Abstract
Background:
Vulvovaginitis has a known association with urinary tract infections (UTIs) in girls. We hypothesize that vulvovaginitis is a major contributor to UTIs in prepubertal girls by increasing periurethral colonization with uropathogens.
Methods:
Periurethral swabs and urine specimens were obtained from a total of 101 girls (58 with vulvovaginitis and 43 without vulvovaginitis). Specimens were cultured for bacterial growth. The dominant organism in the periurethral swabs and urine cultures was recorded and antibiotic sensitivity profiles were compared.
Results:
Periurethral swabs from children with vulvovaginitis were associated with a statistically significant increase in uropathogenic bacteria (79% Enterococcus species or Escherichia coli) as the dominant culture compared with swabs from girls without vaginitis (18%) (p < 0.05). In children with vulvovaginitis, 52% of the urine cultures were positive for UTIs, and the dominant organism in the urine cultures matched the species and antibiotic sensitivity profile of the corresponding periurethral swab. Only 11% of the urine cultures from girls without vulvovaginitis were positive for UTIs.
Conclusions:
Vulvovaginitis may cause UTIs by altering the perineal biome such that there is increased colonization of uropathogens.
Keywords: bacteriuria, perineal biome, preadolescent, urinary tract infection, vulvovaginitis
Introduction
Pediatric urology clinics encounter preadolescent females with urinary tract infections (UTIs) of a wide variety of etiologies, including internal and external anatomical aberrancies, dysfunctional voiding leading to urinary retention in either the bladder and/or vagina, and poor vaginal and vulvar hygiene. One commonly overlooked association is the presence of concurrent vulvovaginitis, which generally refers to an inflammatory process involving the vagina, vulva, perineum, or any combination thereof. Vulvovaginitis is the most common gynecological condition in the premenarchal female [Jaquiery et al. 1999], and represents the greatest indication for referral to a pediatric gynecological specialist [Van Eyk et al. 2009].
Since atrophic vaginitis in postmenopausal females has been shown to change the type of bacteria that binds to vaginal epithelial cells and increases the risk of UTIs [Stamm and Raz, 1999], we wondered whether there might be a link between vulvovaginitis and UTIs in premenarchal females. It is possible that a low estrogen state in premenarchal females can also be associated with vulvovaginitis and resultant periurethral inflammation that can increase localized bacterial colonization. This may lead to urethral, and subsequently, bladder colonization, ultimately culminating in an infection of the genitourinary tract. We therefore sought to determine if prepubescent girls with suspected UTIs are more likely to have concurrent vulvitis.
Methods
Consecutive girls presenting with a clinical suspicion of a UTI were enrolled into this institutional review board-approved study. Patients’ inclusion criteria included at least one of the following: frequency, urgency, dysuria, hesitancy, or nocturia. Only toilet-trained prepubescent, premenarchal children (Tanner stage I) [Marshall and Tanner, 1969, 1970] were asked to participate. Exclusion criteria included: children with a history of known genitourinary tract abnormalities, including vesicoureteral reflux, duplicated collecting system, neurogenic bladder, as well as malignancies or diabetes mellitus, and nonidiopathic vulvitis, such as that related to lichen sclerosis or psoriasis. Children with a history or suspicion of sexual abuse were also excluded. Thus, we did not test for the presence of Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis. Vulvitis was defined as an intense, diffuse erythematous, labial/perineal rash, with or without pruritus.
Each child underwent a sterile periurethral swab and provided a clean-catch urine specimen. Cultures were sent for bacterial growth to the microbiology laboratory. Urine cultures were considered positive if they grew more than 100,000 colony-forming units, while swab cultures were considered positive if any organism grew. The dominant organism (based on semiquantitative streaking on an agar plate) from each culture, as well as the specific antibiotic sensitivity profiles, were recorded and compared. Cultures were grouped into known uropathogens and nonuropathogens. Chi-square tests were used to assess differences between outcome groups with significance set at p < 0.05; if the observed p value was < 0.05, odds ratios (ORs) and the associated 95% confidence intervals (CIs) were calculated for the group comparisons.
Results
A total of 101 prepubescent girls with suspected UTIs were enrolled into the study (age range: 2–8 years, mean age: 5 years); 58 (57.4%) had signs of concurrent vulvitis. Of the 101 patients, 35 had culture-proven UTIs, 30 (85.7%) of which were in the vulvitis cohort.
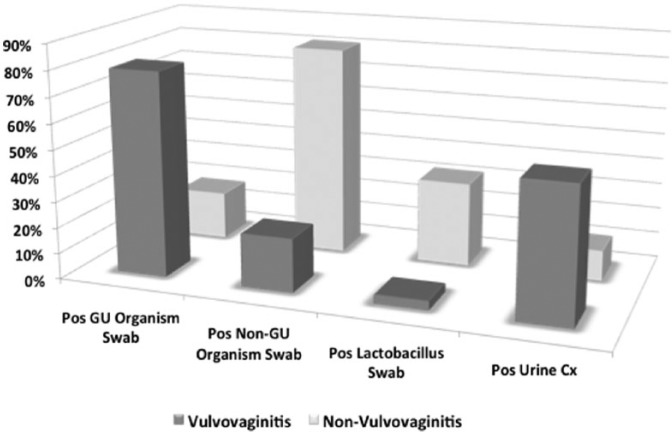
A total of 46 (79.2%) girls with vulvitis grew uropathogenic bacteria (organisms commonly associated with UTIs) as the dominant culture compared with 8 girls (18.6%) without vulvitis (OR: 16.8, 95% CI: 6.19–45.4, p < 0.0001). All 101 periurethral swabs grew a dominant organism. Both groups predominantly grew Enterococcus species and Escherichia coli, with the vulvitis patients having two cases of Enterococcus and one case of Pseudomonas. Conversely, girls without vulvitis were significantly more likely to grow nonuropathogenic bacteria than girls with vulvitis (81% versus 21%, respectively; OR: 16.77, 95% CI: 6.19–45.4, p < 0.0001), with the majority being Staphylococcus or Streptococcus species in both groups. Lactobacillus species were significantly more prevalent in the nonvulvitis than in the vulvitis groups (14 girls versus two girls, 32.6% versus 3.5%, respectively; OR: 13.5, 95% CI: 2.87–63.6, p < 0.0001). In addition, 30 girls (51.7%) with vulvitis had a positive urine culture compared with five girls (11.6%) without vulvitis, indicating a significantly higher UTI rate in the former (OR: 8.14, 95% CI: 2.81–23.6, p < 0.0001). Of note, the dominant organism in the urine cultures matched the species and antibiotic sensitivity profile of the corresponding periurethral swab in all cases. Complete culture data are listed in Table 1 while a summary of the findings is listed in Figure 1.
Table 1.
Culture results by organism.
| Organism | Vulvovaginitis | Nonvulvovaginitis | p value | |
|---|---|---|---|---|
| Swab dominant culture | ||||
| Uropathogen | Enterococcus | 23 | 4 | 0.001 |
| Escherichia coli | 20 | 4 | < 0.01 | |
| Enterobacter | 2 | - | NS | |
| Pseudomonas | 1 | - | NS | |
| Total uropathogen | 46 | 8 | < 0.0001 | |
| Nonuropathogen | ||||
| Staphylococcus/Streptococcus | 10 | 20 | < 0.01 | |
| Lactobacillus | 2 | 14 | < 0.001 | |
| Corynebacterium | - | 1 | NS | |
| Total nonuropathogen | 12 | 35 | < .00001 | |
| Total swab dominant culture | 58 | 43 | < 0.05 | |
| Urine dominant culture | ||||
| Uropathogen | Enterococcus | 16 | 1 | NS |
| Escherichia coli | 14 | 4 | NS | |
| Total urine dominant culture | 30 | 5 | < 0.0001 |
NS, not significant.
Figure I.
Summary of culture results based on the presence or absence of vulvovaginitis.
Cx, culture; GU, genitourinary.
Discussion
This study compares the presence or absence of vulvitis in preadolescent girls presenting with clinical suspicion of a UTI. Most strikingly, we found that girls with vulvitis were more than eight times as likely to have a culture-diagnosed UTI as girls without vulvitis. In addition, these patients were more likely to grow uropathogenic bacteria from periurethral swabs than their nonvulvitis correlates. In all cases, the organisms grown from urine culture matched the organisms obtained from periurethral swab, both by species and antibiotic sensitivities. Conversely, girls without vulvitis were more likely to grow nonuropathogenic bacteria than girls with vulvitis (81% versus 21%, respectively, p < 0.0001), including Lactobacillus species (32.6% versus 3.5%, respectively). Notably, Lactobacillus species inhibit the growth of uropathogenic E. coli. Overall, these data suggest that vulvitis is associated with the presence of a UTI, and may serve as a risk factor for its development.
This study is especially important since there is little previous pathogenesis insight into vulvovaginitis and UTIs in preadolescents. Some of the earliest work by Sigel and colleagues [Sigel et al. 1975] noted that girls with periurethral, perineal, and paravaginal erythema had higher rates of culture-proven UTIs than girls without erythema (15% versus 6%, respectively). Although the authors did not perform statistical analyses, using their data we found that the difference in UTIs between these cohorts was indeed statistically significant (p = 0.001 using the Fisher’s exact test). A small study published in 1983 compared vaginal and urine cultures in girls presenting with vulvovaginitis [Fede, 1983]. A total of 11 girls (24%) had culture-proven UTIs with E. coli, Enterococcus, and Proteus (in order of descending frequency), and similar to our findings, these organisms were identical to the ones cultured from vaginal secretum. Pierce and Hart noted that E. coli and other bowel flora comprised the majority of positive vaginal cultures in girls with vulvovaginitis, and of those who also had urine positive cultures, E. coli was the most common organism isolated [Pierce and Hart, 1992]. In addition, Jaquiery and colleagues found that the two girls with E. coli-positive vulvovaginitis also had concurrent UTIs [Jaquiery et al. 1999]. Our study is unique in that we focused specifically on the presence or absence of vulvitis and obtained periurethral, not vaginal, cultures. Nevertheless, similarly to these other studies, we noted that these girls were significantly more likely to have UTIs with bowel flora, including Enterococcus species and E. coli.
To understand better the pathogenesis of this process, a review of the vaginal bacterial milieu is prudent. This environment is complex and involves various species interacting with one another to form an ecological community, a concept referred to as microbiocoenosis [Matytsina et al. 2010]. Hammerschlag and colleagues obtained vaginal cultures from 100 healthy girls ranging from 2 months to 15 years in an attempt to better characterize this [Hammerschlag et al. 1978]. They cultured a variety of aerobic and facultative anaerobic organisms, including diphtheroids (78%), Staphylococcus epidermidis (73%), a-hemolytic streptococci (39%), lactobacilli (39%), and E. coli (34%). However, in the presence of vulvovaginitis, there appears to be a perturbation of this delicate flora. Gerstner and colleagues performed vaginoscopy and vaginal cultures on girls aged 3 months to 16 years with vaginal discharge and/or vulvovaginitis compared with normal controls [Gerstner et al. 1982]. They noted that Lactobacillus was significantly lower in the study group (11.1% versus 38.7%), while anaerobic bacteria, including Peptococcus and Peptostreptococcus species as well as Bacteriodes species were concurrently higher. Paradise and colleagues looked at 54 vaginal cultures in premenarchal girls (median age: 5.8 years, range: 5–12.6 years) using similar cohorts as the previous study [Paradise et al. 1982]. Our own statistical analysis of their data reveals that girls without vulvovaginitis were significantly more likely to have only ‘normal’ vaginal flora than those with vulvovaginitis, regardless of the presence or absence of concurrent vaginal discharge (42% versus 17%, respectively, p < 0.001). More recently, Jaquiery and colleagues compared premenarchal girls with and without vulvovaginitis and noted, once again, that the former were more likely to have higher levels of vaginal anaerobes (p < 0.01) [Jaquiery et al. 1999]. Our study suggests that this alteration in flora is not limited to the vagina in girls with vulvovaginitis, but can be seen periurethrally as well. Although we did not perform cultures for anaerobic organisms, we did note higher periurethral uropathogenic bacterial colonization (i.e. Enterococcus and E. coli) and less nonuropathogenic bacterial colonization (i.e. Lactobacillus and Staphylococcus/Streptococcus species).
So what is the origin of these organisms? Given the close proximity of the anus to the vagina, fecal flora represents an attractive candidate. Indeed, our data confirmed that girls with vulvitis were significantly more likely to harbor Enterococcus species and E. coli periurethrally, both of which are aerobic bowel flora and can act as opportunistic pathogens. This observation has been mirrored previously in girls with vulvovaginitis [Fede, 1983; Pierce and Hart, 1992]. Alternate bacterial sources have been proposed and include pathogenic respiratory and cutaneous organisms such as Streptococcus pyogenes, Haemophilus influenzae, and Staphylococcus aureus [Pierce and Hart, 1992; Stricker et al. 2003; Muller and Schmitt, 2004].
Regardless of their origin, these girls appear to have a predilection for overlying infection with pathogenic bacterial organisms and concurrent UTIs. We propose that the local inflammation of the vulva, vagina, and perineum associated with vulvovaginitis initiates this process. The premenarchal female population is likely to have higher perigenital inflammation at baseline for several reasons. First, the thin vulvar and vaginal tissues are more susceptible to insult, especially since they lack anatomical protection from pubic hair and labial fat pads. Second, the close proximity of the genital area to the anus, continuous exposure to irritants such as bubble baths, urine, and feces, all combined with suboptimal hygiene characteristic of this age group further contribute to a proinflammatory environment. Interestingly, constipation has also been associated with UTIs [Cayan et al. 2001], and one reason may be increased fecal soiling of the perigenital area. Anecdotally, we have seen a significant association with constipation and not only UTIs and vulvovaginitis, but also urinary incontinence, including nocturnal enuresis as well as lower urinary tract symptoms such as dysuria, frequency, and urgency. In addition, these children have a neutral to alkaline vaginal pH at baseline due to the absence of tissue estrogenization and subsequent colonization by acid-producing organisms such as lactobacilli [Altchek, 1995; Matytsina et al. 2010]. As the inflammation progresses, levels of lactobacilli diminish even further. Ultimately, this proinflammatory environment results in a perturbation of the local bacterial flora, which may allow competing uropathogenic bacteria an opportunity to colonize. As these organisms replicate and spread to the periurethral region, they can then ascend via the urethra to the bladder (urethrovesical reflux), causing a UTI. We believe that this algorithm explains how vulvitis or vulvovaginitis can predispose the preadolescent female to the development of UTIs.
Weaknesses
This study has several weaknesses that should be addressed. First, we did not send periurethral swabs for fungal or anaerobic bacterial cultures. However, candidal infection appears to be rare in this population, despite the fact that many girls with vulvovaginitis receive antifungal treatment. Numerous studies have failed to show a correlation between candidal infections and vulvovaginitis in prepubertal/premenarchal cohorts [Paradise et al. 1982; Jaquiery et al. 1999; Sikanic-Dugic et al. 2009]. This is likely to be due to the low estrogen vaginal environment coupled with a high anaerobic bacterial colonization rate that impedes yeast growth [Banerjee et al. 2004]. Factors such as prior antibiotic use, however, may predispose this population to infection with Candida. Second, based on this study we cannot conclude if vulvitis causes UTIs or vice versa. We suggest that the local inflammatory state associated with vulvitis predisposes to pathogenic bacterial colonization and UTI development. However, we cannot exclude that the etiology of vulvitis is recurrent exposure of the vulva to contaminated urine. This could also account for the identical organisms cultured from both the urine and periurethral area. A possible method to distinguish between these pathways could involve further observations of the girls that have vulvitis with uropathogenic organisms but no UTI; if they were to develop a UTI in the future with the previously identified organism, then it would strengthen our argument pertaining to urethral reflux of these organisms causing UTIs. Third, we did not look for sexually transmitted disease organisms, which could have been present because parents did not admit to their children having been exposed to sexual abuse.
Conclusion
In this prospective study, we observed that preadolescent girls with symptoms of UTI were significantly more likely to actually have a UTI in the presence of vulvitis. Further, girls with vulvitis were more likely to have an altered perineal biome with a higher periurethral growth of uropathogenic organisms and lower concentrations of Lactobacillus compared with girls without vulvitis. Although this study does not establish a causal relationship between vulvitis and UTIs, the presence of the former undoubtedly increases the risk of having the latter. Given this association, we recommend that practitioners always assess these patients for the presence of vulvitis, and if present, work diligently with parents and patients alike to treat and prevent its recurrence.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: SJH is a speaker for Salix Pharmaceuticals, Inc (Raleigh, NC, USA) and shareholder in Akesian Health Products, LLC (Winston-Salem, NC, USA).
Contributor Information
Ilya Gorbachinsky, Department of Urology, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Robert Sherertz, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Gregory Russell, Department of Biostatistical Sciences, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
L Spencer Krane, Department of Urology, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Steve J. Hodges, Department of Urology, Wake Forest University School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
References
- Altchek A. (1995) Pediatric and adolescent gynecology. Compr Ther 21: 235–241. [PubMed] [Google Scholar]
- Banerjee K., Curtis E., de San Lazaro C., Graham J. (2004) Low prevalence of genital candidiasis in children. Eur J Clin Microbiol Infect Dis 23: 696–698. [DOI] [PubMed] [Google Scholar]
- Cayan S., Doruk E., Bozlu M., Duce M., Ulusoy E., Akbay E. (2001) The assessment of constipation in monosymptomatic primary nocturnal enuresis. Int Urol Nephrol 33: 513–516. [DOI] [PubMed] [Google Scholar]
- Fede T. (1983) Vulvovaginitis in children. Clin Exp Obstet Gynecol 10: 118–119. [PubMed] [Google Scholar]
- Gerstner G., Grunberger W., Boschitsch E., Rotter M. (1982) Vaginal organisms in prepubertal children with and without vulvovaginitis. A vaginoscopic study. Arch Gynecol 231: 247–252. [DOI] [PubMed] [Google Scholar]
- Hammerschlag M., Alpert S., Rosner I., Thurston P., Semine D., McComb D., et al. (1978) Microbiology of the vagina in children: normal and potentially pathogenic organisms. Pediatrics 62: 57–62. [PubMed] [Google Scholar]
- Jaquiery A., Stylianopoulos A., Hogg G., Grover S. (1999) Vulvovaginitis: clinical features, aetiology, and microbiology of the genital tract. Arch Dis Child 81: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W., Tanner J. (1969) Variations in pattern of pubertal changes in girls. Arch Dis Child 44: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W., Tanner J. (1970) Variations in the pattern of pubertal changes in boys. Arch Dis Child 45: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matytsina L., Greydanus D., Gurkin Y. (2010) Vaginal microbiocoenosis and cytology of prepubertal and adolescent girls: their role in health and disease. World J Pediatr 6: 32–37. [DOI] [PubMed] [Google Scholar]
- Muller W., Schmitt B. (2004) Group A beta-hemolytic streptococcal vulvovaginitis: diagnosis by rapid antigen testing. Clin Pediatr (Phila) 43: 179–183. [DOI] [PubMed] [Google Scholar]
- Paradise J., Campos J., Friedman H., Frishmuth G. (1982) Vulvovaginitis in premenarcheal girls: clinical features and diagnostic evaluation. Pediatrics 70: 193–198. [PubMed] [Google Scholar]
- Pierce A., Hart C. (1992) Vulvovaginitis: causes and management. Arch Dis Child 67: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S., Siegel B., Sokoloff B. (1975) Perineal erythema: a sign of urinary tract infection in girls? Clin Pediatr (Phila) 14: 1029–1030. [DOI] [PubMed] [Google Scholar]
- Sikanic-Dugic N., Pustisek N., Hirsl-Hecej V., Lukic-Grlic A. (2009) Microbiological findings in prepubertal girls with vulvovaginitis. Acta Dermatovenerol Croat 17: 267–272. [PubMed] [Google Scholar]
- Stamm W., Raz R. (1999) Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clin Infect Dis 28: 723–725. [DOI] [PubMed] [Google Scholar]
- Stricker T., Navratil F., Sennhauser F. (2003) Vulvovaginitis in prepubertal girls. Arch Dis Child 88: 324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyk N., Allen L., Giesbrecht E., Jamieson H., Kives S., Morris M., et al. (2009) Pediatric vulvovaginal disorders: a diagnostic approach and review of the literature. J Obstet Gynaecol Can 31: 850–862. [DOI] [PubMed] [Google Scholar]