Abstract
Objectives:
The aim of the study was to increase the efficiency of treatment for severe symptoms of overactive bladder (OAB) with antimuscarinic drugs in elderly men and women.
Methods:
A total of 341 patients over 65 years of age (average age 69.9; 186 women and 155 men) with severe symptoms of OAB (frequency of incontinence episodes [IEs] ≥ 3/day) underwent examination. Patients were distributed into three main groups: A (n = 58; trospium 60 mg/day + solifenacin 20): three cycles, each cycle 8 weeks, with an 8-week interval; B (n = 55; trospium 30 mg/day + solifenacin 10), regimen was the same as in group A; C (n = 62; trospium 30 mg/day + solifenacin 10) daily during 1 year.
Results:
The most successful treatment for the clinical and urodynamic symptoms of OAB was observed in group A, without an increase in the quantity or intensity of side effects (IEs = 4.8 (0.9) → 1.4 (0.8); p ≤ 0.01). Groups B and C also demonstrated positive effects for most of the markers for lower urinary tract state with statistical significance p ≤ 0.01. Nonparametric correlation between decrease in IEs and relative number of patients who accurately fulfilled prescriptions was in group A, r = 0.53, p ≤ 0.05; in group B, r = 0.61; p ≤ 0.05; in group C, r = 0.55, p ≤ 0.05.
Conclusions:
Cyclic therapy with two different spectrum antimuscarinics appears to be effective for controlling severe OAB in elderly patients. One-year cyclic therapy with a trospium and solifenacin combination provides a high compliance level (76–84%). However, continuous therapy with standard doses of trospium and solifenacin results in low adherence and high rates of treatment withdrawals (≥ 66%) despite satisfactory clinical and urodynamic results.
Keywords: elderly, overactive bladder, solifenacin, trospium
Introduction
It is well known that the main symptoms of overactive bladder (OAB), that is, incontinence, urgency, and frequent urination, cause suffering for people of any age, but elderly men and women have particularly poor tolerance of these symptoms [Kraus et al. 2010; Yoo et al. 2011; Natalin et al. 2013]. The prevalence of OAB increases after 40 years of age. Recent epidemiological studies in the USA showed that the prevalence of OAB among people aged over 40 years is 26–33% for men and 27–46% for women (with race differences); among people aged over 65 years it reaches 40.4% and 46.9% for men and women, respectively [Sexton et al. 2011; Coyne et al. 2012; Griebling, 2013; Milsom et al. 2013].
Manageability of OAB symptoms in elderly people has increased significantly recently. This is due to the introduction of new antimuscarinic drugs, which enable good therapeutic results to be obtained and avoid many of the undesirable effects that are common for first-generation drugs [Erdem and Chu, 2006; Wagg et al. 2007]. Of course, drugs with other mechanisms of action on the detrusor, such as onabotulinumtoxin A and antagonists of beta-3 adrenoreceptors (mirabegron) are also being investigated [Igawa et al. 2010; Andersson, 2013; Chapple et al. 2014], but the current range of antimuscarinic drugs still has potential for increasing effectiveness, and optimization of treatment regimen for OAB in elderly patients remains an important issue. Studies of rational approaches to the application of antimuscarinic drugs in elderly patients with due consideration of ‘old’ bladder and behavioral stereotypes, which are common for elderly people, are still performed [Geoffrion, 2012; Veenboer and Ruud Bosch, 2014; Sicras-Mainar et al. 2014].
Previously we studied the effectiveness of management of OAB symptoms in elderly men and women with combined high doses of antimuscarinic drugs. It was established that administration of trospium and solifenacin over 5 weeks significantly improved the urodynamics of the lower urinary tract (LUT) without increasing side effects [Kosilov et al. 2013].
This study aimed to compare the effectiveness and safety of cyclic and permanent 1-year treatment for OAB symptoms in elderly patients with consideration of the problems related to the accuracy of fulfilling prescriptions in cases of long-term administration of drugs [Noe et al. 2004; Shaya et al. 2005; D’Souza et al. 2008].
Methods
A longitudinal, randomized study was performed from 1 March 2012 to 1 June 2013 in the neurourological department of Primorsky Regional Diagnostic Center. A total of 341 patients over 65 years of age (average age 69.9 years; 186 women and 155 men) were enrolled. All participants were outpatients. The total patient number was 516, of whom 175 were excluded at the preliminary stage. The basic characteristics of the patients are shown in Table 1. Research was performed in accordance with the Declaration of Helsinki, international rules and standards for urodynamic examinations [Brown et al. 2013; Gurpreet et al. 2010; Schafer et al. 2002; Schroder et al. 2009; Singh et al. 2010]. Patients with severe symptoms of OAB (frequency of incontinence episodes [IEs] ≥ 3/day) were included in the study [Rovner, 2005; Dmochowski et al. 2009; Wu et al. 2011]; patients with chronic active diseases and patients with intolerance to antimuscarinic drugs were excluded. Patients had not taken antimuscarinics for at least 1 year prior to study entry. Patients with exacerbations of chronic diseases as well as individuals with antimuscarinic intolerance, central nervous system organic lesions, and a history of long QT syndrome or ventricular flutter were excluded from the study. Generation of random-number sequences during patient selection was performed using the median of squares method. Stratified randomization was used during group formation adjusting for important characteristics influencing the treatment results such as sex and OAB severity. Strata included individuals according to week days. All study participants (patients and staff) were blinded to treatment allocation: all medications (antimuscarinics and placebo) had a similar appearance, taste, and dosage regimen.
Table 1.
Main baseline characteristics of patients (n = 341).
| Groups | Group A | Group A1 | Group B | Group B1 | Group C | Group C1 |
|---|---|---|---|---|---|---|
| Number of patients | 58 | 53 | 55 | 49 | 62 | 64 |
| Mean age in years | 69.1 (5.3) | 70.2 (6.7) | 68.9 (5.1) | 68.4 (7.0) | 71.7 (4.6) | 67.9 (9.5) |
| Median incontinence episodes/day (n) | 4.8 (0.9) | 5.3 (1.1) | 5.6 (0.9) | 5.1 (2.0) | 4.9 (0.7) | 5.5 (1.3) |
| Median urgency episodes/day (n) | 6.7 (2.1) | 5.9 (1.5) | 6.1 (0.9) | 5.5 (1.6) | 7.0 (2.4) | 5.8 (1.3) |
| Median number of urination/day (n) | 11.2 (1.4) | 9.2 (2.5) | 10.2 (1.7) | 10.9 (2.7) | 8.1 (2.6) | 9.4 (2.1) |
| Median volume voided/once (ml) | 116.6 (17.1) | 121.2 (25.4) | 114.5 (12.3) | 127.5 (27.8) | 121.2 (21.7) | 99.1 (14.2) |
The algorithm of clinical and urodynamic examination of patients is shown in Figure 1. The aim of the study was to determine the efficacy of a cyclic regimen of trospium and solifenacin in elevated and standard doses versus continuous regimens in standard doses for the control of severe OAB in elderly patients. The study endpoints were LUT clinical and urodynamic parameters after 12 months of treatment as well as the percentage of compliant patients.
Figure 1.
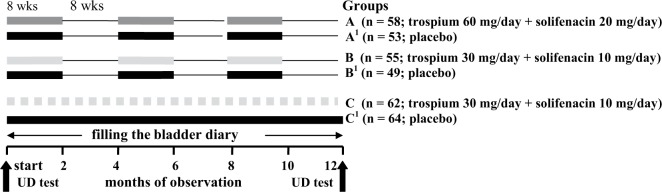
Algorithm of observation and treatment of elderly patients with severe symptoms of overactive bladder (n = 341).
In accordance with the guidelines for administration of drugs patients were distributed into the following groups: A (n = 58; trospium 60 mg/day + solifenacin 20): three cycles of drug therapy, 8 weeks each, with an 8-week interval during 1 year; B (n = 55; trospium 30 mg/day + solifenacin 10), the algorithm is the same as in group A; C (n = 62; trospium 30 mg/day + solifenacin 10), daily over 1 year. In control groups A1 (n = 53), B1 (n = 49), C1 (n = 64) patients received placebo. In our study all medications were free for our patients in accordance with the national obligatory medical insurance program.
The primary outcome measures were decrease in IEs to ≥ 1.5/day and optimization of LUT urodynamic parameters, that is, at least 30% increase in median reflex volume, bladder capacity, and detrusor compliance against background. The above changes in IEs and urodynamic parameters suggest significantly effective therapy [Rovner, 2005; Dmochowski et al. 2009; Wu et al. 2011]. Criteria for accuracy of prescription fulfillment were observation of dosage instruction and time of administration not less than six times a week. Urodynamic examination was performed at the beginning and at the end of the twelfth month of the study. All patients filled standard OAB questionnaires recommended by the International Continence Society at the beginning and end of the follow-up period, and during the year they kept daily bladder diaries where they recorded data concerning frequency of IEs, volume of single urination, quantity of urinations and urgencies per day, and they also marked the time and dose of administered drugs [Amundsen et al. 2006; Parsons et al. 2007; Tissot et al. 2008].
The program JMP SAS Statistical Discovery 8.0.2 (SAS Institute, Cary, NC, USA) was used for the statistical processing of data. To reveal a 5% difference in significance level with 80% power and assumed for such study type standard deviation we needed to recruit 54 patients per treatment and control groups. Patient numbers were somewhat increased to take into account any drop outs. Thus the total number of patients was 341. p < 0.05 was considered statistically significant. The Wilcoxon and Kruskal–Wallis tests were used to compare results in each group; the Kruskal–Wallis rank test was used for control of equality of medians from different groups. One-way analysis of variance with Tukey–Kramer honestly significant difference was used to compare effects in three and more groups, and Spearman’s correlation coefficient was applied to study dependence between processes.
Results
The results of urodynamic examination in group A (Table 2) demonstrate a notable improvement in state of the LUT. Differences are significant in all cases, statistical significance of change of postvoid residual (14.1 (2.9) →31.3 (3.4)), reflex volume (116.7 (19.3) →178.4 (21.9)), and bladder capacity (166.2 (27.2) →248.1 (21.4)) is p ≤ 0.01. Improvement of indexes in group C was also noted (p ≤ 0.05). Cross-sectional analysis of urodynamic parameters during control examination demonstrated significant differences between treatment and control groups for most paired comparisons. The exception was for postvoid residual in A–A1 and B–B1 pairs due to high within-group variation. Reflex volumes were significantly different in A–A1 and B–B1 pairs (178.4 (21.9) versus 136.0 (14.6); p < 0.05, and 188.1 (24.2) versus 121.3 (41.4); p < 0.05, respectively). The most prominent difference between treatment and control groups was revealed for bladder capacity: A–A1: 248.1 (21.4) versus 145.8 (35.8); p < 0.01; B–B1: 241.0 (34.7) versus 147.7 (29.4); p < 0.01. Detrusor compliance values were also markedly higher after treatment in the A1 group (p < 0.01).
Table 2.
The results of urodynamic studies (before treatment n = 341).
| Parameters of the lower urinary tract | Indicators of urodynamics (± standard deviation) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Postvoid residual (ml) |
Reflex volume (ml) |
Bladder capacity (ml) |
Detrusor compliance (ml/cm H2O) |
|||||
| Before treat | After treat | Before treat | After treat | Before treat | After treat | Before treat | After treat | |
| Group A (n = 58) | 14.1 (2.9) | 31.3 (3.4) ** | 116.7 (19.3) | 178.4 (21.9) ** | 166.2 (27.2) | 248.1 (21.4)** | 12.9 (3.5) | 25.9 (5.5)* |
| Group A1 (n = 53) | 17.9 (8.8) | 21.9 (13.2) | 128.4 (22.6) | 136.0 (14.6) | 140.7 (23.7) | 145.8 (35.8) | 13.7 (3.5) | 14.2 (4.2) |
| Group B (n = 55) | 18.4 (5.5) | 28.7 (4.7)* | 136.1 (22.7) | 188.1 (24.2) | 173.4 (28.7) | 241.0 (34.7) * | 15.1(6.5) | 24.2 (5.7) |
| Group B1 (n = 49) | 15.1 (8.5) | 22.2 (12.0) | 125.4 (25.8) | 121.3 (41.4) | 156.8 (33.5) | 147.7 (29.4) | 13.6 (5.6) | 16.6 (9.9) |
| Group C (n = 62) | 20.4 (4.8) | 28.2 (6.8) | 98.6 (12.7) | 156.7 (19.5)* | 164.7 (15.8) | 230.5 (15.8)* | 13.9 (3.7) | 22.9 (5.4)* |
| Group C1 (n = 64) | 17.4 (7.2) | – | 131.5 (19.6) | – | 168.9 (32.7) | – | 16.7(5.8) | – |
< 0.05; ** < 0.001. ‘Before treat’, amounts taken at onset of study and considered baseline; ‘after treat’, 12 months from the start of the study.
Frequency of IEs (Figure 2, Table 3) in group A decreased to 3.3/day (p ≤ 0.01) after the second month of follow up; by the end of the sixth month this index decreased to 3.8 /day (p ≤ 0.01) from initial data, reached 1.3/day in absolute terms, and remained consistently low during all of the follow-up period. Decrease in frequency of daily urinations and urgencies was related to the value of IEs (r = 0.65 (p ≤ 0.05) и r = 0.59 (p ≤ 0.05)).
Figure 2.
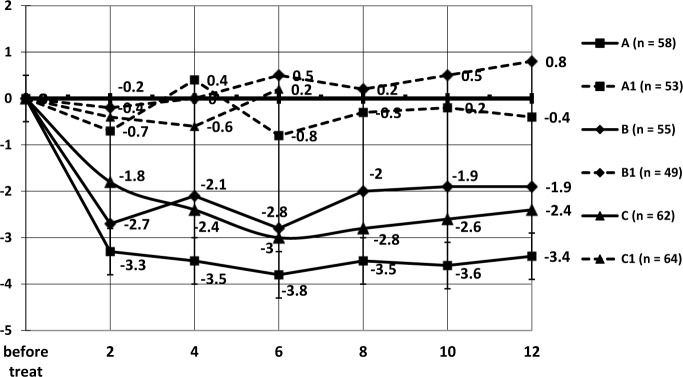
Dynamics of the decrease in frequency of incontinence episodes in elderly patients according to group. y-axis, median of absolute values from baseline in number of incontinence episodes per day. x-axis, months of observation.
Table 3.
Results of clinical assessment (n = 341).
| Parameters of the lower urinary tract |
Incontinence episodes/ day |
Median urgency episodes/day (n) |
Median number of urination/day (n) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Time of study | Before treat | After treat1 | After treat2 | Before treat | After treat1 | After treat2 | Before treat | After treat1 | After treat2 |
| Group A (n = 58) | 4.8 (0.9) | 1.3 (0.6)** | 1.4 (0.5)** | 6.7 (2.1) | 3.3 (0.7)* | 2.5 (1.3)* | 11.2 (1.4) | 5.6 (1.9)** | 4.8 (2.3)** |
| Group A1 (n = 53) | 5.3 (1.1) | 4.5 (0.5) | 4.9 (3.1) | 5.9 (1.5) | 6,5 (2.1) | 5.6 (2.6) | 9,2 (2.5) | 10,4 (2.6) | 9.5 (2.7) |
| Group B (n = 55) | 5.6 (0.9) | 2.5 (1.3) * | 3.7 (1.7) | 6.1 (0.9) | 3.9 (0.7)* | 2.4 (2.0)* | 10.2 (2.7) | 4.7 (1.8)* | 5.5 (3.6)* |
| Group B1 (n = 49) | 5.1 (2.0) | 5.5 (2.2) | 5.9 (2.7) | 5.5 (1.6) | 5.0 (1.6) | 6.1 (1.3) | 10.9 (2.7) | 9.7 (4.2) | 11.0 (5.1) |
| Group C (n = 62) | 4.9 (0.7) | 1.9 (0.9)* | 2.5 (1.1)* | 7.0 (1.4) | 4.1 (1.5)* | 3.9 (1.8)* | 8.1 (2.6) | 5.5 (1.1)* | 4.8 (2.5)* |
| Group C1 (n = 64) | 5.5 (1.3) | 5.7 (4.1) | – | 5.8 (1.3) | 5.6 (2.5) | – | 9.4 (2.1) | 10.4 (3.1) | – |
< 0.05; ** < 0.001. ‘Before treat’, amounts taken at the onset of study and considered baseline; ‘after treat1’, 6 months from the start of the study; ‘after treat2’,12 months from the start of the study.
Cross-sectional analysis of clinical urination parameters suggested that the number of daily IEs in group A after 12 months of follow up was significantly different from that in group B (1.4 (0.5) and 3.7 (1.7), p < 0.05). However this parameter was not significantly different between groups A and C, neither after 6 nor 12 months (p ≥ 0.05). Data obtained throughout the study showed that daily values of median urgency episodes and median number of urinations were changing synchronously without significant differences between groups A, B, and C (p ≥ 0.05). Paired comparisons of these parameters at 6 and 12 months after treatment initiation demonstrated significant differences in treatment and placebo groups (p < 0.05 for all comparisons).
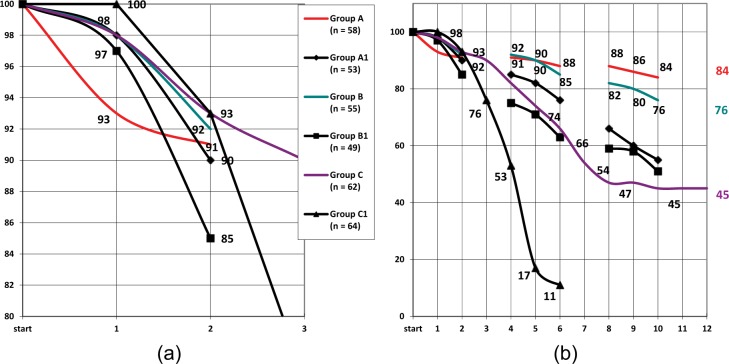
The dynamics of the percentage of patients who accurately followed drug instructions is shown in Figure 3. In group A the number of patients who fulfilled all prescriptions at the end of the study decreased for nine persons in comparison with the beginning of the study and reached 85.5%, which was not significantly different from the results in group B (76.5% (p ≥ 0.05). At the beginning of the third cycle the percentage of patients who followed all instructions in control groups A1 and B1 (66.0% and 59.2%) decreased significantly in comparison with groups A and B (87.9% and 81.8%) (p ≤ 0.05 and p ≤ 0.05, respectively), and almost did not differ between themselves (p ≥ 0.05).
Figure 3.
Changes in number of elderly patients who accurately followed instructions for the administration of drugs, in percentage (a) during first 3 months of follow up, (b) throughout the year. y-axis, percentage of patients; x-axis, months of observation.
The percentage of patients who accurately followed all instructions in group C demonstrated a steady decreasing trend from the beginning of the study, and after the fifth month of follow up it was significantly different from that in groups A and B (p ≤ 0.05 with both groups). The decreasing trend continued and at the end of follow up the percentage of such patients was 30.6%. The number of patients who failed to fulfill prescriptions in control group C1 grew rapidly, and by the end of the third month the percentage of patients who followed instructions was 76.5%, by the end of fourth month, 53.1%, by the end of fifth month, only 17.1%, and after 6 months a majority of patients refused to continue the treatment (89.1%).
During the first 2-month cycle of follow up the percentage of patients who failed to follow instructions or refused to continue the therapy in the main groups was insignificant (6.6–8.6%). At the end of the sixth month, however, the difference between groups A (87.9%) and B (85.4%), on the one part, and C (66.1%), on the other part, became statistically significant (p ≤ 0.05).
Correlation of decreasing trend for number of patients who accurately fulfilled prescriptions in groups A and B was high: r = 0.89 (p ≤ 0.01). In group C the accuracy of prescription fulfillment decreased steadily, and by the end of the fifth month the percentage of such patients was significantly different from the initial data (72.5%; p ≤ 0.05), by the end of the sixth month, from results in group A (87.9) and group B (85.4) with p ≤ 0.05. Calculation of nonparametric correlation between frequency of IEs and the relative number of patients who accurately fulfilled prescriptions, demonstrated moderate direct correlation in groups A (r = 0.53; p ≤ 0.05), B (r = 0.61; p ≤ 0.05), and C (r = 0.55; p ≤ 0.05).
Seven patients (12.1%) in group A reported side effects, and among them one patient (1.7%) refused to continue the treatment due to the side effects. In group B side effects were also reported by seven patients (12.7%), but none stopped treatment for this reason. In group C 12 patients (19.3%) noted side effects during the treatment and 3 patients (4.8%) refused to continue the treatment. The reason for discontinuation of the treatment in all cases was intolerably dry mouth. Other side effects included dry and itching skin (six patients), dizziness and disorder of coordination (five), headache (one), nausea (one), and flatulence (one). In total, four patients discontinued treatment due to adverse events; three patients (all from group C) withdrew due to lack of treatment efficacy; four patients discontinued treatment for other reasons. Data on withdrawals and adverse events are shown in Table 4.
Table 4.
Cases of side effects and discontinuation of treatment (n = 341).
| Group A (n = 58) | Group A1 (n = 53) | Group B (n = 55) | Group B1 (n = 49) | Group C (n = 62) | Group C1 (n = 64) | Total | |
|---|---|---|---|---|---|---|---|
| Dry mouth | 7(1) | – | 7(0) | – | 12(3) | – | 26(4) |
| Other breach of health | 2(0) | 1(0) | 3(0) | 2(0) | 4(4) | 2(0) | 14(4) |
| Discontinuation due to side effects | 1 | – | – | – | 3 | – | 4 |
| Discontinuation due to unsatisfactory outcome | – | – | – | 3 | – | – | 3 |
| Discontinuation due to other causes | – | 2 | – | – | – | 2 | 4 |
| Stopped treatment due to violation of regulations | 9 | 25 | 9 | 25 | 34 | 57 |
The figures in parentheses indicate the number of patients who refused further therapy because of side effects.
Discussion
Treatment-effect studies of two antimuscarinics have already been carried out before by our group as well as by other investigators [Diokno et al. 2001; Kosilov et al. 2013]. Combination of two antimuscarinics affecting different bladder receptors has been proved to be an effective and safe method for long-lasting and therapy-resistant OAB.
Continuous monotherapy or combination therapy with antimuscarinics for up to 1 year or more for OAB is recommended by many authors [Dmochowski et al. 2009; Wu et al. 2011; Andersson, 2013; Veenboer and Ruud Bosch, 2014]. However, long-term treatment is associated with the risk of patient noncompliance [Zinner et al. 2008; Yi et al. 2013]. In our study we compared patient compliance in the groups of cyclic and continuous antimuscarinic treatment over a 1-year period.
As a result of the study it was established that the urodynamic indexes for the LUT changed the most rapidly in the group of elderly patients treated with high-dose trospium and solifenacin in cyclic form. In group A significant differences in median values of urodynamic indexes were observed. In groups B and C the urodynamic state also improved in the majority of patients, but not all indexes demonstrated significant differences from the initial data.
The frequency of IEs in patients from group A rapidly decreased by the second month of follow up (-3.3, p ≤ 0.01) and remained steadily low (-3.4; -3.8) during the course of the follow up. In group C the pattern of improvement in frequency of IEs was smoother; in group B the frequency of IEs decreased by the end of the cycle and somewhat increased (insignificantly) when patients stopped administration of antimuscarinic drugs.
Thus we determined that continuous treatment with two antimuscarinics resulted in synchronic change of clinical outcome measures (IEs, median urgency episodes, and median number of urinations), those being significantly different versus baseline and controls. The majority of urodynamic parameters were significantly different in treatment and control groups both at baseline and at the end of the study. Similarly most urodynamic parameters in each treatment group differed from the same parameters in the control group. This provides evidence that in elderly patients with severe OAB the combination of two antimuscarinics affecting different receptors in standard doses is an effective method regardless of treatment schedule, either continuous or cyclic.
Quite a different situation occurs with the adherence to cyclic and continuous treatment regimens (the second study endpoint). In general, under cyclic therapy patients, who were treated with standard-dose and high-dose antimuscarinic drugs, demonstrated a high level of discipline in fulfillment of prescriptions. In other words, the accuracy of prescription fulfillment in groups with cyclic treatment was steadily high during the year of follow up. On the contrary, in the continuous treatment group, in spite of satisfactory therapeutic effect, compliance decreased below the critical threshold at the study midpoint, retaining less than one third of patients by the end of study.
Such results let us suppose that short-term treatment with high-dose antimuscarinic drugs in elderly patients with severe symptoms of OAB is more effective because it keeps at least some of the patients, who have problems with operative memory and possibly lack of will to follow instructions accurately, focused on the treatment and helps to increase motivation for accurate fulfillment of prescriptions. Discontinuity of cyclic therapy provides some effect of novelty and positive expectations at the beginning of the next course, which makes such an algorithm more effective in comparison with long continuous treatment with antimuscarinics [Diokno et al. 2001; Horstmann et al. 2006; Amend et al. 2008; Chapple et al. 2008; Smith et al. 2011].
However, if elderly patients do not receive a treatment effect in a short timescale, motivation to adhere to treatment declines, resulting in poor compliance with incorrect schedules, missing doses, and self-administered dose changes as well as treatment discontinuation.
Conclusion
Cyclic therapy with two different spectrum antimuscarinics appears to be effective for controlling severe OAB in elderly patients. One-year cyclic therapy with trospium and solifenacin combination provides a high compliance level (76–84%). However, continuous therapy with standard doses of trospium and solifenacin results in low adherence and high rates of treatment withdrawals (≥ 66%), despite satisfactory clinical and urodynamic results.
Footnotes
Funding: Funding study researches were conducted without the involvement of third-party grants, solely on the own funds of the authors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Kirill V. Kosilov, Department of Adaptive Medicine, Humanities Institute of Social Sciences, Far Eastern Federal University, Ayax 10, corp. F-733, Vladivostok, Russian Federation
Sergay A. Loparev, Department of Urology, City Polyclinic No. 3, Vladivostok, Russian Federation
Marina A. Ivanovskaya, Far Eastern Fisheries University, Vladivostok, Russian Federation
Liliya V. Kosilova, Department of the Functional Methods of Examination, Medical Association No. 2 of Vladivostok-sity, Vladivostok, Russian Federation
References
- Amend B., Hennenlotter J., Schäfer T., Horstmann M., Stenzl A., Sievert K. (2008) Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 53: 1021–1028. [DOI] [PubMed] [Google Scholar]
- Amundsen C., Parsons M., Cardozo L., Vella M., Webster G., Coats A. (2006) Bladder diary volume per void measurements in detrusor overactivity. J Urol 176: 2530–2534. [DOI] [PubMed] [Google Scholar]
- Andersson K. (2013) New developments in the management of overactive bladder: focus on mirabegron and onabotulinumtoxin. ATher Clin Risk Manag Ther Clin Risk Manag 9: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Krlin R., Winters J. (2013) Urodynamics: examining the current role of UDS testing. What is the role of urodynamic testing in light of recent AUA urodynamics and overactive bladder guidelines and the VALUE study? Curr Urol Rep 14: 403–408 [DOI] [PubMed] [Google Scholar]
- Chapple C., Cardozo L., Nitti V., Siddiqui E., Michel M. (2014) Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn 33: 17–30. [DOI] [PubMed] [Google Scholar]
- Chapple C., Khullar V., Gabriel Z., Muston D., Bitoun C., Weinstein D. (2008) The effects of antimuscarinic treatments in overactive bladder, an update of a systematic review and metaanalysis. Eur Urol 54: 543–562. [DOI] [PubMed] [Google Scholar]
- Coyne K., Margolis M., Kopp Z., Kaplan S. (2012) Racial differences in the prevalence of overactive bladder in the United States from the epidemiology of LUTS (EpiLUTS) study. Urology 79: 95–101. [DOI] [PubMed] [Google Scholar]
- Diokno A., Lee P., Zorn B., Lenderking W., Grossman M., Bull S., et al. (2001) Factors associated with clinical assessment of overactive bladder and selection of treatment. Clin Ther 23: 1542–1551. [DOI] [PubMed] [Google Scholar]
- Dmochowski R., Larson-Peters A., Aronstein W., Seifu Y. (2009) Efficacy of darifenacin in patients with varying baseline symptom. UroToday Int J 2: 1944–5784. [Google Scholar]
- D’Souza A., Smith M., Miller L., Doyle J., Ariely R. (2008) Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm 14: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem N., Chu F. (2006) Management of overactive bladder disease and urge urinary incontinence in the elderly patient. Am J Med 119: 29–36. [DOI] [PubMed] [Google Scholar]
- Geoffrion R. (2012) Treatments for overactive bladder: focus on pharmacotherapy. J Obstet Gynaecol Can 34: 1092–1101. [DOI] [PubMed] [Google Scholar]
- Griebling T. (2013) Overactive bladder in elderly men: epidemiology, evaluation, clinical effects, and management. Curr Urol Rep 14: 418–425. [DOI] [PubMed] [Google Scholar]
- Gurpreet S., Malcolm L., Lucia D., Stephanie K., Carmel R., Philip T. (2010) Minimum standards for urodynamic practice in the UK. Neurourol Urodyn 29: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Horstmann M., Schaefer T., Aguilar Y., Stenzl A., Sievert K. (2006) Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol Urodyn 25: 441–445. [DOI] [PubMed] [Google Scholar]
- Igawa Y., Aizawa N., Homma Y. (2010) Beta3-adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol 51: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosilov K., Loparev S., Ivanovskaya M., Kosilova L. (2013) Management of overactive bladder (OAB) in elderly men and women with combined, high-dosed antimuscarinics without increased side effects. UroToday Int J 6: art 47. [Google Scholar]
- Kraus S., Bavendam T., Brake T., Griebling T. (2010) Vulnerable elderly patients and overactive bladder syndrome. Drugs Aging 27: 697–713. [DOI] [PubMed] [Google Scholar]
- Milsom I., Coyne K., Nicholson S., Kvasz M., Chen C., Wein A. (2013) Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol 65: 79–95. [DOI] [PubMed] [Google Scholar]
- Natalin R., Lorenzetti F., Dambros M. (2013) Management of OAB in those over age 65. Curr Urol Rep 14: 379–385. [DOI] [PubMed] [Google Scholar]
- Noe L., Sneeringer R., Patel B., Williamson T. (2004) The implications of poor medication persistence with treatment for overactive bladder. Manag Care Interface 17: 54–60. [PubMed] [Google Scholar]
- Parsons M., Amundsen C., Cardozo L., Vella M., Webster G., Coats A. (2007) Bladder diary patterns in detrusor overactivity and urodynamic stress incontinence. Neurourol Urodyn 26: 800–806. [DOI] [PubMed] [Google Scholar]
- Rovner E. (2005) Tolterodine for the treatment of overactive bladder: a review. Expert Opin Pharmacother 6: 653–666. [DOI] [PubMed] [Google Scholar]
- Schafer W., Abrams P., Liao L., Mattiasson A., Pesce F., Spangberg A., et al. (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21: 261–274. [DOI] [PubMed] [Google Scholar]
- Schroder A., Abrams P., Andersson K., Artibani W., Chapple C., Drake M., et al. (2009) Guidelines on Urinary Incontinence. Arnhem: European Association of Urology. [Google Scholar]
- Sexton C., Coyne K., Thompson C., Bavendam T., Chen C., Markland A. (2011) Prevalence and effect on health-related quality of life of overactive bladder in older Americans: results from the epidemiology of lower urinary tract symptoms study. J Am Geriatr Soc 59: 1465–1470. [DOI] [PubMed] [Google Scholar]
- Shaya F., Blume S., Gu A., Zyczynski T., Jumadilova Z. (2005) Persistence with overactive bladder pharmacotherapy in a Medicaid population. Am J Manag Care 11(4 Suppl.): S121–S129. [PubMed] [Google Scholar]
- Sicras-Mainar A., Rejas J., Navarro-Artieda R., Aguado-Jodar A., Ruiz-Torrejón A., Ibáñez-Nolla J., et al. (2014) Antimuscarinic persistence patterns in newly treated patients with overactive bladder: a retrospective comparative analysis. Int Urogynecol J 25: 485–492. [DOI] [PubMed] [Google Scholar]
- Singh G., Lucas M., Dolan L. (2010) Minimum standards for urodynamic practice in the UK. Neurourol Urodyn 29(8):1365–1372. [DOI] [PubMed] [Google Scholar]
- Smith A., Nissim H., Le T. (2011) Misconceptions and miscommunication among aging women with overactive bladder symptoms. Urology 77: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot W., Amundsen C., Diokno A., Webster G., Coats A. (2008) Bladder diary measurements in asymptomatic males: frequency, volume per void, and 24-hr volume. Neurourol Urodyn 27: 198–204. [DOI] [PubMed] [Google Scholar]
- Veenboer P., Ruud Bosch J. (2014) Long-term adherence to antimuscarinic therapy in everyday practice: a systematic review. J Urol 191: 1003–1008. [DOI] [PubMed] [Google Scholar]
- Wagg A., Cardozo L., Chapple C., De Ridder D., Kelleher C., Kirby M., et al. (2007) Overactive bladder syndrome in older people. BJU Int 99: 3. [DOI] [PubMed] [Google Scholar]
- Wu J., Fulton R., Amundsen C., Knight S., Kuppermann M. (2011) Patient preferences for different severities of and treatments for overactive bladder. Female Pelvic Med Reconstr Surg 17: 184–189. [DOI] [PubMed] [Google Scholar]
- Yi J., Jeong S., Chung M., Park H., Lee S., Doo S., et al. (2013) Efficacy and tolerability of combined medication of two different antimuscarinics for treatment of adults with idiopathic overactive bladder in whom a single agent antimuscarinic therapy failed. Can Urol Assoc J 7: E88–E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo E., Kim B., Kim D., Oh S., Kim J. (2011) The impact of overactive bladder on health-related quality of life, sexual life and psychological health in Korea. Int Neurourol J 15: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner N., Kobashi K., Ebinger U., Viegas A., Egermark M., Quebe-Fehling E., et al. (2008) Darifenacin treatment for overactive bladder in patients who expressed dissatisfaction with prior extended-release antimuscarinic therapy Int J Clin Pract 62: 1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]