Abstract
Atypical motor behaviors are common among children with Autism Spectrum Disorders (ASD). However, little is known about onset and functional implications of differences in early motor development among infants later diagnosed with ASD. Two prospective experiments were conducted to investigate motor skills among six-month-olds at increased risk (high-risk) for ASD (N1 = 129; N2 = 46). Infants were assessed using the Mullen Scales of Early Learning (MSEL) and during toy play. Across both experiments, high-risk infants exhibited less mature object manipulation in a highly structured (MSEL) context and reduced grasping activity in an unstructured (free play) context than infants with no family history of ASD. Longitudinal assessments suggest that between six and ten months, grasping activity increases in high-risk infants.
Keywords: Autism, Grasping, Fine Motor Development, Endophenotype
Autism Spectrum Disorders (ASD) are a group of neurodevelopmental disorders characterized by impairments in social functioning and communication, and by the presence of restricted and stereotyped behaviors and interests (American Psychiatric Association, 2013). ASD prevalence has increased in recent years and affect an estimated 1 in 68 children (CDC, 2014). The majority of ASD cases are diagnosed after the age of four years (CDC, 2014; Mandell et al., 2010; Noterdaeme & Hutzelmeyer-Nickels, 2010), which is late from a developmental perspective. To improve outcomes of children with ASD, earlier ASD detection and enrollment into intervention programs are important priorities (Dawson et al., 2010; Landa, Holman, O'Neil, & Stuart, 2011).
ASD Risk Indicators and Endophenotypes
There is growing evidence that ASD has a prodromal phase lasting through late infancy, during which the diagnostic signs of ASD are not clearly observable (Elsabbagh & Johnson, 2010; Landa, Gross, Stuart, & Faherty, 2013). ASD indicators during this phase may involve biological signs such as atypical brain development (Wolff et al., 2012), and subtle behavioral signs in the attention or motor domains (e.g., Bhat, Galloway, & Landa, 2012; Elsabbagh et al., 2013; Flanagan, Landa, Bhat, & Bauman, 2012; Pierce, Conant, Hazin, Stoner, & Desmond, 2011). Family-affectedness is currently the best ASD risk indicator in early childhood, as younger siblings of an affected child are more likely to develop ASD themselves than members of the general population (Ozonoff et al., 2011). Identification of a genetic marker for ASD (e.g., susceptibility genes) may allow for earlier and more definite detection of children at risk. However, due to the large heterogeneity and wide range of different symptoms involved, no reliable genetic marker for ASDs has been identified yet (Szatmari et al., 2007). Biological or behavioral traits that occur in both affected and unaffected children with a family-history of ASD (i.e., genetic relatedness) are referred to as endophenotype (Szatmari et al., 2007). Identification of ASD endophenotypes may facilitate detection of genetic markers because sample sizes in genetic studies could be increased to include unaffected relatives. Subtle differences in motor skill development have been noted in children with ASD, but it remains unclear whether these differences also exist in unaffected siblings and whether they are a core characteristic or an endophenotype of ASD (Hilton, Zhang, Whilte, Klohr, & Constantino, 2012).
Motor Delay in ASD
Atypical motor behaviors are common in ASD and were noted in the first published reports on autism and Asperger's syndrome (Asperger, 1944; Kanner, 1943). Current studies suggest that a large proportion of children with ASD show motor deficits such as hypotonia and motor apraxia (Ming, Brimacombe, & Wagner, 2007) and that motor coordination deficits are common in ASD (for review see Fournier, Hass, Naik, Lodha, & Cauraugh, 2010). Results from retrospective home videos or interviews suggest that motor delays in ASD may manifest during infancy and could serve as an early marker for ASD risk (Gernsbacher, Sauer, Geye, Schweigert, & Hill Goldsmith, 2008; Teitelbaum, Teitelbaum, Nye, Fryman, & Maurer, 1998). However, these findings are difficult to evaluate and replication attempts have been mixed. For example, a retrospective study of 9- to 12-months-old infants identified motor delays that distinguished infants with typical development (TD) from those later diagnosed with ASD or non-ASD developmental delays (DD), but motor delays were common in both ASD and DD infants (Baranek, 1999). Ozonoff and colleagues (2008) also failed to find ASD-specific motor abnormalities using retrospective home videos of 12-month-olds and concluded that early motor abnormalities are common in children with DD but are not ASD-specific. Similarly, studies with older children (21- to 41-month-olds) report that both children with ASD and children with DD show significant motor delays (Provost, Lopez, & Heimerl, 2007). These findings suggest that early motor delays are present in ASDs but may not be specific to the disorder.
A growing number of prospective studies have re-visited the question whether motor delays manifest during infancy in ASD and have identified motor markers that are associated with later communication and social delays, including ASD. For example, head-lag at 6 months during assisted postural transition from supine to sitting (Flanagan et al., 2012), and unusual and repetitive object exploration strategies at 12 months (Ozonoff, Macari, et al., 2008) have been associated with ASD at 36 months. Postural instability and delayed posture development have also been observed in infants at high-familial risk for ASD (Nickel, Thatcher, Keller, Wozniak, & Iverson, 2013) and may interfere with infants’ grasping attempts. However, no studies to date have investigated grasping behaviors in infants at risk for ASD.
The growing number of reports on early motor disruptions in ASD (e.g., Flanagan et al., 2012; Gernsbacher et al., 2008; Landa, 2008; Landa & Garrett-Mayer, 2006; Lloyd, MacDonald, & Lord, 2013; Maestro et al., 2005) together with the increasingly acknowledged importance of motor skills for subsequent social, cognitive and communicative development (e.g., Bhat et al., 2012; Cashon, Ha, Allen, & Barna, 2013; Libertus & Needham, 2011, 2014) highlight the need for a closer focus on the developing motor system in children at increased familial risk for ASD. Early motor impairments may have far-reaching implications by compromising infants’ manual exploration of objects and social interaction experiences. For example, a failure to reach for and grasp objects may interfere with infants’ learning about object properties (Lederman & Klatzky, 2009) and with their practicing of postural control skills (Harbourne, Lobo, Karst, & Galloway, 2013). Impaired postural control may delay the onset of crawling or walking and has been linked to delayed language development (Bhat et al., 2012; Iverson, 2010). Delayed walking may affect the nature of infants’ self-initiated exchanges with their caregivers (Bornstein, Tamis-LeMonda, Hahn, & Haynes, 2008). In particular, the onset of walking allows infants to access distant objects, carry them, and share them with a caregiver (Karasik, Tamis-LeMonda, & Adolph, 2011, in press). Hence, delayed walking would limit infants’ opportunities to learn from such actions and social exchanges. These critical functions of early motor skills for overall development warrant a closer examination of motor abilities in children at risk for ASD.
The Current Study
The current study reports two experiments that investigate early motor development in infants at high familial risk (HR) for ASD and in low-risk infants (LR, no family history of ASD). Participants in both experiments include infant siblings of a child with ASD; these infants are at increased risk for ASD or developmental delays (Landa, Holman, & Garrett-Mayer, 2007; Ozonoff et al., 2011). Experiment 1 compares motor development in six-month-old HR infants subsequently diagnosed with ASD, HR infants subsequently diagnosed with non-ASD developmental delays, unaffected HR infants, and unaffected LR infants using the Mullen Scales of Early Learning (MSEL; Mullen, 1995). We predict that HR infants subsequently diagnosed with ASD will show relatively lower performance in both gross motor and fine motor domains when compared to LR controls (Bhat et al., 2012; Flanagan et al., 2012; Lloyd et al., 2013). Further, we predict that no other developmental domains as measured by the Mullen will differentiate between these groups during a prodromal phase of ASD around six months of age (Landa & Garrett-Mayer, 2006; Landa et al., 2013). Experiment 2 reports on performance of a separate sample of HR and LR infants on the MSEL and an additional experimental object-exploration task. We predict that HR infants will show lower fine motor performance on the MSEL and reduced grasping success during independent exploration of toys.
Experiment 1
Method
Participants
A total of 129 infants (61 females) participated in Experiment 1. Participants included infant siblings of children with confirmed ASD diagnosis (n = 107; 48 females; 95 Caucasian, 7 Asian, 2 African-American, 2 more than one race, 1 unknown) and infants without family history of ASD (n = 22; 13 females; 20 Caucasian, 2 unknown). At the time of testing, all participants were aged around six months (see Table 1) and subsequently followed longitudinally. Only infants who completed a diagnostic assessment at age 36 months using the MSEL, clinical judgment, and Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000) were included in this sample. Infants were divided into four groups based on their ASD family history and results of their 36-month assessment: low-risk infants without ASD (LR Non, n = 22); high-risk infants without ASD, language, or social delays (HR Non, n = 57); high-risk infants showing language or social delays but not ASD (HR DD, n = 28); and high-risk infants with autism or ASD diagnosis (HR ASD, n = 22). All children in the HR ASD group met DSMIV diagnostic criteria according to expert clinical judgment and exceeded ASD thresholds on the ADOS-G. An additional 16 children of the HR DD group and 2 children of the HR Non group met ADOS-G criteria for ASD but did not meet DSM-IV diagnostic criteria according to expert clinical judgment. These groups maintain the integrity of the family design (rather than forming a group of “unaffected” individuals that includes both LR and HR infants). The recurrence rate for ASD in our sample (20.56%) is similar to that of previous reports (Ozonoff et al., 2011). Group details are summarized in Table 1.
Table 1.
Participant Characteristics
| Variable | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|
| LR Non (n = 22) | HR Non (n = 57) | HR DD (n = 28) | HR ASD (n = 22) | LR (n = 19) | HR (n = 23) | |
| Age | 6.18 (0.38) | 6.11 (0.61) | 6.37 (0.46) | 6.04 (0.56) | 6.79 (0.45) | 6.52 (0.72) |
| # Females | 13 (59%) | 33 (58%) | 10 (36%) | 5 (23%) | 11 (58%) | 10 (43%) |
| SES | 56.40 (6.87) | 56.77 (8.31) | 57.16 (10.11) | 59.26 (9.06) | 51.08 (11.39) | 54.28 (8.97) |
| MSEL GM | 50.95 (10.41) | 46.65 (10.31) | 46.36 (11.20) | 48.86 (8.83) | 50.89 (9.13) | 47.39 (9.67) |
| MSEL VR | 52.68 (8.77) | 50.60 (10.93) | 48.14 (9.34) | 52.00 (12.09) | 51.21 (7.25) | 50.65 (8.21) |
| MSEL FM | 51.23 (8.11) | 45.53 (10.02) | 41.89 (10.45) | 44.18 (9.90) | 54.53 (9.74) | 48.83 (8.65) |
| MSEL RL | 53.41 (6.70) | 51.70 (8.24) | 52.25 (7.02) | 49.00 (8.26) | 53.53 (9.13) | 53.43 (8.02) |
| MSEL EL | 48.55 (7.74) | 47.25 (8.30) | 43.79 (7.16) | 45.82 (8.26) | 48.53 (10.17) | 45.30 (6.66) |
| ADOS CSS | 2.05 (1.36) | 2.11 (1.22) | 4.64 (2.73) | 6.59 (1.94) | --- | --- |
Note. Values represent group means with standard deviation given in parentheses; the number of females is reported as total count with group proportion given in parentheses. #Females = number of females; SES = Socioeconomic Status (Hollingshead, 1975); MSEL = Mullen Scales of Early Learning; GM= Gross Motor; VR = Visual Reception; FM = Fine Motor; RL = Receptive Language; EL = Expressive Language; LR = low-risk infants; HR = high-risk infants; LR Non = low-risk infants without Developmental Delays (DD) or Autism Spectrum Disorder (ASD); HR Non = high-risk infants without DD or ASD; HR DD = high-risk infants with language or social delays but without ASD diagnosis; MSEL scores are reported as standardized T-scores with mean of 50 and standard deviation of 10. ADOS CSS = Autism Diagnostic Observation Schedule Calibrated Severity Score (Shumway et al., 2012).
Exclusion criteria for all participants were: primary language exposure (>70% of time) other than English; birth-weight < 2500g; prenatal drug or excessive alcohol exposure; severe birth trauma; head injury; known genetic disorders that would confer increased risk for ASD (e.g., fragile X; the father of one child in the HR Non group was diagnosed with ADHD, this case did not affect statistical results); and severe birth deficits. Prematurity was not an exclusionary criterion and 11 participants were born preterm (M = 34.91 weeks gestation, SD = 1.11; 1 LR Non, 5 HR Non, 3 HR DD, 2 HR ASD). Age for preterm infants was adjusted as detailed in the MSEL assessment manual. An additional seven LR infants were tested but excluded due to signs of general developmental delays in more than one domain on the MSEL or ASD concerns on the ADOS-G. There were seven HR DD infants tested but excluded from this report because they were older than six months at the time of their assessment (M = 8.78 months, SD = 0.60). The Johns Hopkins Medical IRB approved this study and written informed consent was obtained from all families prior to participation.
Measures
All infants completed the MSEL at age six months, and ASD classifications were made at 36 months using the ADOS-G and clinical judgment. Trained Master's or Ph.D. level professionals, blind to previous diagnoses or family ASD history of the child, administered all standardized assessments. The MSEL is a standardized assessment yielding T-scores from five scales: Gross Motor (GM); Fine Motor (FM); Visual Reception (VR); Receptive Language (RL); and Expressive Language (EL). Overall, all groups scored within the normal range on the MSEL at age six months (see Table 1). The ADOS is a play-based assessment with standardized assessment and scoring schema, and is the most widely used ASD diagnostic instrument (Lord et al., 2000). Either ADOS Module 1 (minimal to no language, 29 children) or Module 2 (non-echoed phrase speech, 100 children) was administered.
Analysis
MSEL scores were compared across groups using Analysis of Variance (ANOVA) followed by planned comparisons between the LR Non group and all HR groups. Additionally, means of the HR Non, HR DD, and HR ASD groups were compared using post-hoc comparisons (Tukey HSD). Main analyses were performed on MSEL standardized T-scores. MSEL raw scores and item-level data were examined following significant MSEL T-score results. Measures of effect size are reported as Cohen's d or η2.
Results
Preliminary analyses did not reveal effects of gender on MSEL T-scores in any of the five domains at age six months (all ps > .22). Data were collapsed across gender in all subsequent analyses.
T-Scores
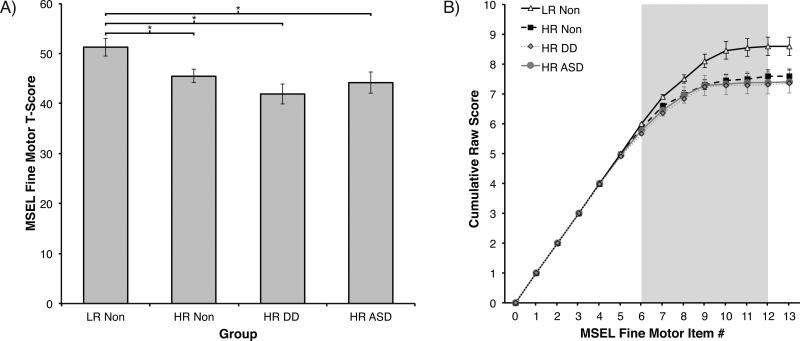
Separate ANOVAs with Group (4) as the between-subjects factor revealed no effects of Group on MSEL T-scores in the GM, VR, EL, or RL domains (all ps > .15). In the FM domain, ANOVA revealed a significant effect of Group, F(3, 125) = 3.90, p = .01, η2 = .09. This effect remained significant even after controlling for non-verbal problem solving skills (VR scores) using Analysis of Co-Variance (ANCOVA), F(3, 124) = 3.71, p = .02, η2 = .06. Planned comparisons revealed significantly higher FM scores in the LR Non group than in either the HR Non group (p = .03, d = .61), the HR DD group (p < .01, d = 1.00), or the HR ASD group (p = .01, d = .80). Post-hoc comparisons indicated no differences between the three HR groups (p > .38; see Figure 1a and Table 1).
Figure 1.
Mullen Scales of Early Learning (MSEL) scores in Experiment 1. A) Standardized T-scores on the Fine Motor (FM) scale of the MSEL by outcome group. B) Cumulative raw scores from the MSEL FM scale by outcome group. Group differences emerge between items 6 to 12 (gray shaded area, see Table 2). Cumulative raw scores at item 13 represent participants’ full raw scores for this scale (corresponding to T-scores shown in A). LR Non = low-risk infants without Developmental Delays (DD) or Autism Spectrum Disorder (ASD); HR Non = high-risk infants without DD or ASD; HR DD = high-risk infants with language or social delays but without ASD diagnosis; HR ASD = high-risk infants with confirmed ASD diagnosis. Error bars are SEM. *p < .05.
Item-Level Data
To identify the level of FM skill when the three groups begin to diverge, we examined raw scores of the MSEL FM Scale. Cumulative raw scores were used where each FM item included its own score plus the sum of all preceding scores. This exploratory analysis allowed us to examine when differences between groups first emerge and when these differences begin to stabilize. Benefits of this approach are that it maintains the ordered structure of the MSEL, and that it does not rely on individual MSEL raw scores, which are mostly scored using dichotomous 0 or 1 ratings and thus have limited power to discriminate between groups. A short explanation of MSEL items 1 through 13 is provided in Table 2. The MSEL uses a baseline and ceiling approach and not all items are administered to each child. For a six-month-old infant, item administration on the MSEL begins at item 4. Items 1-3 receive a passing score without being administered unless the child fails item 4, 5, or 6. Administration continues until the child fails three consecutive items.
Table 2.
Mullen Scales of Early Learning: Definition of Select Fine Motor Scale items
| Item | Description | Score |
|---|---|---|
| 1 | Arms held close to body with hands in fists | 0 or 1 |
| 2 | Holds a ring reflexively (involuntary grasp) | 0 or 1 |
| 3 | Brings fist to mouth | 0 or 1 |
| 4 | Brings hands together at midline | 0 or 1 |
| 5 | Keeps hands open and fingers loose | 0 or 1 |
| 6 | Ulnar palmar grasp of a small peg (not reflexive) | 0 or 1 |
| 7 | Reaches for and grasps a block using radial-palmar grasp | 0 or 1 |
| 8 | During play with blocks, child transfers a block between hands, bangs, or drops a block (need to see 2 behaviors) | 0 or 1 |
| 9 | Reach and grasp block using refined radial-digital grasp | 0 or 1 |
| 10 | Pick up small object using partial or refined pincer grasp | 0 – 2 |
| 11 | Bangs two blocks together at midline | 0 or 1 |
| 12 | Takes out or puts blocks into a metal can | 0 – 3 |
| 13 | Uses two hands together during object manipulation | 0 or 1 |
Note. Administration uses standardized sets of objects. For more information and detailed item definitions, please see the documentation provided with the MSEL test kit (Mullen, 1995). Shaded items are used in the object manipulation composite.
Visual examination of cumulative FM scores across MSEL items reveals that the LR Non group starts to separate from the HR groups around item 6 and that these differences stabilize around item 12 (see Figure 1b). Exploratory between-group Kruskal-Wallis tests on the cumulative raw score also confirm this observation and indicate significant between-group differences starting with item 6, X2(3) = 8.13, p = .04. Due to the cumulative nature of our analyses, this difference persists on all subsequent items (all ps < .04), but statistical results do not change further following item 12. Comparing only LR Non and HR ASD groups, exploratory analyses using the Mann-Whitney test also reveal between-group differences emerging at item 6 (Z = 2.07, p = .04).
Discussion
The results reported here extend prior work suggesting the presence of subtle behavioral signs for ASD risk during infancy, a prodromal period for ASD (Landa & Garrett-Mayer, 2006; Landa et al., 2013). Four recent prospective studies have reported motor atypicalities during the first year in infants at high risk for ASD. Poor gross motor skills on the Alberta Infant Motor Scale (AIMS) have been observed in HR infants (without outcome information) at three and six months of age (Bhat et al., 2012). Reduced postural control has been observed in six-month-old infants subsequently diagnosed with ASD and milder communication or social delays at age 36 months (Flanagan et al., 2012) and in a separate prospective sample of six-month-old HR infants without outcome information (Nickel et al., 2013). And finally, low gross motor scores on the MSEL have been reported in seven-month-olds later diagnosed with ASD (Leonard, Elsabbagh, Hill, & the BASIS team, 2013). The current study extends these findings and suggests differences in the fine motor domain between LR and HR infants – especially on grasping and object-exploration related items of the MSEL. This observation suggests early neurodevelopmental vulnerabilities in HR infants – regardless of developmental outcomes – that may represent an endophenotype associated with ASD and could be meaningful for genetic studies (Szatmari et al., 2007).
It is important to note that all four groups in Experiment 1 scored within the typical range of MSEL (T-scores >35), thus none of the groups showed a clinical delay in their overall fine motor development at age six months. Consequently, it is unclear whether the observed statistical differences on the MSEL would also translate into actual differences in infant's day-to-day behaviors. For example, would HR infants show differences in grasping activity during naturalistic play activities? And if they do, would HR infants eventually catch-up to their LR peers over time? Experiment 2 addresses these questions in a new sample of infants by combining the standardized MSEL with an experimental procedure anchored in the literature on typical development in infancy.
Experiment 2
In Experiment 2, a second group of LR and HR six-month-old infants was assessed to examine whether grasping behavior, which distinguished the LR and HR groups based on items within the MSEL FM scale in Experiment 1, would also distinguish LR and HR infants in a naturalistic, free-play context. Further, a sub-set of infants were tested again at ten months of age to examine the developmental trajectories of grasping activity in LR and HR infants.
In Experiment 2, a naturalistic free-play task was employed to provide infants the opportunity to independently explore objects. A standardized set of perceptually rich toys was presented to each infant. We hypothesized that six-month-old HR infants would exhibit reduced levels of grasping to explore toys on their own when compared to LR infants. We reasoned that if the HR infants had amassed less sophisticated grasping skills than the LR group, as indicated by the MSEL FM scale data in Experiment 1, they would exhibit decreased engagement with objects during free play by grasping them and initiating an exploratory sequence of events. Further, we predicted that HR infants would continue to show reduced grasping at age ten months.
Method
Participants
A total of 42 infants (21 females) participated in Experiment 2. Similar to Experiment 1, participants included infant siblings of children with ASD (n = 23; 10 females; 19 Caucasian, 1 African-American, 3 more than one race) and infants without family history of ASD (n = 19; 11 females; 18 Caucasian and 1 African American). In Experiment 2, outcome evaluations at age 36 months were not yet available. Therefore, the focus of Experiment 2 is on comparing HR vs. LR infants. Four additional infants were tested but received a preliminary diagnosis of ASD and were excluded from the final sample. At the time of initial testing, participants were aged around six months (M = 203 days, SD = 18.97). Participant details and MSEL scores at age six months are shown in Table 1.
Of the 42 infants assessed at age six months, 26 returned for a follow-up assessment at age ten months (M = 318 days, SD = 19.57; 13 females; 14 HR infants). The remaining 16 infants were either not invited back for a ten-month assessment because they passed this age before the ten-month-visit was added to our protocol (n = 6), or were invited back but failed to return for that age visit (n = 10). The final longitudinal sample includes 12 infants in the LR group and 14 infants in the HR group.
Measures
The MSEL was administered as described in Experiment 1. During an additional free-play task, infants were seated in a stable infant chair or on a parent's lap at a rectangular table. The experimenter was seated across from or next to the infant and placed a standardized set of toys on the table within reach of the infant, briefly drawing attention to each toy. Infants were permitted to independently explore the toys for one minute. Neither experimenter nor parent engaged the infant during this time but they responded to social smiles, sharing bids, or requests of the child. The experimenter replaced toys that fell off the table. At age six months, toys used were a nubby ball, a small slinky, and a teething toy. Different toys were used at age ten months to account for the increased play and exploration abilities of this age group. The toys used at age ten months were the same small slinky as at age six months, an infant shape sorter with rattle bugs and net, and three nesting cars. At both ages, the toys offered a range of both textured and smooth surfaces to explore.
Trained observers (blind to group membership and clinical impressions) scored infants’ object exploration behavior during the free-play assessment from video recordings using frame-by-frame coding software. Touching and grasping actions were defined as in previous studies on manual exploration in young infants (e.g., Libertus & Needham, 2010, 2011; Needham, Barrett, & Peterman, 2002). Touching events were coded as any manual contact between hands and an object. Grasping events were coded when the infant was touching an object and lifting at least one corner of the object off the table. This definition of grasping is appropriate for young infants and focuses on action consequences (i.e., toy off the table) rather than means (i.e., particular type of grasp). Inter-coder reliability was assessed on 14 (33%) randomly selected participants and was high (Touching: Cronbach's Alpha = .97; Grasping: Cronbach's Alpha = .95).
Analysis
Based on Experiment 1, Experiment 2 focused only on the FM domain of the MSEL. Dependent measures from the MSEL were T-scores and a fine motor composite isolating object manipulation related items from the FM scale by summing raw scores across items 6-12. This object manipulation composite was motivated by the exploratory item-analysis of Experiment 1 (see Figure 1b). Since this composite score only includes MSEL items that discriminated between LR and HR infants in Experiment 1 and that are beyond the baseline range (items 4-6) for six-month-old infants, this measure is likely to have better discriminative power than the full MSEL FM scale. Dependent measures from the free-play task were the proportion of time infants engaged in touching or grasping actions. Comparisons between LR and HR infants were conducted using between-group ANOVAs. Longitudinal analyses were performed using mixed-design ANOVAs with Group (LR vs. HR) as the between-subjects factor and Age (6 vs. 10 months) as a within-subjects factor.
Results
Age Six Months Analyses
Preliminary analyses revealed no effects of gender on infants’ touching or grasping durations during the free-play task (all ps > .70). An effect of gender was observed for the MSEL FM scale with females showing slightly higher scores than males using both T-scores, t(40) = 2.67, p = .01, d = .84 (MFemale = 55.05, SD = 6.86; MMale = 47.76, SD = 10.47) and using the object manipulation composite score from the MSEL, t(40) = 2.33, p = .03, d = .74 (MFemale = 4.62, SD = 1.56; MMale = 3.43, SD = 1.75). Closer examination of this effect revealed gender differences in the LR group, t(17) = 2.16, p = .05, d = 1.05 (MFemale = 58.27, SD = 7.28; MMale = 49.38, SD = 10.77), but not in the HR group (p = .20). Together with our negative findings in Experiment 1, these patterns cast doubt on the validity of this effect. For consistency, data were collapsed across gender as in Experiment 1. Additional analyses including Gender as a factor were performed for the MSEL scores for completeness.
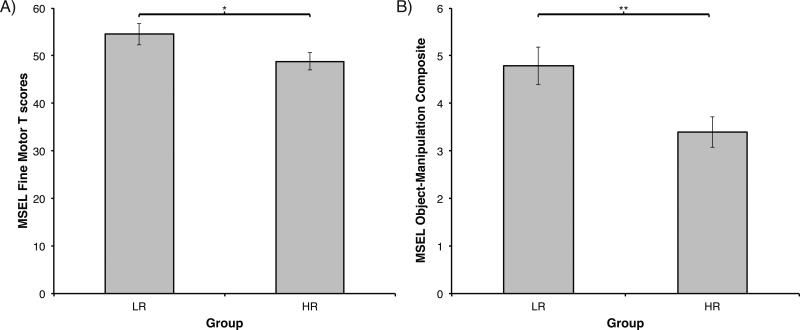
1) Mullen Scales of Early Learning: As in Experiment 1, an ANOVA with Group (2) as the between-subjects factor on the FM T-scores revealed a significant effect of Group, F(1, 40) = 4.03, p = .05, η2 = .09 (Figure 2a). Similarly, an effect of Group was also observed when comparing LR and HR infants on the object manipulation composite we generated from relevant MSEL FM items, F(1, 40) = 7.78, p < .01, η2 = .16 (Figure 2b). Including Gender as factor alters results slightly. A Group (2) x Gender (2) ANOVA using FM T-scores now revealed a non-significant effect of Group, F(1, 38) = 2.97, p = .09, η2 = .06, a significant effect of Gender, F(1, 38) = 6.28, p = .02, η2 = .13, and no Group × Gender interaction (p > .44) . On the object manipulation composite, inclusion of Gender did not change the results and ANOVA still revealed a significant effect of Group, F(1, 38) = 6.43, p = .02, η2 = .13 (MLR = 4.79, SD = 1.72; MHR = 3.39, SD = 1.53), an effect of Gender, F(1, 38) = 4.53, p = .04, η2 = .09, but no Group × Gender interaction (p > .50).
Figure 2.
Mullen Scales of Early Learning (MSEL) at age six months in Experiment 2. A) Standardized T-scores on the Fine Motor (FM) scale of the MSEL by risk group. B) Object-manipulation composite scores (items 6-12 from MSEL Fine Motor Scale) by risk group. LR = low-risk infants; HR = high-risk infants. Error bars are SEM. *p = .05; ** p < .01.
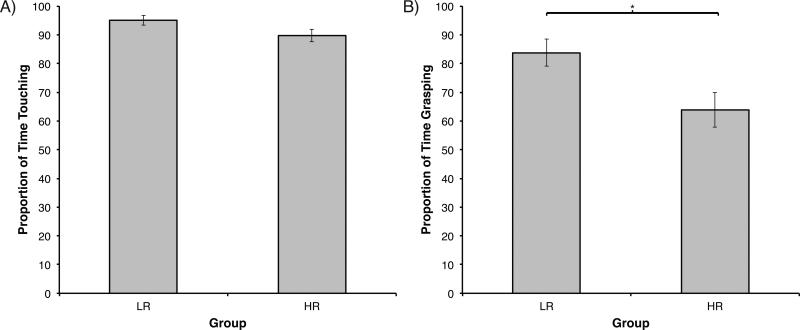
2) Free-play Task: Separate ANOVAs with Group (2) as the between-subjects factor revealed non-significant differences among infants’ overall touching of the toys during free play, F(1, 40) = 3.62, p = .06 (MLR = 94.99, SD = 7.30; MHR = 89.70, SD = 10.11; Figure 3a). With regard to grasping activity, ANOVA revealed significantly longer durations in LR compared to HR infants, F(1, 40) = 6.32, p = .02, η2 = .14 (MLR = 83.76, SD = 20.85; MHR = 63.86, SD = 28.79; Figure 3b). While overall object contact was comparable across groups, HR infants showed less grasping activity than LR infants.
Figure 3.
Free play assessment at age six month in Experiment 2. A) Proportion of touching duration by risk group. B) Proportion of grasping duration by risk group. LR = low-risk infants; HR = high-risk infants. Error bars are SEM. *p = .02.
Longitudinal analysis
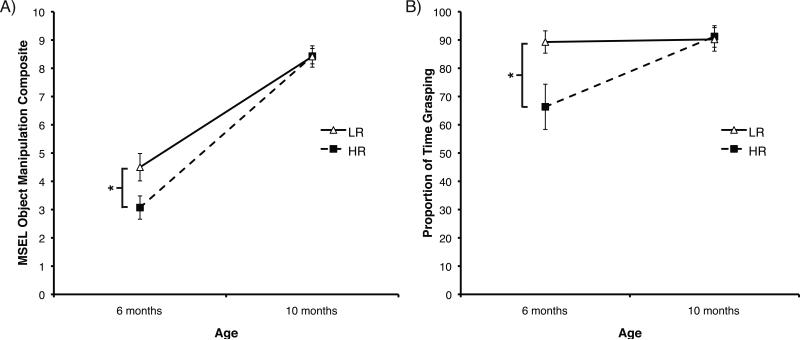
Longitudinal scores for the MSEL FM object manipulation score and for grasping duration within the free-play task at both six and ten months of age were available for 26 infants. A mixed-model ANOVA on the MSEL object manipulation scores with Group (2) as between-subjects factor and Age (2) as repeated factor revealed a significant effect of Age, F(1, 24) = 302.22, p < .01, η2 = .89 (M06m = 3.73, SD = 1.73; M10m = 8.42, SD = 1.14), a significant Age × Group interaction, F(1, 24) = 7.29, p = .01, η2 = .02, but no main effect of Group (p = .16). Further investigating the Age × Group interaction, we observed significant differences between the LR and HR groups at age six months, t(24) = 2.26, p = .03, d = .89 (MLR = 4.50, SD = 1.68; MHR = 3.07, SD = 1.54), but not at age ten months, t(24) = -0.26, p = .98 (MLR = 8.42, SD = 1.31; MHR = 8.43, SD = 1.02; Figure 4a).
Figure 4.
Longitudinal analyses from six to ten months of age in Experiment 2. A) Mullen Scales of Early Learning (MSEL) object manipulation composite scores by risk group. B) Free-play assessment proportion of grasping duration by risk group. LR = low-risk infants; HR = high-risk infants. Error bars are SEM. *p <.05.
Results for infants’ grasping activity during the free-play assessment mirrored the findings for the MSEL object manipulation score. An Age (2) by Group (2) repeated-measures ANOVA revealed a significant effect of Age, F(1, 24) = 5.28, p = .03, η2 = .14 (M06m = 76.93, SD = 26.15; M10m = 90.76, SD = 14.06), an effect of Group, F(1, 24) = 4.20, p = .05, η2 = .10, η2 = .12, and an Age × Group interaction, F(1, 24) = 4.55, p = .04. Follow-up analyses of this interaction indicated significant differences between the LR and HR groups at age six months, t(24) = 2.44, p = .02, d = .96 (MLR = 89.28, SD = 13.67; MHR = 66.35, SD = 29.92), but not at age ten months, t(24) = -0.18, p = .86 (MLR = 90.20, SD = 14.43; MHR = 91.24, SD = 14.27; Figure 4b). Together, these results indicate relatively lower grasping activity in HR compared to LR infants at six months of age. However, by age ten months, the simple act of grasping in the HR group rises to a level comparable to that displayed by age-peers in the LR group. It is likely that grasping duration during our free-play task is at ceiling in both groups at this age. Qualitative differences in how objects are grasped needs to be assessed to determine whether differences in grasping behavior between HR and LR infants continue to be present later ages.
Discussion
The results reported in Experiment 2 suggest reduced grasping and object exploration activity in infants at high familial risk for ASD. Together with Experiment 1, our results are in agreement with other studies that have observed differences in early motor skill development among HR infants (e.g., Bhat et al., 2012; Flanagan et al., 2012; Leonard et al., 2013; Nickel et al., 2013). Overall, the MSEL FM T-score results observed in Experiment 2 show a similar pattern as in Experiment 1, but statistical results are somewhat weakened by an effect of gender in the LR sample (see below). Focusing only on object manipulation-related items of the MSEL (derived in Experiment 1) seems to offer a reliable measure to detect differences between HR and LR infants (see Figure 2). Finally, reduced grasping activity in HR infants was also observed activity during an unstructured free-play task in Experiment 2 and provides additional evidence for the patterns observed in Experiment 1.
Following a sub-set of infants longitudinally indicates that group differences in grasping behavior as measured here seem to attenuate with age. LR infants show high levels of grasping behavior at both six and ten months of age. In contrast, HR infants show lower grasping activity than LR infants at age six months, but significantly increase their grasping activity over the next four months. It is possible that these differences disappear by age ten months because infants’ grasping activity reaches ceiling (i.e., is above 90% in both groups). In contrast, others have noted a progressive worsening of motor skills with increasing age in children with ASD (Landa & Garrett-Mayer, 2006; Lloyd et al., 2013). A more detailed analysis of grasping quality (i.e., how are the objects grasped and explored) may reveal differences between ten-month-old LR and HR infants and should be conducted in the future.
Gender differences in motor development
Experiment 2 – but not Experiment 1 – revealed an effect of gender in six-month-old infants whereby girls scored slightly better on the MSEL FM scale and object manipulation scores than boys. This effect was only observed in the LR group and prior reports of effects of gender on infants’ hand functions are rare. Some studies suggest that gender differences in infancy exist with regard to infants’ motor activity level, with boys generally showing higher levels of activity (Campbell & Eaton, 1999; Eaton & Enns, 1986), and with regard to bimanual hand use, with girls showing higher levels of bimanual synchrony (Piek, Gasson, Barrett, & Case, 2002). Thus, based on previous studies it is unclear whether one would expect to find gender differences on a broad measure such as the MSEL. The current study identified no effects of Gender in Experiment 1 or on the free-play task used in Experiment 2, casting doubt on the validity of the effect of Gender observed among LR infants on the MSEL in Experiment 2. Future research is needed to more closely examine the effects of Gender on infant's fine motor skills at age six months before this result can be interpreted. However, results of our free-play task and our exploratory object-manipulation composite both suggest that differences between LR and HR infants’ fine motor and grasping skills exists regardless of the potential influences gender may have on these behaviors.
General Discussion
Previous studies have reported atypical gross motor skills among HR infants as early as three months of age (e.g. Bhat et al., 2012; Esposito, Venuti, Maestro, & Muratori, 2009; Flanagan et al., 2012; Nickel et al., 2013). The current study extends these findings by systematically investigating fine motor skills in infants at high-familial risk for ASD using both the standardized MSEL and an experimental free-play task. Across two separate experiments, our results provide converging evidence for weaker fine motor and decreased grasping activity in six-month-old HR compared to LR infants. These findings inform our understanding of the developmental trajectories of HR infants, as studies with typically developing infants indicate that grasping skills and experiences are critical for infants’ active exploration of their environment and facilitate learning about object properties and observed actions (Gerson & Woodward, 2013; Skerry, Carey, & Spelke, 2013).
Experiment 1 revealed lower fine motor scores on the MSEL in six-month-old HR infants compared to LR infants, although both groups scored within the typical range on this measure. Differences between HR and LR infants were evident even in HR infants who did not receive a diagnosis of ASD or other delays by age 36 months, suggesting that lower fine motor scores on the MSEL are characteristic of infants at high familial risk for ASD regardless of whether they fully manifest the ASD phenotype later (meeting one definition of an endophenotype, see below). An exploratory item-by-item analysis of the FM scale revealed that HR infants scored lower than LR peers on object exploration and grasping related items of the MSEL. Experiment 2 examined fine motor and grasping skills in HR infants more closely using a naturalistic grasping assessment and by examining developmental trajectories of grasping activity between six and ten months of age. Results from Experiment 2 confirmed the patterns observed in Experiment 1 and indicate lower grasping activity during naturalistic object exploration and lower scores on object exploration related items of the MSEL in HR infants.
Role of grasping experiences
Successful grasping actions typically emerge around four to six months of age as infants make the transition into independent grasping (Berthier & Keen, 2006). Engaging in successful grasping results in new experiences influencing infant's motor, cognitive, and social development (Gerson & Woodward, 2013; Libertus & Needham, 2011; Skerry et al., 2013). Further, successful grasping provides new opportunities to initiate social bids and may change the kind of stimulation and feedback provided by the parent (Bornstein et al., 2008). A more recent longitudinal study with typically developing infants demonstrates the potential long-term influences of early motor skills by showing that object exploration at age five months predicts academic achievement at age 14 years (Bornstein, Hahn, & Suwalsky, 2013). Connections between motor and cognitive development also have been reported in infants at high familial risk for ASD. For example, fine motor skills between 12-24 months of age were found to predict expressive language skills at age 36 months in HR infants (LeBarton & Iverson, 2013). These observations suggest that object exploration experiences may be part of a developmental cascade that facilitates social and cognitive growth (Bornstein et al., 2013; Gottlieb, 1991). Our results revealed reduced fine motor and grasping activity in HR infants and suggest that closer attention to these object exploration related motor skills may be warranted in infants at high familial risk for ASD.
Implications
The lower fine motor and grasping scores reported here were not specific to infants later diagnosed with ASD, but seem to be part of an ASD endophenotype. Endophenotype or intermediate phenotype refers to a trait (here less mature FM and lower grasping activity in early infancy) that occurs more commonly in affected and unaffected family members of a risk group than in the general population. Thus, endophenotypes are heritable characteristics that may have a genetic relation to ASD without predicting full diagnosis (Szatmari et al., 2007). Despite not offering an early marker for ASD, identifying ASD endophenotypes has important implications for studies related to the cosegregation of alternative ASD phenotypes and may improve our understanding of the neurobiology and genetics of ASDs (Szatmari et al., 2007; Viding & Blakemore, 2007). Findings from other studies with older children (4 years of age or older) suggest that motor impairments may constitute a core characteristic of ASD rather than an endophenotype (Hilton et al., 2012). While the fine motor differences observed in the HR sample in this study cannot be construed as a motor deficit, we have noted other motor deficits in 6-month-old HR infants (e.g., Flanagan et al., 2012). Further, we have previously reported a worsening of fine motor performance over the first three years of life in infants later diagnosed with ASD (Landa et al., 2013) as well as in a latent class consisting of HR children with and without ASD who later manifested fine motor delay (Landa et al., 2012).
To better understand the role of a weakness in FM or grasping skills in HR infants, several important questions need to be addressed. First of all, are there potential confounding factors that may explain our observed findings in the HR population? For example, to rule out that the interactions with an affected sibling influence infants’ FM and grasping engagement one would have to study infant sibling of children with developmental delays but not ASD. Second, the role of lower FM and grasping activity in early infancy for later emerging developmental delays in language, social, or communicative domains needs to be better understood (Bhat et al., 2012; Iverson, 2010; LeBarton & Iverson, 2013). And finally, what is the value of targeting early FM and grasping skills via interventions in HR infants? While all infants in the current study scored within the normal range on the MSEL FM scale, it is possible that infants at HR for ASD may benefit from early motor intervention targeting the development of grasping and object exploration related skills. Previous studies suggest that early grasping related motor skills can be facilitated via cost-effective, parent-guided training (Libertus & Needham, 2010). The value of such enrichment for HR infants should be explored in future research.
Limitations
While the findings reported here are interesting and address a clinically and theoretically relevant question, several limitations need to be considered when interpreting our results. First of all, while lower fine motor scores were observed in Experiment 1, overall scores on this measure where within the range of typical development for all three groups. Thus, it is not the case that HR infants later diagnosed with ASD show a general fine motor delay at six months of age. Rather, differences in motor behavior seem subtle and likely involve qualitative aspects of motor behavior that are difficult to quantify on standardized tests. Other findings also suggest that developmental differences in affected infants seem to reach clinical significance only after the first year of life (Landa, Gross, Stuart, & Bauman, 2012). Using a selection of object exploration-focused items from the MSEL Fine Motor scale seems to improve statistical power to detect motor differences in six-month-old infants, but the validity of this truncated MSEL score needs to be examined more carefully before conclusions should be based on this exploratory measure alone.
Experiment 2 offers what seems to be a more sensitive measure for fine motor-related differences between LR and HR infants, but these findings are limited by the lack of outcome diagnoses. Future studies should examine grasping behavior in detail using HR infants with confirmed ASD outcome. Further, only a one-minute exploration task of a limited selection of toys was used. This is a limitation of our design and future studies should investigate how HR infants respond in a free-play situation with a wider selection of toys and more time to engage with them. It is also possible that factors related to sensory preferences influenced HR infants’ comparatively reduced grasping activity in Experiment 2. While our results suggest largely comparable amounts of toy contact across both LR and HR groups, we cannot fully rule out this possibility. And finally, our failure to observe differences in grasping behavior at age ten months in our longitudinal sample highlights the need for caution when interpreting negative findings. Previous studies have reported progressive slowing in the acquisition of motor skills in ASD (Landa & Garrett-Mayer, 2006; Landa et al., 2013; Lloyd et al., 2013). By ten months of age, most infants in the present study seem to have increased their grasping activity (i.e., above 90% of the time), reaching ceiling performance within our one-minute assessment. A more complex and age-appropriate measure on grasping quality or diversity and complexity of object exploration strategies may be required to identify differences at age ten months among LR and HR infants.
Conclusions
Together, the two experiments reported here provide converging evidence for reduced grasping and fine motor activity among six-month-old infants at increased familial risk for ASD. Our results have important implications for our understanding of ASDs. Subtle lags in object exploration-related motor skills in early infancy may present an ASD endophenotype and further our understanding of the genes involved in the disorder. In addition, our findings call for a critical evaluation of the potential of motor training or enrichment in families with ASD history. In particular, training strategies that focus on promoting self-produced grasping activity during the first six months of life should be examined (e.g., Libertus & Needham, 2010). Future studies are needed to examine our preliminary findings more closely and to determine if both quantitative and qualitative aspects of early object exploration activity are affected in infants at HR for ASD.
Acknowledgements
We thank the families for their generous participation in and commitment to this research, and the staff at Kennedy Krieger Institute Center for Autism and Related Disorders for their efforts and help in executing this work.
Funding for this study was provided by the National Institute of Mental Health grant MH59630 awarded to Rebecca Landa (PI). Support for Klaus Libertus was partially provided by a postdoctoral fellowship from the Autism Science Foundation and ROAR for Autism. Klaus Libertus's current affiliation is Learning Research and Development Center, University of Pittsburgh (he was affiliated with the other indicated institutions during research, writing and review of this paper.)
Contributor Information
Klaus Libertus, Center for Autism and Related Disorders, Kennedy Krieger Institute; and Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine.
Kelly A. Sheperd, Center for Autism and Related Disorders, Kennedy Krieger Institute; and Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine
Samuel W. Ross, Center for Autism and Related Disorders, Kennedy Krieger Institute
Rebecca J. Landa, Center for Autism and Related Disorders, Kennedy Krieger Institute; and Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5 ed. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Asperger H. Die autistischen Psychopathen im Kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117:76–136. [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. doi: 10.1023/A:1023080005650. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Keen R. Development of reaching in infancy. Experimental Brain Research. 2006;169:507–518. doi: 10.1007/s00221-005-0169-9. doi: 10.1007/s00221-005-0169-9. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa R. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior & Development. 2012;35:838–846. doi: 10.1016/j.infbeh.2012.07.019. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Suwalsky JT. Physically developed and exploratory young infants contribute to their own long-term academic achievement. Psychological Science. 2013;24:1906–1917. doi: 10.1177/0956797613479974. doi: 10.1177/0956797613479974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Hahn CS, Haynes OM. Maternal responsiveness to young children at three ages: Longitudinal analysis of a multidimensional, modular, and specific parenting construct. Developmental Psychology. 2008;44:867–874. doi: 10.1037/0012-1649.44.3.867. doi: 10.1037/0012-1649.44.3.867. [DOI] [PubMed] [Google Scholar]
- Campbell DW, Eaton WO. Sex differences in the activity level of infants. Infant and Child Development. 1999;8:1–17. doi: 10.1002/(sici)1522-7219(199903)8:1<1::aid-icd186>3.0.co;2-o. [Google Scholar]
- Cashon CH, Ha OR, Allen CL, Barna AC. A U-shaped relation between sitting ability and upright face processing in infants. Child Development. 2013;84:802–809. doi: 10.1111/cdev.12024. doi: 10.1111/cdev.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Prevalence of Autism Spectrum Disorder among children aged 8 Years. Morbidity and Mortality Weekly Report - Surveillance Summaries. 2014;63:1–21. [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WO, Enns LR. Sex differences in human motor activity level. Psychological Bulletin. 1986;100:19–28. doi: 10.1037/0033-2909.100.1.19. [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH, BASIS Team Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry. 2013;74:189–194. doi: 10.1016/j.biopsych.2012.11.030. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Maestro S, Muratori F. An exploration of symmetry in early autism spectrum disorders: analysis of lying. Brain and Development. 2009;31:131–138. doi: 10.1016/j.braindev.2008.04.005. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. The American Journal of Occupational Therapy. 2012;66:577–585. doi: 10.5014/ajot.2012.004192. doi: 10.5014/Ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry. 2008;49:43–50. doi: 10.1111/j.1469-7610.2007.01820.x. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson SA, Woodward AL. Learning from their own actions: The unique effect of producing actions on infants' action understanding. Child Development. 2013;85:264–277. doi: 10.1111/cdev.12115. doi: 10.1111/cdev.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development - Results. Developmental Psychology. 1991;27:35–39. [Google Scholar]
- Harbourne RT, Lobo MA, Karst GM, Galloway JC. Sit happens: Does sitting development perturb reaching development, or vice versa? Infant Behavior & Development. 2013;36:438–450. doi: 10.1016/j.infbeh.2013.03.011. doi: 10.1016/j.infbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, Constantino J. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism. 2012;16:430–441. doi: 10.1177/1362361311423018. doi: 10.1177/1362361311423018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language. 2010;37:229–261. doi: 10.1017/S0305000909990432. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Transition from crawling to walking and infants' actions with objects and people. Child Development. 2011;82:1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Crawling and walking infants elicit different verbal responses from mothers. Developmental Science. doi: 10.1111/desc.12129. in press. doi: 10.1111/desc.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Clinical Practice Neurology. 2008;4:138–147. doi: 10.1038/ncpneuro0731. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psycholgy and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby sibs of children with autism. Journal of Child Psychology and Psychiatry. 2012;53:986–989. doi: 10.1111/j.1469-7610.2012.02558.x. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Faherty A. Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Development. 2013;84:429–442. doi: 10.1111/j.1467-8624.2012.01870.x. doi: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O'Neil AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton ES, Iverson JM. Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental Science. 2013;16:815–827. doi: 10.1111/desc.12069. doi: 10.1111/desc.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Haptic perception: A tutorial. Attention Perception & Psychophysics. 2009;71:1439–1459. doi: 10.3758/APP.71.7.1439. doi: 10.3758/app.71.7.1439. [DOI] [PubMed] [Google Scholar]
- Leonard HC, Elsabbagh M, Hill EL, the BASIS team Early and persistent motor difficulties in infants at-risk of developing autism spectrum disorder: A prospective study. European Journal of Developmental Psychology. 2013;11:1–18. doi: 10.1080/17405629.2013.801626. [Google Scholar]
- Libertus K, Needham A. Teach to reach: the effects of active vs. passive reaching experiences on action and perception. Vision Research. 2010;50:2750–2757. doi: 10.1016/j.visres.2010.09.001. doi: 10.1016/j.visres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Needham A. Reaching experience increases face preference in 3-month-old infants. Developmental Science. 2011;14:1355–1364. doi: 10.1111/j.1467-7687.2011.01084.x. doi: 10.1111/j.1467-7687.2011.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Needham A. Encouragement is nothing without control: Factors influencing the development of reaching and face preference. Journal of Motor Learning and Development. 2014;2:16–27. doi: 10.1123/jmld.2013-0019. [Google Scholar]
- Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013;17:133–146. doi: 10.1177/1362361311402230. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cesari A, Cavallaro MC, Paziente A, Pecini C, Sommario C. Course of autism signs in the first year of life. Psychopathology. 2005;38:26–31. doi: 10.1159/000083967. doi: 10.1159/000083967. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Morales KH, Xie M, Lawer LJ, Stahmer AC, Marcus SC. Age of diagnosis among Medicaid-enrolled children with autism, 2001-2004. Psychiatric Services. 2010;61:822–829. doi: 10.1176/appi.ps.61.8.822. doi: 10.1176/appi.ps.61.8.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in Autism Spectrum Disorders. Brain and Development. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen: Scales of Early Learning (AGS Edition) American Guideline Service Inc; Circle Pines, MN: 1995. [Google Scholar]
- Needham A, Barrett T, Peterman K. A pick-me-up for infants' exploratory skills: Early simulated experiences reaching for objects using 'sticky mittens' enhances young infants' object exploration skills. Infant Behavior & Development. 2002;25:279–295. doi: 10.1016/S0163-6383(02)00097-8. [Google Scholar]
- Nickel LR, Thatcher AR, Keller F, Wozniak RH, Iverson JM. Posture development in infants at heightened versus low risk for autism spectrum disorders. Infancy. 2013;18:639–661. doi: 10.1111/infa.12025. doi: 10.1111/infa.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noterdaeme M, Hutzelmeyer-Nickels A. Early symptoms and recognition of pervasive developmental disorders in Germany. Autism. 2010;14:575–588. doi: 10.1177/1362361310371951. doi: 10.1177/1362361310371951. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12:457–472. doi: 10.1177/1362361308096402. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:e488–495. doi: 10.1542/peds.2010-2825. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Rogers SJ. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38:644–656. doi: 10.1007/s10803-007-0430-0. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek JP, Gasson N, Barrett N, Case I. Limb and gender differences in the development of coordination in early infancy. Human Movement Science. 2002;21:621–639. doi: 10.1016/s0167-9457(02)00172-0. [DOI] [PubMed] [Google Scholar]
- Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry. 2011;68:101–109. doi: 10.1001/archgenpsychiatry.2010.113. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37:321–328. doi: 10.1007/s10803-006-0170-6. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Shumway S, Farmer C, Thurm A, Joseph L, Black D, Golden C. The ADOS calibrated severity score: relationship to phenotypic variables and stability over time. Autism Research. 2012;5:267–276. doi: 10.1002/aur.1238. doi: 10.1002/aur.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry AE, Carey SE, Spelke ES. First-person action experience reveals sensitivity to action efficiency in prereaching infants. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18728–18733. doi: 10.1073/pnas.1312322110. doi: 10.1073/pnas.1312322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Maziade M, Zwaigenbaum L, Mérette C, Roy M-A, Joober R, Palmour R. Informative phenotypes for genetic studies of psychiatric disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:581–588. doi: 10.1002/ajmg.b.30426. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Blakemore S-J. Endophenotype approach to developmental psychopathology: Implications for autism research. Behavior Genetics. 2007;37:51–60. doi: 10.1007/s10519-006-9105-4. doi: 10.1007/s10519-006-9105-4. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]