Abstract
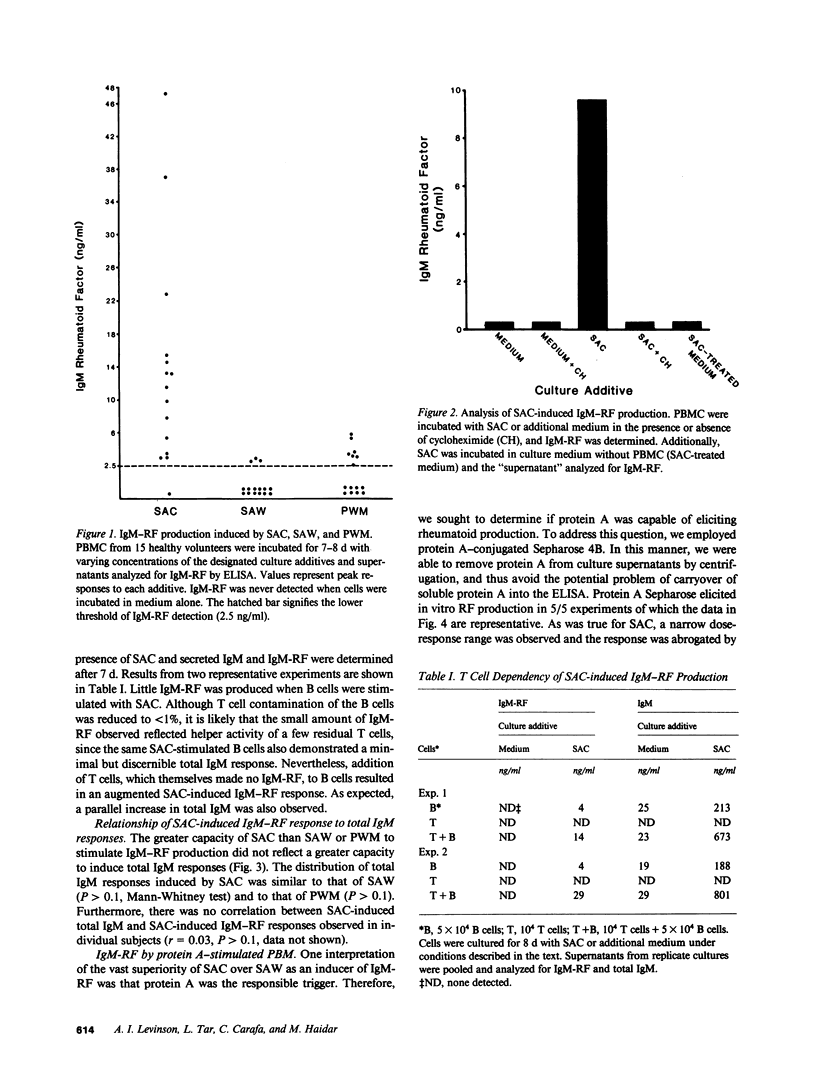
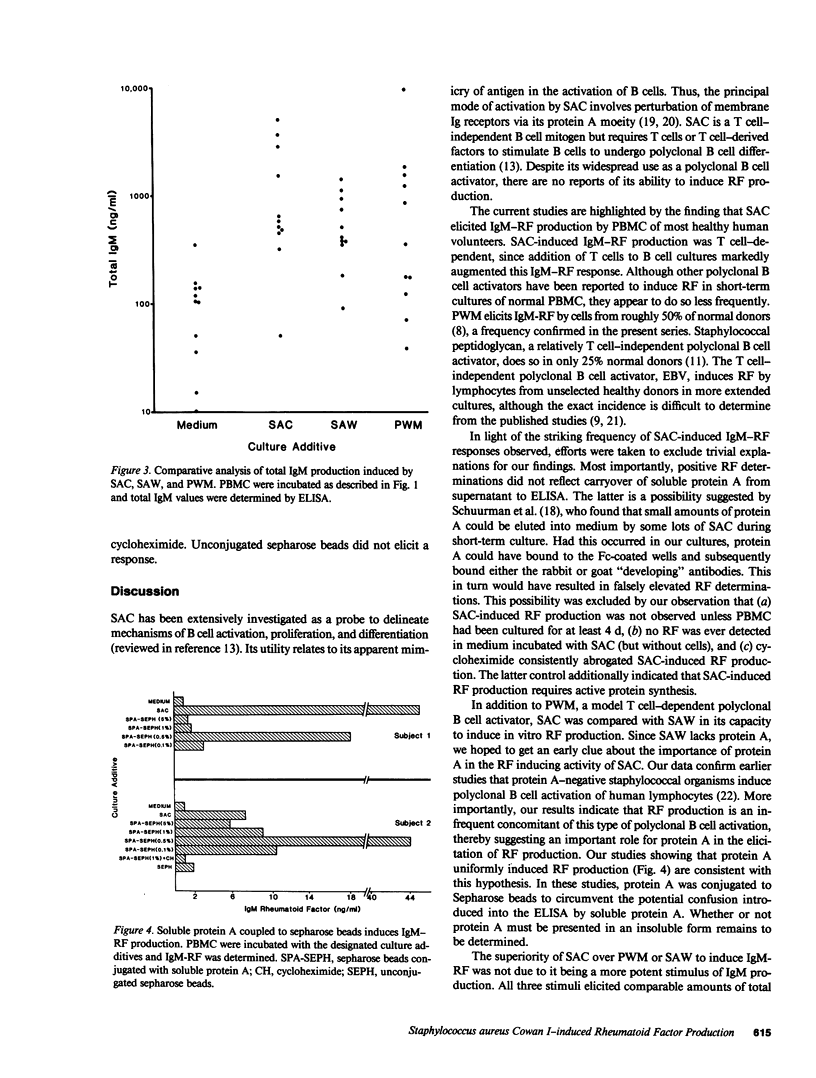
These studies demonstrate that Staphylococcus aureus Cowan I (SAC), a protein A-positive Staphylococcal strain, is a potent and consistent inducer of IgM rheumatoid factor production by normal human peripheral blood mononuclear cells. The frequency and magnitude of this response greatly exceeded that of parallel cultures stimulated with pokeweed mitogen or the protein A-negative S. aureus Wood strain, although all three agents induced a similar amount of total IgM. Cell fractionation studies indicated that SAC-induced IgM rheumatoid factor is T cell-dependent. The striking ability of SAC to induce IgM rheumatoid factor may relate to its protein A content, since cultures stimulated with protein A-coupled sepharose beads also consistently produced this autoantibody. Thus SAC is a new probe of in vitro IgM rheumatoid factor production and its use has provided further evidence that most healthy individuals harbor precursors of IgM rheumatoid factor secreting cells. Unlike other polyclonal activators, SAC is unique in its capacity to bind immunoglobulin, a property that may account for its prominent anti-IgG inducing capacity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dziarski R. Anti-immunoglobulin autoantibodies are not preferentially induced in polyclonal activation of human and mouse lymphocytes, and more anti-DNA and anti-erythrocyte autoantibodies are induced in polyclonal activation of mouse than human lymphocytes. J Immunol. 1984 Nov;133(5):2537–2544. [PubMed] [Google Scholar]
- Egeland T., Munthe E. The role of the laboratory in rheumatology. Rheumatoid factors. Clin Rheum Dis. 1983 Apr;9(1):135–160. [PubMed] [Google Scholar]
- Fong S., Tsoukas C. D., Frincke L. A., Lawrance S. K., Holbrook T. L., Vaughan J. H., Carson D. A. Age-associated changes in Epstein-Barr virus-induced human lymphocyte autoantibody responses. J Immunol. 1981 Mar;126(3):910–914. [PubMed] [Google Scholar]
- Fong S., Vaughan J. H., Carson D. A. Two different rheumatoid factor-producing cell populations distinguished by the mouse erythrocyte receptor and responsiveness to polyclonal B cell activators. J Immunol. 1983 Jan;130(1):162–164. [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Goldstein M., Hoxie J., Zembryki D., Matthews D., Levinson A. I. Phenotypic and functional analysis of B cell lines from patients with multiple myeloma. Blood. 1985 Aug;66(2):444–446. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Heterogeneity of B cells: direct evidence of selective triggering of distinct subpopulations by polyclonal activators. Scand J Immunol. 1976;5(1-2):55–69. doi: 10.1111/j.1365-3083.1976.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Heimer R., Wolfe L. D., Abruzzo J. L. IgM and IgG anti-F(ab')2 antibodies in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1298–1306. doi: 10.1002/art.1780251105. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Johnson P. M., Faulk W. P. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol. 1976 Nov;6(3):414–430. doi: 10.1016/0090-1229(76)90094-5. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E. In vitro synthesis of IgM rheumatoid factor by lymphocytes from healthy adults. J Immunol. 1980 Aug;125(2):934–939. [PubMed] [Google Scholar]
- Kronvall G., Frommel D. Definition of staphylococcal protein A reactivity for human immunoglobulin G fragments. Immunochemistry. 1970 Jan;7(1):124–127. doi: 10.1016/0019-2791(70)90036-4. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Lind I., Mansa B. Production of anti-IgGG antibodies by means of Ig adsorbed to Staphylococcus aureus cowan type 1. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):829–834. doi: 10.1111/j.1699-0463.1974.tb02380.x. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Butler J. L., Fauci A. S. Regulation of human B-cell activation, proliferation, and differentiation by soluble factors. J Clin Immunol. 1984 Sep;4(5):337–347. doi: 10.1007/BF00917136. [DOI] [PubMed] [Google Scholar]
- Nardella F. A., Teller D. C., Barber C. V., Mannik M. IgG rheumatoid factors and staphylococcal protein A bind to a common molecular site on IgG. J Exp Med. 1985 Dec 1;162(6):1811–1824. doi: 10.1084/jem.162.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo I., Carafa C., Dziarski R., Levinson A. I. Analysis of in vitro polyclonal B cell differentiation responses to bacterial peptidoglycan and pokeweed mitogen in rheumatoid arthritis. Clin Exp Immunol. 1984 May;56(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- Pasquali J. L., Fong S., Tsoukas C. D., Slovin S. F., Vaughan J. H., Carson D. A. Different populations of rheumatoid factor idiotypes induced by two polyclonal B cell activators, pokeweed mitogen and Epstein--Barr virus. Clin Immunol Immunopathol. 1981 Nov;21(2):184–189. doi: 10.1016/0090-1229(81)90207-5. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., del Prete G., Maggi E., Biagiotti R., Almerigogna F., Ricci M. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J Immunol. 1982 Aug;129(2):596–602. [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto K., Cooper N. R., Johnson J. S., Vaughan J. H. Complement fixation by rheumatoid factor. J Clin Invest. 1975 Mar;55(3):437–445. doi: 10.1172/JCI107949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Agnello V., Kunkel H. G. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970 May;6(5):689–706. [PMC free article] [PubMed] [Google Scholar]



