.117 | t(16.55) =−1.66, p=.115 |
| LEARN Post | r(59) = −.231, p=.078 | r(59) = −.288,p=.027 | t(57) = −1,21, p = .231 | t(57) = −1.84, p = .071 |
Note: MIC=Motivational Interviewing and Internet Condition. NPC=Nutrition Psychoeducation and Internet Condition. PWL=Percent Weight Loss. Post=Post-Treatment Assessment. 3-month=3-month Follow-up Assessment. Tracking Post=Frequency of Tracking in Livestrong.com in 28 days Prior to Post-Treatment Assessment. Tracking 3-month=Frequency of Tracking in Livestrong.com in 28 days Prior to 3-month Follow-up Assessment. LEARN Post= Total Number of Chapters Read from the Weight Loss Manual: Lifestyle, Exercise, Attitudes, Relationships, Nutrition during Treatment.
BED versus patients without BED
Two t-tests (MIC/NPC combined) showed no significant difference in percent weight loss between patients with and without BED at post-treatment, t(87)= −0.656, p=.513, or 3-month follow-up, t(87)= −0.754, p=.453.
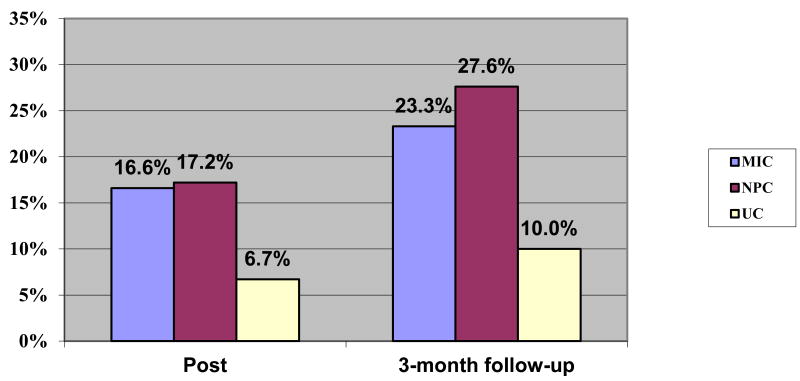
Patients reaching at least 5% loss of initial body weight did not differ significantly by condition based on Fisher's tests (Figure 3). The percentages, however, suggest that approximately twice the number of MIC and NPC patients reached this goal at post-treatment and 3-month follow-up assessments than UC.
Figure 3. Percent of participants reaching at least 5% loss of initial weight.
Note. MIC= Motivational Interviewing and Internet Condition. NPC= Nutrition Psychoeducation and Internet Condition. UC=Usual Care.
Patients (MIC/NPC combined) reaching at least 5% weight loss by post-treatment or 3-month follow-up assessment tracked significantly more frequently in the 28 days prior to post but not 3-month follow-up when compared to those who did not. The total number of LEARN chapters read by post-treatment was not significantly different between participants who met or did not meet this goal (Table 5).
Physical and Metabolic Assessments
Table 3 shows the descriptive data for the physical measures and repeated measures ANOVAs. There was a significant decrease in systolic blood pressure over time, the time-by-condition interaction was not significant. The triglycerides time-by-condition interaction was significant. A follow-up ANCOVA (controlling for baseline triglycerides) was significant, F(2,85)=3.92, p=.024, partial eta2=.084. LSD post-hoc analyses showed that post-treatment triglycerides in the NPC were significantly lower compared to UC (p=0.008) and trended towards significance for MIC (p=0.057). There were no significant differences between MIC and UC (p=0.423).
Psychological and Motivation Assessments
Table 4 shows descriptive data for the psychological measures and repeated measures ANOVAs. There were significant decreases in disordered eating symptoms (EDE-Global) and motivation (AM) scores overtime, the time-by-condition interactions were not significant. The BDI time-by-condition interaction was significant. A follow-up ANCOVA (controlling for baseline BDI) examining post-treatment assessment BDI was significant, F(2,85)=3.60, p=.032, partial eta2=.078. LSD post-hoc analyses showed that NPC BDI scores at post-treatment assessment were significantly lower when compared to UC (p=0.009) but not MIC (p=0.195). There were no significant differences between MIC and UC (p=0.168). A second ANCOVA (controlling for baseline BDI) examining 3-month follow-up assessment BDI scores trended towards significance, F(2,85)=2.97, p=.057, partial eta2=.065.
Discussion
The current study tested scalable weight loss treatments in PC. Findings suggest that a low-burden weight loss intervention, supported with free and widely available web resources, can be implemented effectively in primary care. NPC was more effective than MIC when compared to UC and appears to have conferred additional weight, metabolic, and psychological benefits. Weight loss was related to treatment adherence and did not differ for BED patients. Both interventions, augmented by a web-supported resource, were well-received and utilized by patients. Fidelity ratings indicate that MAs can be trained to deliver MI effectively.
To our knowledge, this is the first MI weight loss intervention in PC to include an attention-control intervention comparison group. Somewhat surprisingly, only the NPC intervention showed clear and consistent benefits over UC, with NPC patients continuing to lose weight in the three months beyond treatment cessation. These findings raise doubt about the potential relative benefit of MI for obesity in PC settings. Notably, MI was delivered with at least adequate fidelity and dose.32 Reasons why NPC outperformed MIC are uncertain. One possibility is that the effects of MI may have been attenuated in the current study because the patients were already highly motivated upon treatment entry. For example, patients averaged 6.6-6.7 on the Autonomous Motivation subscale at baseline, with 7 being the highest possible score. Alternatively, individuals' knowledge of basic nutritional facts may be overestimated33 and the relative lack of basic nutritional information in MIC could have hindered weight loss. While preliminary, with NPC we were able to achieve similar, and even additional benefits, with a more scalable and resource-efficient intervention and with similar satisfaction and retention compared to MIC.
Our findings suggest that primary care interventions for obesity may not require specialized MI or web resources. First, our findings, like those of other studies, 34 suggest that the use of adequately delivered MI in PC for weight loss is no more effective than well-implemented behavioral weight loss programs. Second, the current intervention was the first of its kind to test free web resources that are available to the public, resulting in comparable outcomes.14,15
Average weight loss was comparable to similar trials,14,15 though small overall. Nevertheless, while not statistically significant, approximately 25% of intervention participants lost at least 5% of their initial body weight, a goal associated with attenuating weight-related health consequences.35 The lack of statistical significance may be lack of power. The rates of participants reaching this 5% goal was similar to or exceeded those reported in other three-13,14 and six-month15 obese-only trials. The current study addressed a recent call to shift focus to scalability and adherence for weight loss treatment.36 From a public health perspective, by disseminating either treatment, approximately a quarter of physicians' patients could experience at least a 5% weight loss following approximately 2.5 hours of treatment.
Weight loss was related to treatment adherence. While treatment adherence was somewhat better among patients in MIC, this did not translate to more weight loss. Perhaps more targeted, or a stepped-care approach, with MI at critical treatment windows may improve outcomes,37 since it appears that motivation decreased slightly at each assessment point.
The NPC also resulted in specific metabolic and mood improvements. NPC patients' triglyceride levels decreased significantly when compared to UC, while a previous trial reported with no intervention-related metabolic improvements11 and other similar studies did not examining blood-work.13-16 Further, to our knowledge, this is the first study of its kind11,13-16 to examine depression. Relative to UC, NPC patients experienced significant depression decreases.
Our findings add to the mixed literature regarding the impact of BED on weight loss treatment.21 Overall, patients with versus without BED did not differ significantly in weight loss, but our sample size did not allow us to test for moderation effects (i.e., we were unable to examine whether NPC and MIC had differential weight loss effects by BED status). A recent RCT testing behavioral and medication treatments for obese patients found that BED moderated weight loss outcomes.40
This is the first PC MI weight loss study with web-support, to our knowledge, to include MI treatment fidelity. Although a PC MI weight loss study, without web-support, published follow-up fidelity data indicating that MI was not effectively implemented.38 Our findings suggest MAs, without prior training, can learn MI with at least adequate fidelity. The MAs also delivered NPC as intended. Our findings, though preliminary, suggest that organizations such as the Centers for Medicare and Medicaid should be encouraged to provide coverage for lifestyle interventions provided by clinicians other than PC physicians, physician assistants, and nurse practitioners.
The current study has several limitations. While the inclusion/exclusion criteria were meant to mimic typical PC patients, results may not generalize to non-treatment seeking populations or to patients with significant psychological and physical comorbidities. UC patients were proscribed from starting structured/commercial weight loss programs, however, research indicates low PC provider referrals to such programs.39 The sample also was relatively well-educated. Participants were compensated, which may influence retention and therefore outcomes. While recruitment was based on a power-analysis and retention was excellent, the sample size was relatively small. It is important to note also that some of the statistically significant improvements may be of limited or uncertain clinical value. Although the current study included follow-up assessments after treatment completion to fill a major gap in this emerging literature, longer-term follow-up is needed to discern lasting effects.
In summary, the current trial showed that a scalable weight loss intervention has potential to help substantial portions of patients in primary care settings lose enough weight that might be clinically meaningful.. The attention-control intervention, focused solely on nutrition psychoeducation, resulted in more extensive benefits than did MI, when compared to UC. These findings, however, are preliminary and require further examination with longer intervention and follow-up assessments, to the extent that is feasible within PC settings. Longer interventions, with focus on more specific nutritional information related to improving weight-related conditions (e.g., low-sugar to decrease HbA1c) may have more extensive metabolic benefits. Conceivably, MI may best be utilized within PC to encourage enrollment into in-house or external weight loss programs. Additional areas of future research include developing more extensive, and most importantly, free or low cost and accessible web-based weight loss resources available to PC settings that can serve as an adjunct to PC clinician support. Continuing to examine these and other elements of addressing weight loss in PC may further improve outcomes.
What is already known about this subject.
To increase dissemination, an opportune place to incorporate obesity interventions may be in primary care settings.
Motivational interviewing (MI) may result in weight loss and non-specialists can be trained to provide MI.
MI-interventions in primary care may be improved with additional web-supported weight loss resources.
What this study adds.
Scalable MI- and web-supported intervention provided reliably by non-specialist staff, medical assistants.
Includes metabolic variables, fidelity measures, an attention-control condition, follow-up assessments after treatment cessation, and a diverse patient group.
Weight loss did not differ significantly between patients diagnosed with or without binge eating disorder.
Acknowledgments
This study was supported by NIH career development awards, K23-DK092279 for RDB and K24-DK070052 for CMG.
We thank Drs. Inginia Genao, Robin Masheb, Patrick O'Connor, Deborah Tate, and Valentina Ivezaj, the clinicians Jennifer Lovallo, Kari McKinley, Marissa Patterson, Shawntel Payton, Carolyn Perrotti, and Madelyn Rubin, and research assistant Magali Laitem, M.A., for their contributions.
Footnotes
Conflicts of Interest: RDB, MAW, and SM have no conflicts of interest.
CMG reports no relevant conflicts of interest but has received consultant fees from Shire, honoraria from American Psychological Association and American Academy of CME, and book royalties from Guilford Press and Taylor and Francis for academic books.
RDB and CMG conceived the experiment; RDB, SM, and CMG conducted the experiment; RDB, MAW, and CMG analyzed data; all authors were involved in writing and had final approval of the submitted manuscript.
References
- 1.National Center for Health Statistics. Health, United States, 2012: With Special Feature on Emergency Care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- 2.Plourde G, Prud'homme D. Managing obesity in adults in primary care. CMAJ. 2012;184:1039–1044. doi: 10.1503/cmaj.111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai AG, Wadden TA. Treatment of obesity in primary care practices in the United States: A systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns. 2011;82:123–129. doi: 10.1016/j.pec.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malterud K, Ulriksen K. Obesity in general practice: A focus group study on patient experiences. Scand J Prim Health Care. 2010;28:205–210. doi: 10.3109/02813432.2010.526773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostbye T, Yarnall KSH, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 3rd. Guilford Press; New York: 2012. [Google Scholar]
- 8.Madison MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84:16–26. doi: 10.1016/j.pec.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 9.DiLillo V, Smith West D. Motivational Interviewing for weight loss. Psychiatr Clin N Am. 2011;34:861–869. doi: 10.1016/j.psc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Reviews. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich E, Candel MJJM, Schaper NC, de Vries NK. Effect evaluation of a Motivational Interviewing based counselling strategy in diabetes care. Diabetes Res Clin Practice. 2010;90:270–278. doi: 10.1016/j.diabres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Ely AC, Banitt A, Befort C, Hou Q, Rhode PC, Grund C, Greiner A, Jeffries S, Ellerbeck E. Kansas primary care weighs in: A pilot randomized trial of chronic care model program for obesity in 3 rural Kansas primary care practices. J Rural Health. 2008;24:125–132. doi: 10.1111/j.1748-0361.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 13.McDoniel SO, Wolskee P, Shen J. Treating obesity with a novel hand-held device, computer software program, and internet technology in primary care: the SMART motivated trial. Patient Educ Couns. 2010;79:185–191. doi: 10.1016/j.pec.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: A randomized controlled trial. Obesity. 2010;18:308–813. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDoniel SO, Hammond RS. A 24-week randomised controlled trial comparing usual care and metabolic-based diet plans in obese adults. Int J Clin Pract. 2010;64:1503–1511. doi: 10.1111/j.1742-1241.2010.02464.x. [DOI] [PubMed] [Google Scholar]
- 16.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, Emmons KM, Rosner BA, Colditz GA. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Bio Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 19.Johnson JG, Spitzer RL, Williams BW. Health problems, impairment and illnesses associated with bulimia nervosa and binge eating disorder among primary care and obstetric gynaecology patients. Psychol Med. 2001;31:1455–466. doi: 10.1017/s0033291701004640. [DOI] [PubMed] [Google Scholar]
- 20.Marques L, Alegria M, Becker AE, Chen C, Fang A, Chosak A, Diniz JB. Comparative prevalence, correlates of impairment, and service utilization for eating disorders across U.S ethnic groups: Implications for reducing ethnic disparities in health care access for eating disorders. Int J Eat Disord. 2011;44:412–420. doi: 10.1002/eat.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaine B, Rodman J. Responses to weight loss treatment among obese individuals with and without BED: A matched-study meta-analysis. Eat Weight Disorders. 2007;12:54–60. doi: 10.1007/BF03327579. [DOI] [PubMed] [Google Scholar]
- 22.Shephard RJ. PAR-Q: Canadian home fitness test and exercise screening alternatives. Sports Med. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 23.Webber KH, Tate DF, Bowling MJ. A randomized comparison of two motivationally enhanced internet behavioral weight loss programs. Behav Res Ther. 2008;46:1090–1095. doi: 10.1016/j.brat.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. 12th. Guilford Press; New York: 1993. pp. 317–60. [Google Scholar]
- 25.Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord. 2004;35:80–85. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Steer R. Manual for revised Beck Depression Inventory. New York: Psychological Corporation; 1987. [Google Scholar]
- 27.Beck AT, Steer R, Garbin MG. Psychometric properties of the Beck Depression Inventory 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 28.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Ed Res. 2007;22:691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- 29.Brownell KD. The LEARN Program for Weight Management. 10th. American Health Publishing Company; Dallas, TX: 2004. [Google Scholar]
- 30.Ball SA, Martino S, Corvino J, Morganstern J, Carroll KM. Independent tape rater guide. Unpublished psychotherapy tape rating. 2005 [Google Scholar]
- 31.Martino S, Ball SA, Nich C, Frankforter TL, Carroll LM. Community program therapist adherence and competence in motivational enhancement therapy. Drug Alcohol Depend. 2008;96:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke B. Meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Res Soc Work Pract. 2010;20:137–160. [Google Scholar]
- 33.Larzelere M, Marchand S, Chen H, Tillery B, Zoorob R. Basic nutrition: What patients know and don't know. MEJ Fam Med. 2005;3:1–10. [Google Scholar]
- 34.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A Primary care intervention for weight loss: Results of a randomized controlled pilot study. Obesity. 2010;18:1614–1618. doi: 10.1038/oby.2009.457. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3 Suppl 2:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 36.Pagota SL, Appelhans BM. A call for an end to the diet debates. JAMA. 2013;310:687–688. doi: 10.1001/jama.2013.8601. 35. [DOI] [PubMed] [Google Scholar]
- 37.Webber KH, Tate DF, Ward DS, Bowling JM. Motivation and its relationship to adherence to self-monitoring and weight loss in a 16-week Internet behavioral weight loss intervention. J Nutr Educ Behav. 2010;42:161–167. doi: 10.1016/j.jneb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Groeneveld IF, Proper KI, Absalah S, van der Beek AJ, van Mechelen W. An individually based lifestyle intervention for workers at risk for cardiovascular disease: A process evaluation. Am J Health Promot. 2011;25:396–401. doi: 10.4278/ajhp.091001-QUAN-319. [DOI] [PubMed] [Google Scholar]
- 39.Shiffman S, Sweeney CT, Pillitteri JL, Sembower MA, Harkins AM, Wadden TA. Weight management advice: What do doctors recommend to their patients? Prev Med. 2009;49:482–486. doi: 10.1016/j.ypmed.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Grilo CM, White MA. Orlistat with behavioral weight loss for obesity with versus without binge eating disorder: Randomized placebo-controlled trial at a community mental health center serving educationally and economically disadvantaged Latino/as. Behav Res Ther. 2013;51:167–175. doi: 10.1016/j.brat.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]