Abstract
Botanical seed oils reduce the generation of leukotrienes in patients with asthma.
Our objective was to determine the efficacy of a botanical seed oil combination against airflow obstruction in asthma, and to determine the pharmacogenomic effect of the leukotriene C4 synthase (LTC4S) polymorphism A-444C.
We conducted a randomized, double-blind, placebo-controlled, cross-over clinical trial in mild to moderate asthmatics to determine the change in FEV1 after 6 weeks of therapy with borage and echium seed oils versus corn oil placebo. We also examined the effect of the variant LTC4S -444C allele on the change in lung function.
We did not identify a difference in FEV1 in the study cohort as a whole (n = 28), nor in the group of A homozygotes. In the C allele carriers (n = 9), FEV1 improved by 3% after treatment with borage and echium seed oils and declined by 4% after placebo corn oil (p = 0.02). All 9 C allele carriers demonstrated an improvement in their FEV1 on active treatment compared to placebo as compared to only 7 out of 19 A allele homozygotes (p = 0.007). We observed transient differences in ex vivo leukotriene generation from circulating basophils and granulocytes. We did not observe significant differences in urinary LTE4 levels.
We conclude that compared to corn oil, a combination of borage and echium seed oils improves airflow obstruction in mild to moderate asthmatics who carry the variant allele in the LTC4S gene (A-444C). Botanical oil supplementation may have therapeutic potential in asthma if used in a personalized manner.
Trial registration: This trial was registered at http://www.clinicaltrials.gov as NCT00806442.
Keywords: Asthma, Borage oil, Echium oil, Leukotrienes, LTC4 synthase
Background
5-lipoxygenase (5-LO)-dependent oxidative metabolism of arachidonic acid (AA) leads to generation of leukotrienes (LTs), which are associated with airway inflammation in asthma (Peters-Golden and Henderson 2007). Studies have demonstrated that dietary supplementation with marine or botanical seed oils containing omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) can decrease airway inflammation by reducing the generation of leukotrienes and pro-inflammatory cytokines, and attenuating neutrophil function (Barros et al. 2011; Chilton-Lopez et al. 1996; Lee et al. 1985).
Seed oils from the Boraginaceae family of plants, including borage oil (Borago officinalis) and echium oil (Echium plantagineum) contain medium chain omega-6 and omega-3 PUFAs, including γ-linolenic acid (GLA;18:3,n-6), α-linolenic acid (ALA;18:3,n-3) and stearidonic acid (SDA;18:4,n-3) (Figure 1). GLA is efficiently converted by cells and tissues to dihomo γ-linolenic acid (DGLA) that competes with AA for substrate utilization by 5-LO, thus having anti-inflammatory potential in asthma. However, the conversion of ALA to long-chain omega-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (both known to reduce leukotrienes) is poor in humans, which is believed to be a result of the inefficiency of the initial rate-limiting step (Δ-6 desaturase, FADS2 gene) involved in long-chain PUFA biosynthesis. However SDA is downstream of Δ-6 desaturase and is up to five-fold more efficiently converted to EPA than ALA (James et al. 2003). Additionally, SDA has been demonstrated to block leukotriene generation from leukocytes in vitro (Guichardant et al. 1993). Further, our preliminary dose-titration study has suggested that a combination of 1.7 g/day of GLA and 0.8 g/day of SDA obtained from a mixture of borage and echium seed oils can significantly reduce the generation of leukotrienes from AA without impacting circulating AA levels (Arm et al. 2013). However, the physiologic effects of botanical seed oils on improvement in airflow obstruction in asthma are not known. Hence, we conducted a randomized, double-blind, placebo-controlled, cross-over clinical trial in mild to moderate asthmatics comparing the change in forced expiratory volume in one second (FEV1) after 6 weeks of therapy with the botanical oil combination.
Figure 1.
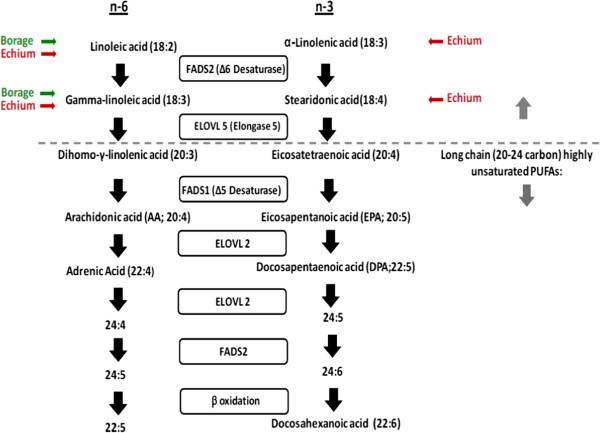
Pathways for metabolism of omega-6 (left) and omega-3 (right) fatty acids in humans. The synthesis of long chain PUFAs from the essential dietary medium chain PUFAs, α-linolenic acid (ω-3) and linoleic acid (ω-6). The fatty acids derived from borage oil (linoleic and gamma-linoleic acids, both ω-6) and echium (stearidonic, ω-3; linoleic and gamma-linoleic acids, both ω-6) would be expected to enter the pathways as indicated.
The ability of inflammatory cells to generate leukotrienes is influenced by polymorphisms in genes involved in leukotriene synthesis (Sampson et al. 2000). Of particular interest is a common A to C single nucleotide polymorphism located 444 base pairs upstream of the gene encoding leukotriene C4 synthase (LTC4S A-444C, rs730012). The dominant variant C allele of the gene for LTC4 synthase has been associated with an increase in cysteinyl LT production, reduced lung function and increased effectiveness of LT receptor antagonists against bronchoconstriction in asthma (Sampson et al. 2000; Silverman et al. 1998; Tantisira and Drazen 2009). To our knowledge, no studies have examined whether the LTC4S variant allele has an impact on the efficacy of PUFA supplementation on physiologic outcomes associated with asthma. Hence we hypothesized that dietary supplementation with the botanical seed oil combination would improve airflow obstruction in asthmatics when compared to placebo, preferentially in those who carried the variant C allele at the LTC4S A-444C locus.
Results
We enrolled 43 participants with mild to moderate asthma and randomized 39, of which 28 completed the study (Figure 2). Nine participants were found to carry at least one copy of the variant C allele in the LTC4S promoter. There were no significant differences in baseline clinical characteristics between the two genotype groups (Table 1).
Figure 2.
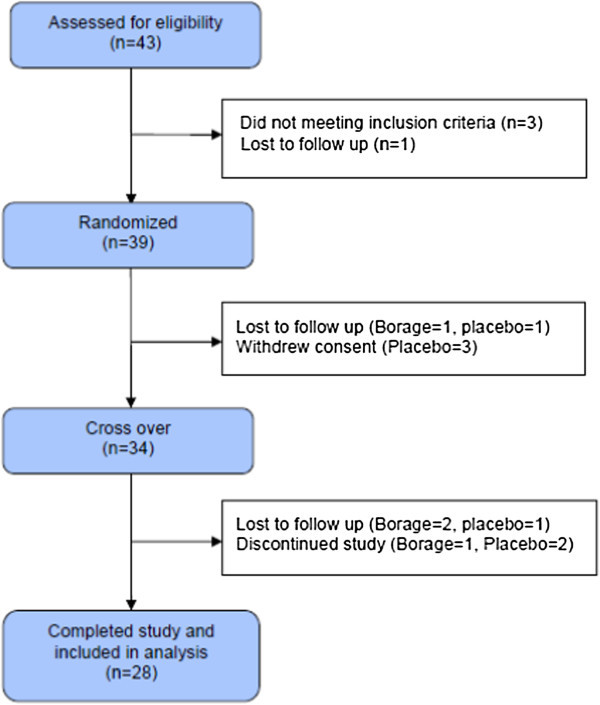
Study enrollment and randomization flow sheet.
Table 1.
Participant’s characteristics and study results
| Characteristics and results | A homozygotes (AA) | C allele carriers (AC/CC) |
|---|---|---|
| N | 19 | 9 (8 AC, 1 CC) |
| Age (years)a | 37 ± 14 (20 – 60) | 29 ± 8 (18 – 39) |
| Sex (female/male) | 14/5 | 8/1 |
| Race (Caucasian/other) | 15/4 | 7/2 |
| Hispanic (yes/no) | 1/18 | 3/6 |
| ICS dose (μg fluticasone)a | 117 ± 250 (0 – 1000) | 85 ± 128 (0 – 267) |
| Baseline rescue inhaler use (puffs/week)a | 5 ± 6 (0 – 18) | 7 ± 7 (0 – 20) |
| Baseline FEV1 (L)a | 2.24 ± 0.51 (1.35 – 3.01) | 2.31 ± 0.47 (1.75 – 3.26) |
| Baseline FEV1 (%)a | 69 ± 12 (51 – 89) | 69 ± 9 (55 – 84) |
| Change in FEV1 after placebo (%) | 1 ± 9 (-21 – 18) | −4 ± 6 (-15 – 3) |
| Change in FEV1 after drug (%)a | 1 ± 5 (-11 – 9) | 3 ± 5 (-3 – 12) |
| Change in FEV1 after drug compared to placebo (%)a* | −1 ± 9 (-13 – 24) | 7 ± 5 (1 – 17) |
*p value for difference between group means =0.02.
aMean ± standard deviation (minimum – maximum).
There was no difference in the change in FEV1 between the botanical oil and placebo arms after 6 weeks of treatment in the study cohort as a whole, and in the group of A homozygotes. In contrast, in C allele carriers (n = 9), the FEV1 improved 3% after treatment with combination botanical oils and declined 4% after placebo corn oil (difference of 7% between drug and placebo, p = 0.02, Figure 3). More importantly, all 9 individuals with the C allele, but only 7 of 19 individuals homozygous for the A allele, showed an improvement in their FEV1 when receiving the botanical oil combination compared to placebo (p = 0.007 for the percentage of responders, Figure 4).
Figure 3.
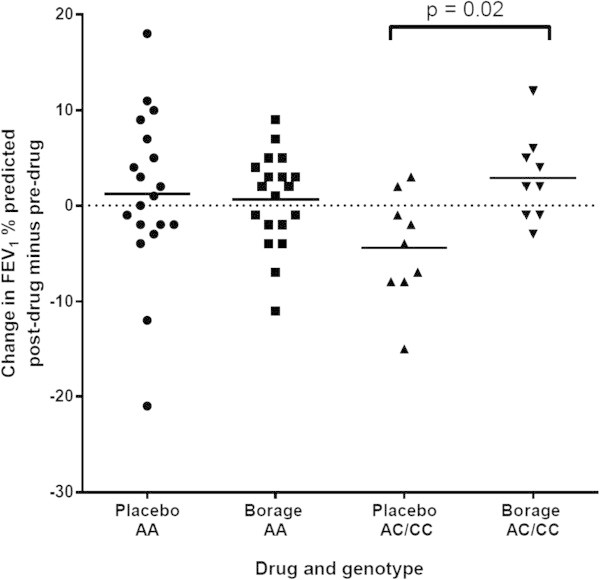
Change in FEV 1 % predicted in participants from both genotype groups after 6 weeks of therapy with drug and placebo. Horizontal bars represent group means.
Figure 4.
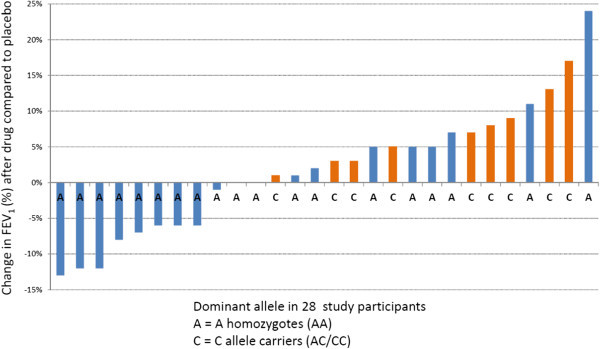
Effect of 6 weeks of dietary supplementation with borage and echium seed oils on FEV 1 compared to corn oil placebo in 28 mild to moderate asthmatics. The letters on the X axis demonstrate their leukotriene C4 synthase promoter polymorphism status (A-444C, rs730012).
Baseline urinary LTE4 levels did not differ between the genotypes and showed no changes in either group on either arm of the study (data not shown). The two genotype groups showed similar levels of LTs generated by basophils (with FcϵRI cross-linkage) and granulocytes (with ionophore stimulation) before randomization. However, generation of ionophore-induced 5-lipoxygenase pathway products was markedly reduced during treatment with the botanical oil combination in the C allele carriers, but not in the A allele homozygotes. This difference was substantial at the first measurement after 3 weeks of therapy (Figure 5a), but lost significance after six weeks. The majority of the products detected were LTB4 and proximal 5-LO pathway products (5-HETE and all-trans LTB4 (Figure 5b). CysLT production as a fraction of the total did not vary by genotype, and trend of CysLT generation tended to follow the overall trend for 5-LO production, but the difference between the treatment arms in the C allele carriers after 3 weeks of treatment did not reach statistical significance (p = 0.06, Figure 5c).
Figure 5.
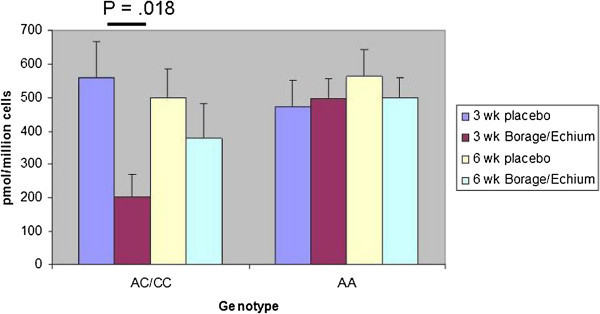
Effects of dietary supplementation with borage and echium seed oils on 5-LO pathway product formation by ionophore-stimulated peripheral blood granulocyte fractions from stable asthmatic subjects with AA and AC/CC LTC4S genotypes. a. Sum of total 5-LO pathway products (5-HETE, all-trans-LTB4, LTB4, and cys-LTs) generated per 1 × 106 ionophore-stimulated granulocytes in each indicated genotype after 3 and 6 weeks of treatment on each arm. b. Non-cys LTs (5-HETE, all-trans-LTB4, LTB4) c. cys-LTs (LTC4, LTD4, and LTE4) were measured for the same samples.
Overall, the treatment was well tolerated. We did not observe liver dysfunction or a significant drop in hemoglobin concentrations, which have been reported with botanical seed oil consumption.
Discussion
This study demonstrates that a combination of omega-3 and omega-6 dietary medium chain PUFAs (containing SDA and GLA, respectively), provided over six weeks in a fixed ratio in the form of borage and echium seed oils, results in a significant difference in FEV1 compared with corn oil placebo in subjects bearing at least one copy of a polymorphic LTC4S allele in a small proof-of-concept crossover study. This is the first study to suggest that botanical oil supplementation can modify lung function and thus have therapeutic potential in asthma if used in a personalized manner.
Dietary supplementation with omega-3 PUFAs from fish oil significantly reduces exercise-induced bronchoconstriction as well as concentrations of CysLTs and prostaglandins in induced sputum in elite athletes (Mickleborough et al. 2006; Tecklenburg-Lund et al. 2010). Omega-3 PUFAS were equivalent to the effects of the CysLT1 receptor antagonist montelukast on blocking bronchoconstriction caused by eucapnic hyperpnea in the latter study (Tecklenburg-Lund et al. 2010). Nagakura et al. (2000) found that dietary supplementation with fish oil rich in omega-3 PUFAs EPA and DHA improves symptoms and airway hyper-responsiveness in children with asthma living in a health care facility with strictly controlled environment in terms of inhalant allergens and diet. In a random sample of adults in The Netherlands studied between 1994 and 1997, McKeever et al. (2008) found that a high intake of omega-3 fatty acids does not protect against asthma, but a high intake of several omega-6 fatty acids is associated with a significant reduction in FEV1. They proposed that the high dietary intake of omega-6 fatty acids, rather than reduced omega-3 intake, may have an adverse effect on lung health. There is a possible detrimental effect of oil and fat consumption in asthma. Wood et al. (2011) examined the effect of a single high-fat meal versus low-fat meal on the bronchodilator response to albuterol in asthmatics. The high fat meal contained 48 g (49% of total energy) total fat, including 20.5 g (21% of total energy) saturated fat. In our study, we did not control the diets our subjects consumed while in the study; hence it is likely they were all are eating a typical modern Western diet which contains about 125 g of fat/day. Our protocol provided 9 g of fat given throughout the day and only a small proportion (~2.5 g) of that were the proposed bio-actives (GLA and SDA). Consequently it is unlikely that providing this small amount of fat on top of such a background diet would have had any impact on the clinical and other parameters examined, based on the mass of the fat alone.
5-lipoxygenase (5-LO)-dependent oxidative metabolism of arachidonic acid (AA) leads to generation of pro-inflammatory leukotrienes (LTs). However, dihomo-gamma-linoleic acid (DGLA), a precursor of AA, suppresses LT generation by competing with AA for substrate utilization by 5-LO. Borage (Borago officinalis) seed oil is a rich source of gamma linolenic acid (GLA), which is the precursor of DGLA (Figure 1). Dietary supplementation with Borage seed oil provides effective inhibition of leukotriene generation as demonstrated by significant attenuation of LT biosynthesis from circulating granulocytes in vitro in cell culture and ex vivo (Henz et al. 1999; Johnson et al. 1997; Ziboh and Fletcher 1992). However, hepatic metabolism of GLA to AA by ∆-5 desaturase increases circulating free AA levels, which has the potential of neutralizing DGLA’s potential as a leukotriene synthesis modifier (Ferretti et al. 1997; Johnson et al. 1997; Kelley et al. 1997; Seyberth et al. 1975). Supplementation of diet with long chain omega-3 fatty acids such as eicosapentaenoic acid (EPA) found in fish oils have been shown to compete with DGLA at the ∆-5 desaturase step preventing elevations in AA as a result of providing GLA in borage oil (Barham et al. 2000; Surette et al. 2008). In the current study, the botanical omega-3 PUFA, stearidonic acid (SDA) from the seed oil of echium (Echium plantagineum) was utilized because it bypasses the rate-limiting ∆-6 desaturase in long chain, omega-3 PUFA biosynthesis and thus is efficiently converted to EPA.
Because the effects of botanical omega-3 and omega-6 PUFAs on asthma had not previously been studied, we sought to determine whether borage and echium oils provided in the ratio defined in our preliminary dose-titration study could impact FEV1 when compared with a placebo in a small, crossover study of stable asthmatics (Arm et al. 2013). In our study, none of the secondary outcomes, including asthma symptoms, rescue inhaler use and peak expiratory flow, improved either in the cohort as a whole or in either genotype group. This is likely because the majority of participants enrolled in the study were relatively well-controlled and the study was likely underpowered to identify these differences.
It is well known that FEV1 measurements show substantial heterogeneity in response to treatments, including to 5-LO inhibitors (Drazen et al. 1999), CysLT1 receptor antagonists (Tantisira and Drazen 2009), and even the gold standard therapy of inhaled glucocorticoids (Tantisira et al. 2011). Since we anticipated that any therapeutic benefit of the botanical omega-3 and omega-6 lipids would reflect a modulatory effect on LT generation, we performed a pre-specified analysis of genotyping at the LTC4S locus for the common A to C variant that has been associated with numerous outcomes reflective of altered CysLT generation (Asano et al. 2002; Acevedo et al. 2007; Sayers et al. 2003). We observed two distinct effects of the variant C allele on the response to the botanical oil supplementation. First, the C allele carriers showed a net difference of 7% in FEV1 between the placebo corn oil and botanical oil arms (Figure 3), and all 9 of them demonstrated their highest FEV1 measurements while on the study botanical combination (Figure 4). In contrast, the A allele homozygotes showed no change in FEV1 at any time during the course of the study. The 7% difference in FEV1 is comparable to the efficacy of montelukast seen in patients with mild persistent asthma with near-normal lung function (Barnes et al. 2001).
Second, the granulocytes from the C allele carriers, but not those from the A allele homozygotes, showed a transient but significant, reduction in overall 5-LO product formation in response to ionophore (Figure 5). In contrast to our preliminary dose-titration study, in which botanical oil effects on LT formation by granulocytes and basophils were compared to pre-treatment levels, the second study involved a corn oil placebo. Thus, we cannot exclude the possibility that the corn oil (containing relatively high levels of the omega-6 PUFA, linoleic acid) could have altered LT generation so as to modify the evident effect of the botanicals. Indeed, this seemed to be the case for FEV1, in which the difference was due to both an increase in FEV1 with the botanical oils and a decrease in FEV1 with the corn oil – an observation that was restricted to the C allele carriers (Figure 5a). Since the effect of the botanicals was significant for total 5-LO pathway products and for non-CysLTs (Figure 5), we suspect that the variant LTC4S allele may alter the response of the 5-LO pathway to dietary omega-3 and omega-6 PUFAs in a manner independent of its primary function of conjugating LTA4 to glutathione, perhaps from an epistatic effect.
In summary, we conclude that as compared to corn oil, a combination of borage and echium seed oils containing 1.7 g/day of γ-linolenic acid and 0.8 g/day of stearidonic acid improves airflow obstruction in mild to moderate asthmatics who carry the variant allele in the LTC4 synthase gene promoter (A-444C). Our findings suggest that botanical oil supplementation can have therapeutic potential in asthma if used in a personalized manner. Further studies examining the effects of botanical oil combinations in asthma are warranted.
Methods
We designed a randomized, double-blind, placebo-controlled, cross-over clinical trial (Figure 6). The protocol was approved by the Partners Human Subjects Research Committee. An investigator-initiated IND was obtained from the US Food and Drug Administration (IND number 74,110). Mild to moderate asthmatics with a physician diagnosed history of asthma and the presence of variable airflow obstruction were recruited. Patients on leukotriene modifiers, oral or high dose inhaled steroids, theophylline or omalizumab (a monoclonal antibody against Immunoglobulin E) were excluded. Medical and respiratory history, a brief physical examination and routine laboratory tests were conducted at the screening visit to exclude the presence of significant co-morbid diseases.
Figure 6.
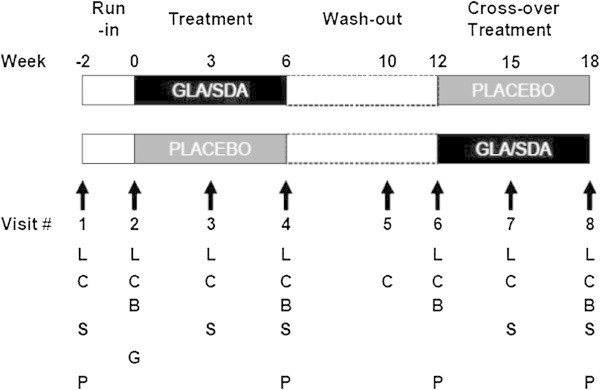
Study design. Illustrates study visits and periods of run-in, treatment, and wash-out. GLA = gamma-linolenic acid; SDA = stearidonic acid; Placebo = corn oil; B = blood draw for measurement of biochemical outcomes; C = provision and review of diary cards; G = blood draw for genomic analysis; L = measurement of lung function by spirometry; P = pregnancy test; S = safety monitoring.
The study design is depicted in Figure 6. The primary outcome was pre-specified to be the difference in the change in forced expiratory volume in one second (FEV1) after three weeks of therapy between combination botanical oil and placebo therapy. Spirometry was performed with the KoKo Spirometry software. Pre-specified secondary analyses included the examination of the effect of A-444C polymorphism of the LTC4S gene on FEV1, rescue inhaler use, daily symptoms documented in diary cards, daily lung function assessed with Jaeger electronic peak flow meters, leukotriene generation from peripheral white blood cells in response to a range of stimulus doses and urine LTE4 measurements. Details of assays used for measurements are provided in the online resource accompanying the manuscript. Blood from participants was collected weekly for assessment of hematological indices, liver and renal function. Medication compliance was monitored by medication diaries, counts of returned capsules, and plasma fatty acids measurements.
Capsules containing a combination of borage and echium seed oils were obtained from Croda Europe Ltd (Leek, Staffordshire, UK). Subjects randomized to receive drug were given nine 1 g capsules (7 with echium and 2 with borage oil) each day, for a total of 1.57 g and 0.87 g gamma-linolenic acid (GLA) and stearidonic acid (SDA), respectively, per day. Subjects randomized to placebo received 9 capsules of corn oil each day. Each 1 gram capsule of corn oil contained no GLA or SDA and 560 mg of linoleic acid (the major fatty acid in corn oil) for a total dosage of 5 g of linoleic acid. The typical Western diet contains very high levels of linoleic acid (6-8% of daily energy) so this supplementation protocol would add a very small percentage of the linoleic acid found in the typical modern Western diet.
We used Student’s t-tests to compare normally distributed data, Wilcoxon rank sum tests for unpaired non-parametric data and Wilcoxon signed rank tests for paired non-parametric data. Categorical data were analyzed using two-sided Fisher’s exact tests. We employed repeated measures mixed model analysis in which repeated measures were taken on each subject at each visit (time) and the group and time interactions were modeled with the main effects. A compound symmetry correlation structure was used since the correlation between pairs of times would be similar and not expected to change across time. Data were analyzed using SAS 9.1 (SAS Institute Inc., Cary, NC, USA) where the significance level is set at 95%. All subjects were genotyped for their LTC4S A-444C polymorphism status at the Harvard Partners Center for Genetics and Genomics (HPCGG) by using either Sequenom MassARRAY system (Sequenom, San Diego, CA) or Taqman analysis on the Applied Biosystems 7900HT system (Applied Biosystems, Forster City, California, USA) based on assay availability in the laboratory. Quality control was assured by running internal and external controls on all genotyping plates.
Funding/Support
Encapsulated seed oils were a generous gift from Croda Europe Ltd (Leek, Staffordshire, UK). The oils were authenticated by the WFUHS Center for Botanical Lipids and Inflammatory Disease Prevention. Representative certificates of analysis are on file. This work was funded by P50 AT002782, U19 AI078908, UL1 RR025758 and KL2 RR025757 from the National Institutes of Health, a bridge grant from the American Academy of Allergy Asthma and Immunology and the Fund to Sustain Research Excellence, Brigham and Women’s Hospital Biomedical Research Institute.
Abbreviations
- 5-HETE
5 hydroxyeicosatetraenoic acid
- 5-LO
5 lipoxygenase
- AA
Arachidonic acid
- CysLT
Cysteinyl leukotriene
- DGLA
Dihomo γ-linolenic acid
- DHA
Docosahexanoic acid
- DPA
Docosapentaenoic acid
- EPA
Eicosapentoenoic acid
- FcϵR1
Fc receptor for IgE
- FEV1
Forced expiratory volume in one second
- FADS
Fatty acid desaturase
- GLA
Gamma-linolenic acid
- LA
Linoleic acid
- LT
Leukotriene
- LTC4S
Leukotriene C4 synthase
- LTE4
Leukotriene E4
- PUFA
Polyunsaturated fatty acid
- SDA
Stearidonic acid.
Footnotes
Competing interests
Shamsah Kazani and Jonathan Arm are currently employees of the Novartis Institutes of Biomendical Research, Inc.. They do not have any conflicts relevant to this study.
Hannah C. Ainsworth, Joshua Boyce, Heng Chhay, Floyd H Chilton, Stefanie Dutile, Usha Govindarajulu, Elliot Israel, Priscilla Ivester, Susan Sergeant and Michael E. Wechsler declare no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Authors’ contributions
JA, JB, PI, HA, SS, FC and EI conceived of the study, participated in its design, execution and coordination and helped to draft the manuscript. HC conducted the ex vivo assays. SK and UG performed the statistical analysis. SD conducted the subject’s clinical visits. SK and MW drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Shamsah Kazani, Email: shamsah.kazani@novartis.com.
Jonathan P Arm, Email: jonathan.arm@novartis.com.
Joshua Boyce, Email: jboyce@rics.bwh.harvard.edu.
Heng Chhay, Email: hchhay@partners.org.
Stefanie Dutile, Email: sdutile@partners.org.
Michael E Wechsler, Email: wechslerm@njhealth.org.
Usha Govindarajulu, Email: ugovindarajulu@partners.org.
Priscilla Ivester, Email: ivester@wakehealth.edu.
Hannah C Ainsworth, Email: hainswor@wakehealth.edu.
Susan Sergeant, Email: ssergean@wakehealth.edu.
Floyd H Chilton, Email: schilton@wakehealth.edu.
Elliot Israel, Email: eisrael@partners.org.
References
- Acevedo N, Vergara C, Mercado D, Jiménez S, Caraballo L. The A-444C polymorphism of leukotriene C4 synthase gene is associated with IgE antibodies to Dermatophagoides pteronyssinus in a Colombian population. J Allergy Clin Immunol. 2007;119:505–507. doi: 10.1016/j.jaci.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Arm JP, Boyce JA, Wang L, Chhay H, Zahid M, Patil V, Govindarajulu U, Ivester P, Weaver KL, Sergeant S, Israel E, Chilton FH. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013;12:141. doi: 10.1186/1476-511X-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K, Shiomi T, Hasegawa N, Nakamura H, Kudo H, Matsuzaki T, Hakuno H, Fukunaga K, Suzuki Y, Kanazawa M, Yamaguchi K. Leukotriene C4 synthase gene A(-444)C polymorphism and clinical response to a CYS-LT1 antagonist, pranlukast, in Japanese patients with moderate asthma. Pharmacogenet Genomics. 2002;12:565–570. doi: 10.1097/00008571-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000;130:1925–1931. doi: 10.1093/jn/130.8.1925. [DOI] [PubMed] [Google Scholar]
- Barnes N, Wei LX, Reiss TF, Leff JA, Shingo S, Yu C, Edelman JM. Analysis of montelukast in mild persistent asthmatic patients with near-normal lung function. Respir Med. 2001;95:379–386. doi: 10.1053/rmed.2001.1052. [DOI] [PubMed] [Google Scholar]
- Barros R, Moreira A, Fonseca J, Delgado L, Castel-Branco MG, Haahtela T, Lopes C, Moreira P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br J Nutr. 2011;106:441–450. doi: 10.1017/S0007114511000328. [DOI] [PubMed] [Google Scholar]
- Chilton-Lopez SME, Swan DD, Fonteh AN, Johnson MM, Chilton FH. Metabolism of gammalinolenic acid in human neutrophils. J Immunol. 1996;156:2941–2947. [PubMed] [Google Scholar]
- Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, Drajesk J. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999;22:168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Nelson GJ, Schmidt PC, Kelley DS, Bartolini G, Flanagan VP. Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids. 1997;34:435–439. doi: 10.1007/s11745-997-0057-5. [DOI] [PubMed] [Google Scholar]
- Guichardant M, Traitler H, Spielmann D, Sprecher H, Finot PA. Stearidonic acid, an inhibitor of the 5-lipoxygenase pathway. A comparison with timnodonic and dihomogammalinolenic acid. Lipids. 1993;28:321–324. doi: 10.1007/BF02536317. [DOI] [PubMed] [Google Scholar]
- Henz BM, Jabolonska S, van de Kerhof PC, Stingl G, Blazczyk M, Vandervalk PG, Veenhuizen R, Muggli R, Raederstorff D. Double-blind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br J Dermatol. 1999;140:685–688. doi: 10.1046/j.1365-2133.1999.02771.x. [DOI] [PubMed] [Google Scholar]
- James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr. 2003;77:1140–1145. doi: 10.1093/ajcn/77.5.1140. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton FH. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–1444. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Makcy BE, Kyle D. Effects of dietary arachidonic acid on human immune response. Lipids. 1997;32:449–456. doi: 10.1007/s11745-997-0059-3. [DOI] [PubMed] [Google Scholar]
- Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, Spur BW, Robinson DR, Corey EJ, Lewis RA, Austen KF. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Cassano PA, Ocké M, Burney P, Britton J, Smit HA. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63:208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR. Leukotrienes. New Eng J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Sampson AP, Siddiqui S, Buchanan D, Howarth PH, Holgate ST, Holloway JW, Sayers I. Variant LTC(4) synthase allele modifies cysteinyl leukotriene synthesis in eosinophils and predicts clinical response to zafirlukast. Thorax. 2000;55(Suppl 2):S28–S31. doi: 10.1136/thorax.55.suppl_2.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers I, Barton S, Rorke S, Beghé B, Hayward B, Van Eerdewegh P, Keith T, Clough JB, Ye S, Holloway JW, Sampson AP, Holgate ST. Allelic association and functional studies of promoter polymorphism in the leukotriene C4 synthase gene (LTC4S) in asthma. Thorax. 2003;58:417–424. doi: 10.1136/thorax.58.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyberth HW, Oelz O, Kennedy T, Sweetman BJ, Danon A, Frölich JC, Heimberg M, Oates JA. Increased arachidonate in lipids after administration to man: effects on prostaglandin biosynthesis. Clin Pharmacol Ther. 1975;18:521–529. doi: 10.1002/cpt1975185part1521. [DOI] [PubMed] [Google Scholar]
- Silverman E, In KH, Yandava C, Drazen JM. Pharmacogenetics of the 5-lipoxygenase pathway in asthma. Clin Exp Allergy. 1998;Suppl 5:164–170. doi: 10.1046/j.1365-2222.1998.028s5164.x. [DOI] [PubMed] [Google Scholar]
- Surette ME, Stull D, Lindemann J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr Med Res Opin. 2008;24:559–567. doi: 10.1185/030079908X273011. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Drazen JM. Genetics and pharmacogenetics of the leukotriene pathway. J Allergy Clin Immunol. 2009;124:422–427. doi: 10.1016/j.jaci.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, Lange C, Lazarus R, Sylvia J, Klanderman B, Duan QL, Qiu W, Hirota T, Martinez FD, Mauger D, Sorkness C, Szefler S, Lazarus SC, Lemanske RF, Peters SP, Lima JJ, Nakamura Y, Tamari M, Weiss ST. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. New Eng J Med. 2011;365:1173–1183. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecklenburg-Lund S, Mickleborough TD, Turner LA, Fly AD, Stager JM, Montgomery GS. Randomized controlled trial of fish oil and montelukast and their combination on airway inflammation and hyperpnea-induced bronchoconstriction. PLoS ONE. 2010;18:e13487. doi: 10.1371/journal.pone.0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, Fletcher MP. Dose-response effects of dietary gamma-linolenic acid-enriched oils on human polymorphonuclear-neutrophil biosynthesis of leukotriene B4. Am J Clin Nutr. 1992;55:39–45. doi: 10.1093/ajcn/55.1.39. [DOI] [PubMed] [Google Scholar]


