Abstract
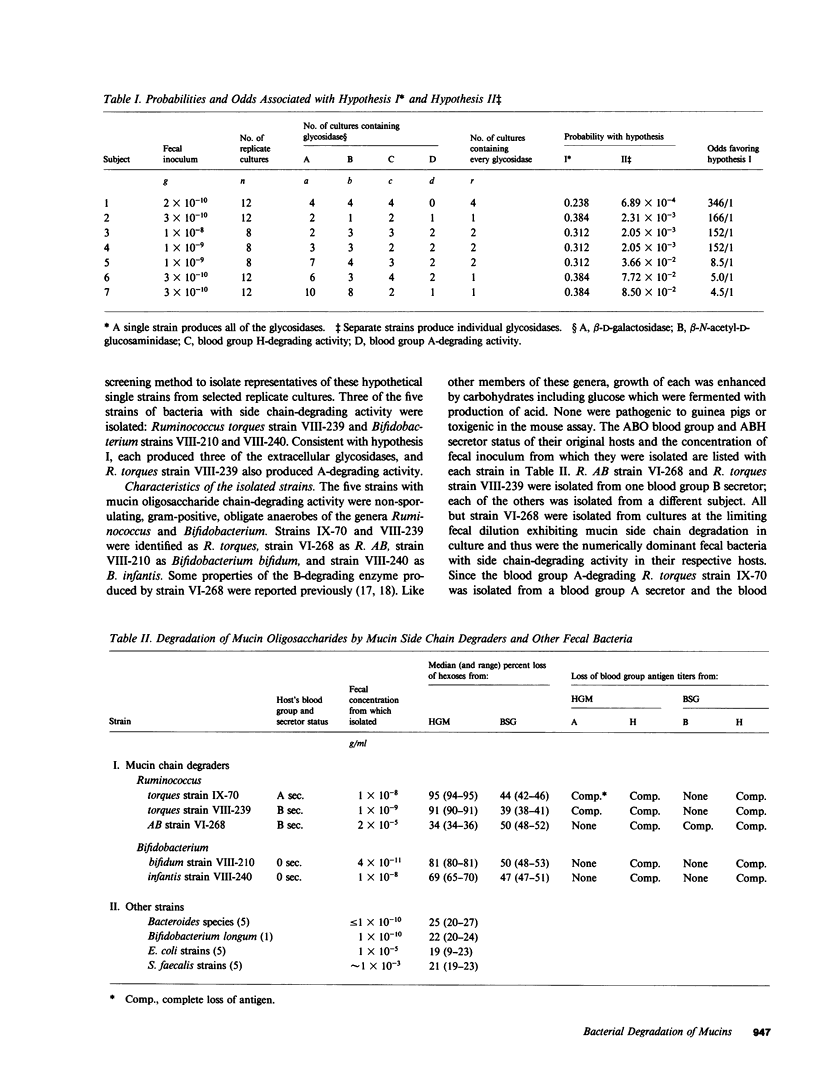
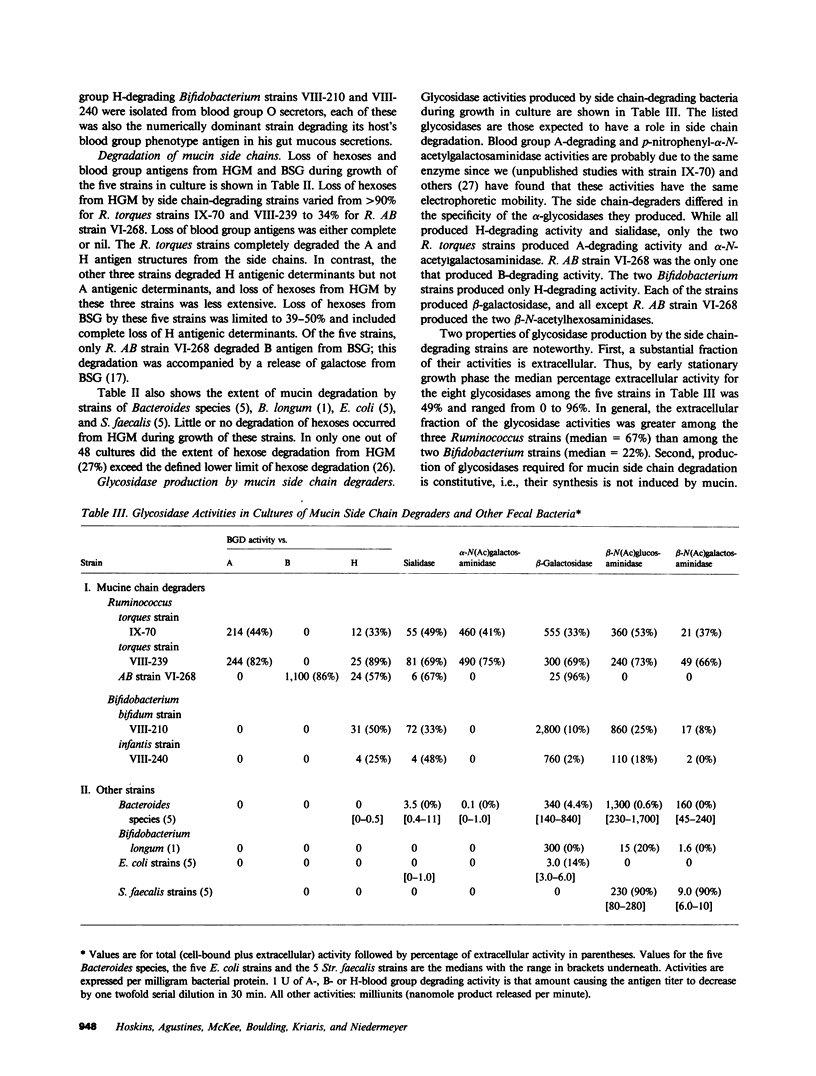
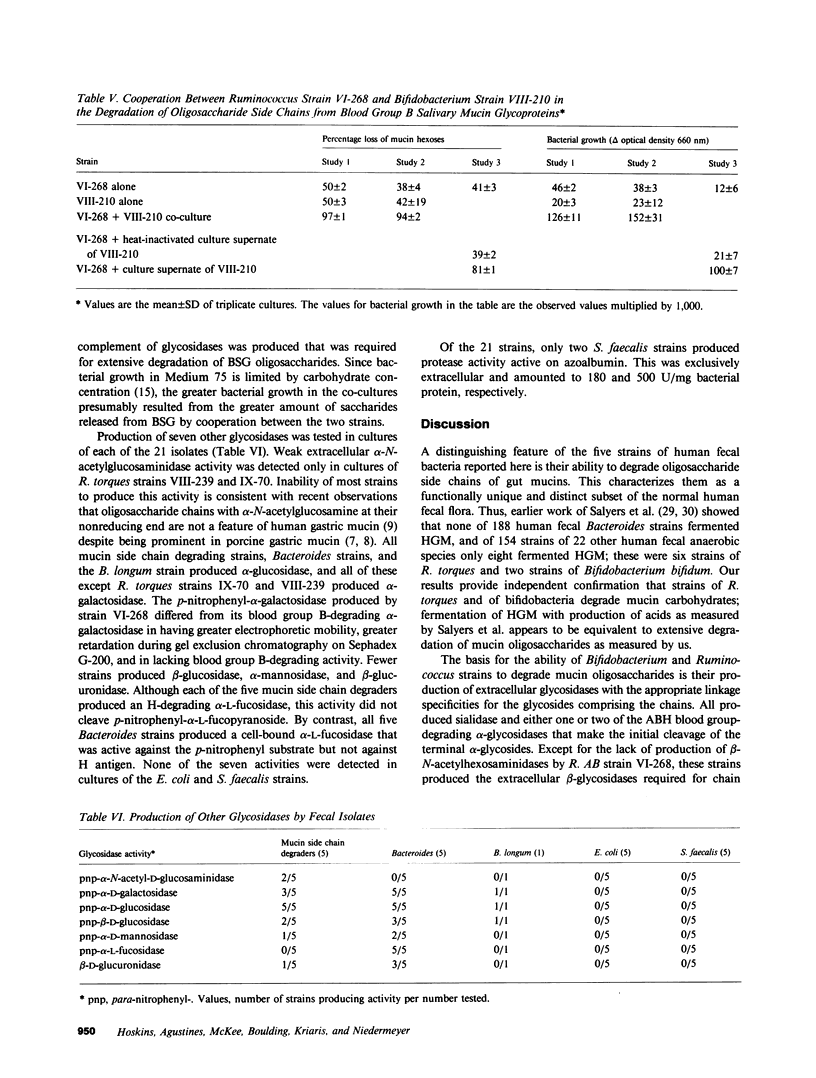
We previously reported that the oligosaccharide chains of hog gastric mucin were degraded by unidentified subpopulations numbering approximately 1% of normal human fecal bacteria. Here we report on the enzyme-producing properties of five strains of mucin oligosaccharide chain-degrading bacteria isolated from feces of four healthy subjects. Four were isolated from the greatest fecal dilutions yielding mucin side chain-degrading activity in culture, and thus were the numerically dominant side chain-degrading bacteria in their respective hosts. Three were Ruminococcus strains and two were Bifidobacterium strains. Two Ruminococcus torques strains, IX-70 and VIII-239, produced blood group A- and H-degrading alpha-glycosidase activities, sialidase, and the requisite beta-glycosidases; these strains released greater than 90% of the anthrone-reacting hexoses from hog gastric mucin during growth in culture. The Bifidobacterium strains lacked A-degrading activity but were otherwise similar; these released 60-80% of the anthrone-reacting hexoses but not the A antigenic structures from hog gastric mucin. Only Ruminococcus AB strain VI-268 produced blood group B-degrading alpha-galactosidase activity, but this strain lacked beta-N-acetylhexosaminidases to complete degradation of B antigenic chains. When this strain was co-cultured with a strain that produced beta-N-acetylhexosaminidases, release of hexoses from blood group B salivary glycoprotein increased from 50 to greater than 90%, and bacterial growth was enhanced. The glycosidases required for side chain degradation were produced by these strains in the absence of mucin substrate, and a substantial fraction of each activity in stationary phase cultures was extracellular. In contrast, none of 16 other fecal Bacteroides, Escherichia coli, Streptococcus faecalis, and Bifidobacterium strains produced ABH blood group-degrading enzymes; other glycosidases produced by these strains were predominantly cell bound except for extracellular beta-N-acetylhexosaminidases produced by the five S. faecalis strains. We conclude that certain Bifidobacterium and Ruminococcus strains are numerically dominant populations degrading mucin oligosaccharides in the human colon due to their constitutive production of the requisite extracellular glycosidases including blood group antigen-specific alpha-glycosidases. These properties characterize them as a functionally distinct subpopulation of normal human enteric microflora comprised of specialized subsets that produce blood group H antigen-degrading glycosidases alone or together with either blood group A- or B-degrading glycosidases.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Allen A., Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980 Mar;21(3):249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff D., Furukawa K. Enzymes that destroy blood group specificity. I. Purification and properties of alpha-L-fucosidase from Clostridium perfringens. J Biol Chem. 1970 Apr 10;245(7):1659–1669. [PubMed] [Google Scholar]
- Brown J. P., Dietrich P. S. Mutagenicity of plant flavonols in the Salmonella/mammalian microsome test: activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. Mutat Res. 1979 Mar;66(3):223–240. doi: 10.1016/0165-1218(79)90083-1. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell C. L., Hoskins L. C. Antigen degradation in human colon ecosystems. Host's ABO blood type influences enteric bacterial degradation of a cell surface antigen on Escherichia coli O86. Gastroenterology. 1977 Jul;73(1):37–41. [PubMed] [Google Scholar]
- Derevitskaya V. A., Arbatsky N. P., Kochetkov N. K. The structure of carbohydrate chains of blood-group substance. Isolation and elucidation of the structure of higher oligosaccharides from blood-group substance H. Eur J Biochem. 1978 May 16;86(2):423–437. doi: 10.1111/j.1432-1033.1978.tb12325.x. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GYORGY P., ROSE C. S., SPRINGER G. F. Enzymatic inactivation of bifidus factor and blood group substances. J Lab Clin Med. 1954 Apr;43(4):543–552. [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J Clin Invest. 1976 Jan;57(1):63–73. doi: 10.1172/JCI108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. II. A gene interaction in man that affects the fecal population density of certain enteric bacteria. J Clin Invest. 1976 Jan;57(1):74–82. doi: 10.1172/JCI108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C. Ecological studies of intestinal bacteria. Relation between the specificity of fecal ABO blood group antigen-degrading enzymes from enteric bacteria and the ABO blood group of the human host. J Clin Invest. 1969 Apr;48(4):664–673. doi: 10.1172/JCI106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- LINDSTEDT G., LINDSTEDT S., GUSTAFSSON B. E. MUCUS IN INTESTINAL CONTENTS OF GERMFREE RATS. J Exp Med. 1965 Feb 1;121:201–213. doi: 10.1084/jem.121.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy G. N., Aminoff D. Purification and properties of alpha-N-acetylgalactosaminidase from Clostridium perfringens. J Biol Chem. 1980 Dec 25;255(24):11737–11742. [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizont R. Degradation of intestinal glycoproteins by pathogenic Shigella flexneri. Infect Immun. 1982 May;36(2):615–620. doi: 10.1128/iai.36.2.615-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizont R. Glycoprotein degradation in the blind loop syndrome: identification of glycosidases in jejunal contents. J Clin Invest. 1981 Feb;67(2):336–344. doi: 10.1172/JCI110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Richmond M. H. Enzymic adaptation in bacteria: its biochemical and genetic basis. Essays Biochem. 1968;4:105–154. [PubMed] [Google Scholar]
- Roberton A. M., Stanley R. A. In vitro utilization of mucin by Bacteroides fragilis. Appl Environ Microbiol. 1982 Feb;43(2):325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg A., Meyer F. A. Structure and function of mucus. Adv Exp Med Biol. 1982;144:53–74. doi: 10.1007/978-1-4615-9254-9_6. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Zdebska E., Slomiany B. L. Structures of the neutral oligosaccharides isolated from A-active human gastric mucin. J Biol Chem. 1984 Dec 10;259(23):14743–14749. [PubMed] [Google Scholar]
- Slomiany B. L., Zdebska E., Slomiany A. Structural characterization of neutral oligosaccharides of human H+Leb+ gastric mucin. J Biol Chem. 1984 Mar 10;259(5):2863–2869. [PubMed] [Google Scholar]
- Tamura G., Gold C., Ferro-Luzzi A., Ames B. N. Fecalase: a model for activation of dietary glycosides to mutagens by intestinal flora. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4961–4965. doi: 10.1073/pnas.77.8.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Halbeek H., Dorland L., Vliegenthart J. F., Kochetkov N. K., Arbatsky N. P., Derevitskaya V. A. Characterization of the primary structure and the microheterogeneity of the carbohydrate chains of porcine blood-group H substance by 500-MHz 1H-NMR spectroscopy. Eur J Biochem. 1982 Sep;127(1):21–29. doi: 10.1111/j.1432-1033.1982.tb06832.x. [DOI] [PubMed] [Google Scholar]
- Variyam E. P., Hoskins L. C. Mucin degradation in human colon ecosystems. Degradation of hog gastric mucin by fecal extracts and fecal cultures. Gastroenterology. 1981 Oct;81(4):751–758. [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]



