Abstract
11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) and type 2 (11β-HSD2) are expressed in rat testis, where they regulate the local concentrations of glucocorticoids. Here, we investigated the expression and localization of 11β-HSD in rat testis during postnatal development, and the regulation of these genes by luteinizing hormone (LH) and androgens. mRNA and protein levels were analyzed by quantitative real-time-polymerase chain reaction and western blotting, respectively, in testes collected from rats at postnatal day (PND) 7, 14, 21, 35, and 90, and from rats treated with LH, 7α-methyl-19-nortestosterone (MENT) and testosterone at PND 21 and PND 90. Immunohistochemical staining was used to identify the localization of the 11β-HSD in rat testis at PND 7, 14, and 90. We found that 11β-HSD1 expression was restricted to the interstitial areas, and that its levels increased during rat testis development. In contrast, whereas 11β-HSD2 was expressed in both the interstitial areas and seminiferous tubules at PND 7, it was present only in the interstitial areas at PND 90, and its levels declined during testicular development. Moreover, 11β-HSD1 mRNA was induced by LH in both the PND 21 and 90 testes and by MENT at PND 21, whereas 11β-HSD2 mRNA was induced by testosterone and MENT in the PND 21 testis and by LH in the PND 90 testis. In conclusion, our study indicates that the 11β-HSD1 and 11β-HSD2 genes have distinct patterns of spatiotemporal expression and hormonal regulation during postnatal development of the rat testis.
Keywords: 11β-hydroxysteroid dehydrogenase type 1, 11β-hydroxysteroid dehydrogenase type 2, development, Leydig cell, testis
INTRODUCTION
The testis is a primary target for glucocorticoids (GCs), which are produced both during normal physiology and in response to stress. For example, it has been demonstrated that GC deficiency impairs Leydig cell steroidogenesis, leading to a delay in the maturation of the testis.1,2,3 Moreover, elevated GC levels, arising either in response to stress or as a result of exogenous dosing, have been reported to suppress testosterone production,4,5,6 induce apoptosis and degeneration of Leydig cells,7,8,9 and promote testicular maturation.10 Interestingly, the effects of GC on testis have been shown to differ during distinct stages of postnatal development, such that plasma testosterone is increased by immobilization stress in prepubertal rats, but is lowered in adult rats under the same conditions.11 The effects of GCs in the testis are known to be mediated, at least in part, by the glucocorticoid receptor, which has been shown to be expressed in the testis during postnatal development.12,13 GC levels in the testis are regulated in part by 11β-hydroxysteroid dehydrogenases (11β-HSDs), which catalyze the interconversion of active GCs and inert GC metabolites, namely, cortisol and cortisone in humans, and corticosterone and 11-dehydrocorticosterone in rodents. Two 11β-HSD isoforms have been identified in vivo: 11β-HSD type 1 (11β-HSD1) and type 2 (11β-HSD2). 11β-HSD1, an oxidoreductase that uses NADPH as a cofactor, is abundantly expressed in rat Leydig cells and we have previously shown that its capacity for oxidation increases during development of these cells.14,15 Testis expression of 11β-HSD2, an exclusive oxidase that uses NAD+ as cofactor,16 is a matter of some controversy, with some studies failing to detect it and others demonstrating its expression in Leydig and Sertoli cells.17,18,19 These discrepant observations have been attributed to differences in the species studied, the sensitivity of the detection methods, and the sampling time selected. Moreover, in our initial report of the expression of 11β-HSD2 in rat Leydig cells in 2005,20 we found that it was expressed at levels ~1000-fold lower than those of 11β-HSD1, which might explain why other studies failed to detect it in the testis.
Given that both 11β-HSD1 and 11β-HSD2 are expressed in rat testis, and coordinately modulate testis GC concentrations, studying the developmental expression patterns of these enzymes throughout the testis may shed light upon the differential effects of GCs during testis development. Accordingly, in the present study, we carried out a comprehensive investigation of the gene expression levels, localization and hormonal regulation of 11β-HSD1 and 11β-HSD2 in the testis of postnatal day (PND) 7 (infant), PND 14, PND 21 (prepubertal), PND 35 (pubertal), and PND 90 (adult) rats.
MATERIALS AND METHODS
Materials
The luteinizing hormone releasing hormone (LHRH) antagonist [Ac-D2Nal1, 4C1DPhe2, D3Pal3, Arg5, DGlu6 (anisole adduct), DAla10]-GnRH (NalGlu) was kindly provided by Dr. Jean Rivier (Salk Institute, San Diego, CA, USA). Ovine luteinizing hormone (LH) was generously supplied by the NIH (oLH-26 AFP-5551B, NIH, Bethesda, MD, USA). 7α-methyl-19-nortestosterone (MENT) was kindly provided by the Upjohn Company (Kalamazoo, MI, USA). Mannitol and testosterone were purchased from Sigma Chemical Co., (St. Louis, MO, USA). Testosterone and MENT were dissolved in cottonseed oil containing 5% ethanol.
Primary anti-11β-HSD1 (ab39364) and anti-β-actin (ab1801) antibodies were purchased from Abcam, Inc., (Cambridge, MA, USA). Primary anti-11β-HSD2 antibody (sc-20176) was from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). The secondary antibody (two-step IHC detection reagent, pv 6001) was goat anti-rabbit IgG antibody coupled to horseradish peroxidase, and was purchased from Beijing ZSBG Biotechnology Co., (Beijing, China).
Male Sprague-Dawley rats were purchased from Charles River Laboratories (Salk Institute). The animal protocol was approved by the Institutional Animal Care and Use Committee of the Rockefeller University (protocol 91200). Studies performed in Wenzhou Medical University Laboratory Animal Center were carried out in an animal laboratory approved by Science and Technology Department of Zhejiang Province (Certificate No. 2203001) using rats purchased from the Shanghai SLAC Laboratory Animal Co., Ltd., (Shanghai, China).
Tissue preparation
PND 7, 14, 21, 35, and 90 male rats were decapitated, after which testes were removed, frozen in liquid nitrogen and stored at -70°C for reverse transcription-polymerase chain reaction (RT-PCR), quantitative real-time PCR (qPCR) or western blot detection. Six rats were analyzed at each age, and between 3 and 6 samples from different rats were analyzed by qPCR, western blot or immunohistochemistry at each time point indicated.
RT-PCR and qPCR
Total RNA was isolated by a single-step method using the TRIzol Reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. 50 mg testis was homogenized in 1 ml TRIzol and extracted with 0.2 ml chloroform. Total RNA was then precipitated with 0.5 ml isopropanol, washed with 70% ethanol, dried in a vacuum, and dissolved in 150 μl diethylpyrocarbonate-treated water. The RNA concentration was measured spectrophotometrically by absorbance at 260 nm.
Primers were synthesized by Life Technologies Corporation (Carlsbad, CA, USA) using the appropriate published sequences.20 Forward and reverse primers were in different exons to minimize the effects of possible genomic DNA contamination. For Hsd11b1 (11β-HSD1), the forward primer was 5’-GAAGAAGCATGGAGGTCAAC-3’ and the reverse primer was 5’-GCAATCAGAGGTTGGGTCAT-3’. For Hsd11b2 (11β-HSD2), the forward primer was 5’-CGTCACTCAAGGGGACGTAT-3’ and the reverse primer was 5’-CGTCACTCAAGGGGACGTAT-3’. For the reference gene, Rps16 (ribosomal protein S16), the forward primer was 5’-AAGTCTTCGGACGCAAGAAA-3’ and the reverse primer was 5’-GCAATCAGAGGTTGGGTCAT-3’.
cDNA fragments were amplified by RT-PCR using total RNA from testes. In brief, total testis RNA (2 μg) was used as the template for avian myeloblastosis virus reverse transcriptase in the presence of random primers, deoxyribonucleotides, RNasin (RNAase inhibitor) and M-MLV reverse transcriptase (Promega Biosciences, Inc., San Luis Obispo, CA, USA) at 37°C for 60 min. The reaction was terminated by heating at 95°C for 15 min.
qPCR was carried out in a 22 μl volume using a 96-well plate format and SYBR Green PCR core reagents (Invitrogen) purchased from Life Technologies Corporation. Fluorescence was detected on an ABI 7700 system (Applied Biosystems, Foster City, CA, USA). Target and reference mRNA levels were expressed as the threshold cycle value (Ct) determined using their standard curves. Standard curves were created using serial dilutions of genes detected in the same plate. The relative mRNA levels of 11β-HSD1 and 11β-HSD2 were expressed as the Ct normalized using Rps16.
Western blotting
Testis tissue was homogenized in 0.5–1 ml phosphate buffered saline (PBS)-sucrose buffer (0.01 mol l-1, pH 7.4 PBS + 0.25 mol l–1 sucrose) and centrifuged at 700 g for 30 min at 4°C. The supernatant was collected and the protein concentration was detected spectrophotometrically by absorbance at 595 nm using a Bio-Rad Protein Assay kit (catalog No. 500–0006; Bio-Rad, Hercules, CA, USA).
Testis protein (60 μg) was mixed with loading buffer (3:1) and boiled for 10 min. SDS polyacrylamide gel electrophoresis was performed at a constant voltage of 60–80 volts, after which proteins in the gel were electrophoretically transferred onto nitrocellulose membranes. After 30 min immersion in 5% nonfat milk to block nonspecific binding, membranes were incubated with a 1:1000 dilution of the primary antibody. Membranes were then washed and incubated with a 1:2000 dilution of secondary antibody conjugated to horseradish peroxidase. The washing step was repeated, after which immunoreactive bands were visualized by chemiluminescence using enhanced chemiluminescence western blot detection reagents (Invitrogen). The protein levels for 11β-HSD1 and 11β-HSD2 were quantified by analyzing luminosity after normalization to β-actin.
Immunohistochemistry
Eighteen male rats (6 each at PND 7, 14 or 90) were used for immunohistochemistry experiments. Rats were anesthetized by chloral hydrate, perfused with PBS and 4% paraformaldehyde, after which the testes were removed. Testes were fixed in 4% paraformaldehyde for at least 24 h, cut into 2–3 cm sections, and then washed and embedded in paraffin wax. Paraffin wax sections were cut into 3–5 μm slices and treated according to the immunohistochemistry protocol recommended by the manufacturer. Anti-11β-HSD1 and anti-11β-HSD2 antibodies were used at dilutions of 1:400 and 1:100, respectively, in primary antibody diluent, and incubated with tissue slices overnight at 4°C, followed by 30 min incubation in secondary antibody.
Hormonal manipulation
Hormonal regulation of 11β-HSD gene expression was analyzed in PND 21 and 90 rats. For each age, five groups of six rats each were established as follows: (a) CON (control). Animals received a daily ip injection of 8% mannitol and a sc daily injection of cottonseed oil containing 5% ethanol; (b) NG. Animals received a daily ip injection of NalGlu (0.3 mg kg−1) plus sc vehicle. (c) NG + LH. Animals received daily ip injections of NalGlu and 0.2 mg kg−1 LH plus sc vehicle. (d) NG + T. Animals received a daily ip injection of NalGlu and testosterone (7.5 mg kg−1) by daily sc injection. (e) NG + MENT. Animals received NalGlu plus MENT (0.7 mg kg−) by daily sc injection. Treatments commenced on PND 17 or PND 86, and the animals were killed on PND 21 and 90 by asphyxiation with CO2. Testes were collected and processed as described above.
Statistics
All data are expressed as the means ± standard error of the mean statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Data from the mRNA and protein analysis of spatiotemporal changes in 11β-HSD expression during development were analyzed by repeated measures in the general linear model. Mauchly's test of sphericity confirmed the relationships among data from different ages (P < 0.05) and Greenhouse-Geisser <0.7, therefore Bonferroni was used to compare results between different ages. Data from mRNA and protein analysis of hormonal regulation of 11β-HSD expression were analyzed by one-way ANOVA with Tukey's post-hoc test. Data were normally distributed. Differences were regarded as statistically significant at P < 0.05. Measurements were repeated 3 times.
RESULTS
mRNA and protein analysis levels of 11β-HSD1 and 11β-HSD2 in rat testis during development
First, we carried out analysis of 11β-HSD1 and 11β-HSD2 in rat testis during development at both the mRNA (Figure 1) and protein (Figure 2) levels.
Figure 1.
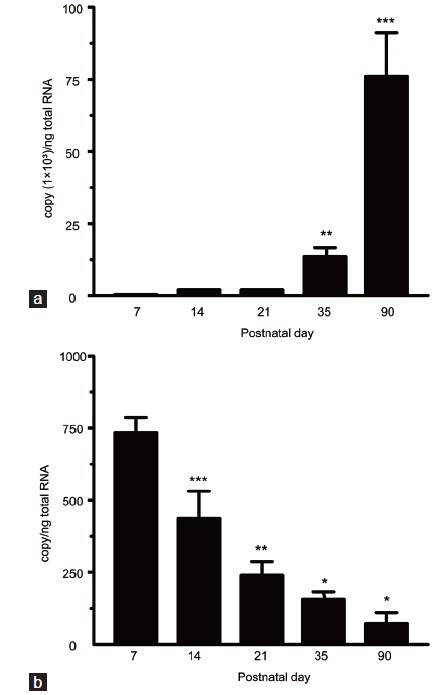
qPCR analysis of developmental fluctuations in 11β-HSD1 and 11β-HSD2 levels in rat testis. Testicular samples from rats at PND 7, 14, 21, 35 and 90 were subjected to qPCR analysis. Relative mRNA levels of 11β-HSD1 (a) and 11β-HSD2 (b) are expressed as the threshold cycle values (Ct) normalized to the reference gene, Rps16 (means ± s.e.m. n = 3–6). Superscript asterisks show the statistical difference (*P < 0.05, **P < 0.01, ***P < 0.001, the repeated measures in the general linear model, Mauchly's test of sphericity followed by pairwise comparisons with Bonferroni). PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2; qPCR: quantitative real-time polymerase chain reaction; Rps16: ribosomal protein S16; s.e.m.: standard error of the mean.
Figure 2.
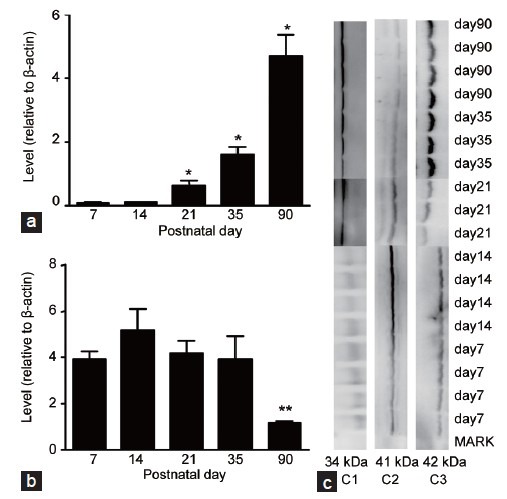
Western blot analysis of developmental fluctuations in 11β-HSD1 and 11β-HSD2 levels in rat testis. Testicular samples from rats at PND 7, 14, 21, 35 and 90 were subjected to western blotting analysis. For each sample, 60 μg protein was loaded. Relative protein levels of 11β-HSD1 (a) and 11β-HSD2 (b) were normalized to β-actin (means ± s.e.m. n = 3–6). The superscript asterisks show the statistical difference (*P < 0.05, **P < 0.01, the repeated measures of the general linear model, Mauchly's test of sphericity followed by pairwise comparisons with Bonferroni). (c) The pattern of immunoreactive proteins for each antibody. C1: 11β-HSD1 at 34 kDa; C2: 11β-HSD2 at 41 kDa; C3: β-actin at 42 kDa. PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2; s.e.m.: standard error of the mean.
11β-HSD1 testis mRNA levels increased between PND 21 and 35 (P < 0.01) and between PND 35 and 90 (P < 0.001) (Figure 1). Similarly, 11β-HSD1 testis protein levels increased with age, with the exception that statistical significance (P < 0.05) was initially observed between PND 14 and 21 (Figure 2). In contrast, 11β-HSD2 testis mRNA levels decreased between PND 7 and 90, with statistical significance (P < 0.001) initially observed between PND 7 and 14 (Figure 1). While 11β-HSD2 testis protein levels were comparable between PND 7 and 35 (P > 0.05), they were markedly decreased at PND 90 (P < 0.01) (Figure 2).
Localization of 11β-HSD1 and 11β-HSD2 during development in rat testis
Results of immunohistochemical staining for 11β-HSD1 and 11β-HSD2 in the testis of PND 7, 14, and 90 rats are shown in Figure 3.
Figure 3.
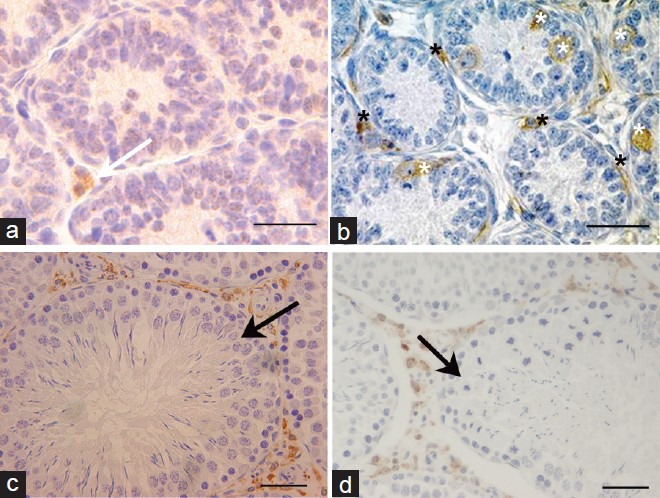
Immunohistochemical analysis of 11β-HSD1 and 11β-HSD2 expression in rat testis. (a) Rat testis at PND 14 (×400). Brown staining represents 11β-HSD1. White arrow points to 11β-HSD1-positive cells in the interstitial areas. (b) Rat testis at PND 7 (×400). Brown staining represents 11β-HSD2. The black stars point to 11β-HSD2-positive cells in interstitial areas and the white stars point to 11β-HSD2-positive cells in the seminiferous tubules. (c) Rat testis at PND 90 (×200). Brown staining represents 11β-HSD1. Black arrow points to the seminiferous tubule, which was 11β-HSD1-negative. (d) Rat testis at PND 90 (×200). Brown staining represents 11β-HSD2. Black arrow points to the seminiferous tubule which was 11β-HSD2-negative. Scale bars = 25 μm. PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2.
While 11β-HSD1-positive cells were sparse in the interstitial areas at PND 14 (white arrow, Figure 3a), they were abundant in the interstitial areas at PND 90. 11β-HSD1 expression was undetectable in the seminiferous tubule at PND 90 (black arrow, Figure 3c).
In contrast, while 11β-HSD2-positive cells were abundant around (black star, Figure 3b) and inside (white star, Figure 3b) the seminiferous tubules at PND 7, by PND 90, 11β-HSD2-positive cells were restricted to the interstitial areas (black arrow, Figure 3d). Based on their morphological characteristics and localization, we speculate that the 11β-HSD2-positive cells at PND 7 are spindle shape mesenchymal stem cells, spermatogonial stem cells or primordial germ cells, although further studies will be required for a more accurate conclusion.
Hormonal regulation on 11β-HSD1 and 11β-HSD2 in developing testis
To enable us to analyze the effect on 11β-HSD gene expression in PND 21 and 90 testes of treatment with exogenous LH, testosterone or MENT, rats were treated with the LHRH antagonist NalGlu to suppress endogenous LH and testosterone concentrations. Figures 4–6 show the effects of treatment with these hormones on 11β-HSD1 and 11β-HSD2 expression.
Figure 4.
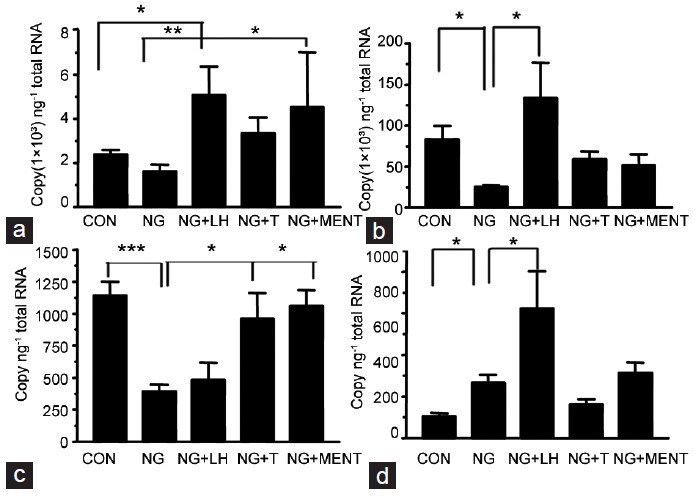
Hormonal regulation of 11β-HSD1 and 11β-HSD2 mRNA levels. The samples were from rats treated with vehicle (CON), NalGlu (NG), NalGlu plus testosterone (NG + T), NalGlu plus LH (NG + LH), or NalGlu plus MENT (NG + MENT). (a) shows the mRNA levels of 11β-HSD1 at PND 21; (b) shows the mRNA levels of 11β-HSD1 at PND 90; (c) shows the mRNA levels of 11β-HSD2 at PND 21; (d) shows the mRNA levels of 11β-HSD2 at PND 90. Relative mRNA levels are expressed as the threshold cycle values (Ct) normalized to Rps16 (means ± s.e.m., n = 3–6). The superscript asterisks show the statistical difference between two groups (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey's post-hoc analysis). PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2; CON: control; NalGlu: [Ac-D2Nal1, 4C1DPhe2, D3Pal3, Arg5, DGlu6 (anisole adduct), DAla10]-GnRH; T: testosterone; LH: luteinizing hormone; MENT: 7α-methyl-nortestosterone; Rps16: ribosomal protein S16; s.e.m.: standard error of the mean.
Figure 6.
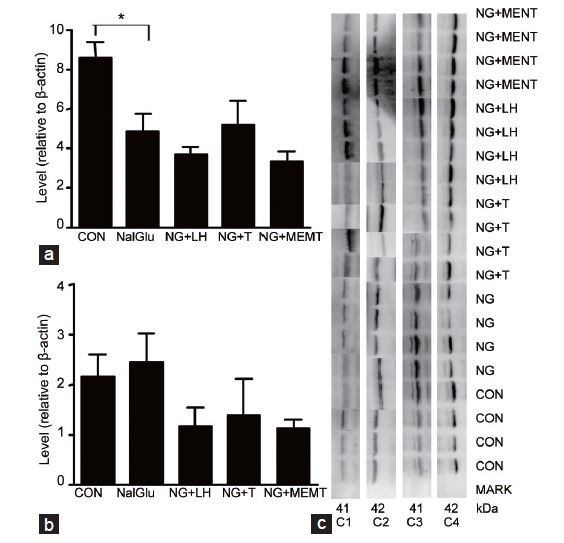
Hormonal regulation of 11β-HSD2 protein levels. The samples were previously treated by vehicle (CON), NalGlu (NG), NalGlu plus testosterone (NG + T), NalGlu plus LH (NG + LH), or NalGlu plus MENT (NG + MENT) (a) shows the protein levels of 11β-HSD2 relative to β-actin at PND 21, and (b) shows the protein levels of 11β-HSD2 relative to β-actin at PND 90 (means ± s.e.m., n = 3–6). The superscript asterisk shows the statistical difference between two groups (*P < 0.05, one-way ANOVA with Tukey's post-hoc analysis). (c) Shows the pattern of immunoreactive proteins recognized by the specific antibodies. C1: 11β-HSD2 of PND 90 at 41 kDa; C2: β-actin of PND 90 at 42 kDa; C3: 11β-HSD2 of PND 21 at 41 kDa; C4: β-actin of PND21 at 42 kDa. PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2; CON: control; NalGlu: [Ac-D2Nal1, 4C1DPhe2, D3Pal3, Arg5, DGlu6 (anisole adduct), DAla10]-GnRH; T: testosterone; LH: luteinizing hormone; MENT: 7α-methyl-nortestosterone; s.e.m.: standard error of the mean.
While NalGlu had no effect on expression of 11β-HSD1 at PND 21 (Figures 4a and 5a), it decreased 11β-HSD1 expression at PND 90 (Figure 4b). Compared with controls, 11β-HSD1 mRNA levels were induced by exogenous LH (P < 0.01) at PND 21 (Figure 4a) and PND 90 (Figure 4b), and by MENT but not testosterone at PND 21 (P < 0.05) (Figure 4a). Similar results were observed at the protein level (P < 0.01) (Figure 5b). NalGlu reduced 11β-HSD2 at the mRNA and protein levels (Figures 4c and 6a) at PND21, whereas at PND 90, it stimulated expression of 11β-HSD2 (Figure 4d). Relative to controls, mRNA levels of 11β-HSD2 at PND 21 were induced by exogenous testosterone (P < 0.05) and MENT (P < 0.05) (Figure 4c), but not by exogenous LH (P > 0.05) (Figure 4c). In contrast, at PND 90, 11β-HSD2 mRNA levels were increased by exogenous LH (P < 0.01) (Figure 4d), but not by exogenous testosterone or MENT (P > 0.05, Figure 4d). Protein levels of 11β-HSD2 were comparable (P > 0.05) in each group (Figure 6b).
Figure 5.
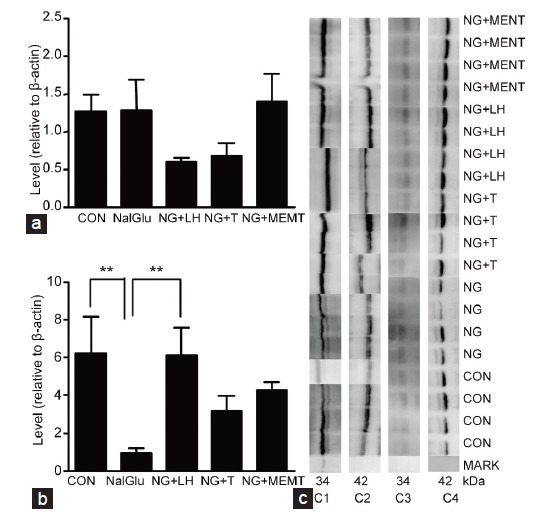
Hormonal regulation of 11β-HSD1 protein levels. The samples were from rats treated with vehicle (CON), NalGlu (NG), NalGlu plus testosterone (NG + T), NalGlu plus LH (NG + LH), or NalGlu plus MENT (NG + MENT). (a) Shows the protein levels of 11β-HSD1 relative to β-actin at PND 21, and (b) shows the protein levels of 11β-HSD1 relative to β-actin at PND 90 (means ± s.e.m., n = 3–6). The superscript asterisks show the statistical difference between two groups (**P < 0.01, one-way ANOVA with Tukey's post-hoc analysis). (c) Shows the pattern of immunoreactive proteins recognized by the specific antibodies. C1: 11β-HSD1 of PND 90 at 34 kDa; C2: β-actin of PND 90 at 42 kDa; C3: 11β-HSD1 of PND 21 at 34 kDa; C4: β-actin of PND21 at 42 kDa. PND: postnatal day; 11β-HSD1: 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2; CON: control; NalGlu: [Ac-D2Nal1, 4C1DPhe2, D3Pal3, Arg5, DGlu6 (anisole adduct), DAla10]-GnRH; T: testosterone; LH: luteinizing hormone; MENT: 7α-methyl-nortestosterone; s.e.m.: standard error of the mean.
DISCUSSION
GCs have distinct effects on rat testis that vary both with postnatal development stage, as well as with specific physiological contexts, such as stress. In this context, 11β-HSDs play important roles in modulating the production of testosterone in response to GC signaling.21 For example, during the prepubertal stage, when circulating levels of GC are low,22 the reducing activity of 11β-HSD1 maintains local GCs at levels required by normal testicular development. Moreover, the oxidative activity of 11β-HSD1 and 11β-HSD2 suppress GC levels to facilitate aldosterone signaling, and to avoid stress-related disruption of testis physiology. These data imply that developmental fluctuations in 11β-HSD1 and 11β-HSD2 expression are linked to age-dependent effects of GC signaling in the testis.
We found that 11β-HSD2 expression declines during postnatal testis development of testis, falling from mRNA and protein level peaks at PND 7 and PND 35, respectively, to their lowest levels at PND 90 for both mRNA and protein. These data are in conflict with our previous results in Leydig cells, which showed that 11β-HSD2 mRNA and activity were higher in adult Leydig cell (ALC) compared with progenitors Leydig cell (PLC) or immature Leydig cells (ILC).23 This discrepancy can be resolved in part by our immunolocalization of 11β-HSD2 expression in the seminiferous tubules at PND 7, implying that Leydig cells are not the sole source of 11β-HSD2 in infant rat testis. The elevated and ubiquitous expression of 11β-HSD2 in rat testis at this stage is in striking contrast to 11β-HSD1, which is undetectable until PND 14 (Figure 3) and consistent with our previous report in Leydig cells,15 is restricted to the ALC. Collectively, these data indicate that of the two isoforms, 11β-HSD2 plays the major role in modulating the effects of GC in the infant testis. Furthermore, given that GC production is very low during the rat stress hyporesponsive period between PND 4 and 12,24 and since 11β-HSD2 is a unidirectional oxidase that catalyzes only the inactivation of GC, we conclude that 11β-HSD2 plays a critical role in strictly limiting the exposure of the infant rat testis to GCs to ensure normal testicular development.
NalGlu, a LHRH antagonist, has been reported to cause reductions in testis weight, seminiferous tubule diameter, Leydig cell volume and testosterone production, as well as repressing expression of LH and LH receptor (LHR).25,26 In this study, NalGlu was used to suppress endogenous LH and testosterone levels to enable us to investigate the effects of exogenous LH, testosterone or the synthetic androgen MENT, on expression of 11β-HSD1 and 11β-HSD2 during postnatal testis development. Testosterone is metabolized by 5α-reductase 1 and 3α-HSD, which is highly active during early stages of rat testis development. Accordingly, the use of MENT, which resists metabolism by to 5α-reductase 1 and which has a higher affinity than testosterone for the androgen receptor (AR),27,28,29 enabled us to evaluate the role of androgen signaling on regulating 11β-HSD expression. Indeed, while 11β-HSD2 mRNA were suppressed at PND 21 by both testosterone and MENT, at a ten-fold lower dose, only MENT suppressed 11β-HSD1 expression (Figure 4a), suggesting that while both ligands are active in rat testis of PND 21, MENT is the more potent of the two.
While the differentiation of mesenchymal precursor cells into PLC between PND 10 and 13 is independent of LH and inhibited by testosterone, both hormones are essential for subsequent processes, including proliferation, preliminary steps in steroidogenesis, and differentiation into of PLC into ILC.30,31 Our experiments on hormonal regulation of 11β-HSD were carried out at PND 17–21, during the prepubertal stage, or PND 86–90, during the adult stage, at which times Leydig cells exist as PLC or ALC, respectively. Relative to PLC, LHR is expressed lower levels in ALC, while AR is expressed at a higher level.32 We found that while 11β-HSD1 in rat testis of PND 21 is not affected by NalGlu, it is repressed by NalGlu and restored by exogenous LH at PND 90 (Figure 4a), indicating that 11β-HSD1 expression in the testis is more sensitive to LH in adults than in prepubertal rats. Moreover, we found that 11β-HSD1 is primarily distributed in ALC and increases during testis development. These data, along with our recent demonstration that Leydig cell numbers are reduced by NalGlu and partially restored by LH or MENT,33 lead us to speculate that fluctuations in 11β-HSD1 levels in adult rat testis in response to NalGlu, LH or androgens, may be secondary effects to the effects of these factors on Leydig cell number.
We found that 11β-HSD2 was robustly expressed in both the interstitial areas and seminiferous tubules in infant rat testis and decreased with increasing age. In contrast to hormonal regulation of 11β-HSD1 expression, 11β-HSD2 levels were reduced by NalGlu and restored by testosterone or MENT at PND 21, and increased by both NalGlu and LH at PND 90 (Figure 4c–4d). These data suggest that 11β-HSD2 is more sensitive to testosterone than LH in the prepubertal rat testis. It has been previously shown that NalGlu reduces seminiferous tubules diameter in rat testis at PND 90,26 during the window of sensitivity of the testis to LH. It may be speculated that development of seminiferous tubules was delayed in NalGlu-treated rats, and that 11β-HSD2-positive cells remained in these animals, which would explain why 11β-HSD2 mRNA levels were unexpectedly increased after treatment with NalGlu. With the exception of the reduction of 11β-HSD2 protein levels by NalGlu at PND 21, our observations at the mRNA level were not recapitulated at the protein level (Figure 6). We attribute the discrepancies between our qPCR and western blot data to the greater sensitivity of qPCR in detecting changes at the transcription level.
In summary, we have shown that 11β-HSD1 and 11β-HSD2 have contrasting expression in rat testis after birth: an age-dependent increase in 11β-HSD1 and an age-dependent decrease in 11β-HSD2. The 11β-HSD1 is mainly expressed in Leydig cells and is more responsive to LH in the adult stage, whereas the 11β-HSD2 is expressed widely in interstitial areas and seminiferous tubules and is more sensitive to testosterone in prepubertal stage. The distinct developmental changes in 11β-HSD2 make it possible to function as a gatekeeper for rat testis in the prepubertal stage of development.
AUTHOR CONTRIBUTIONS
HYZ carried out the molecular genetic studies, performed the statistical analysis, drafted the manuscript and made the major revisions. XXC, HL and ALF carried out the immunoassays and participated in the animal treatment. RSG conceived and designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81373032, 30871434 and 31171425), the Zhejiang Natural Science Foundation (No. LY12H31002) and the Wenzhou Science and Technology Program (No. H20090024, H20100020 and H20090003).
REFERENCES
- 1.Lescoat G, Lescoat D, Garnier DH. Influence of adrenalectomy on maturation of gonadotrophin function in the male rat. J Endocrinol. 1982;95:1–6. doi: 10.1677/joe.0.0950001. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy C, Yuvaraj S, Sivakumar R, Ravi Sankar B, Balasubramanian K. Metyrapone-induced corticosterone deficiency impairs glucose oxidation and steroidogenesis in Leydig cells of adult albino rats. Endocr J. 2002;49:405–12. doi: 10.1507/endocrj.49.405. [DOI] [PubMed] [Google Scholar]
- 3.Brennemann W, Köhler W, Zierz S, Klingmüller D. Testicular dysfunction in adrenomyeloneuropathy. Eur J Endocrinol. 1997;137:34–9. doi: 10.1530/eje.0.1370034. [DOI] [PubMed] [Google Scholar]
- 4.Charpenet G, Taché Y, Bernier M, Ducharme JR, Collu R. Stress-induced testicular hyposensitivity to gonadotropin in rats. Role of the pituitary gland. Biol Reprod. 1982;27:616–23. doi: 10.1095/biolreprod27.3.616. [DOI] [PubMed] [Google Scholar]
- 5.Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, et al. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67:1750–5. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- 6.Hu GX, Lin H, Sottas CM, Morris DJ, Hardy MP, et al. Inhibition of 11beta-hydroxysteroid dehydrogenase enzymatic activities by glycyrrhetinic acid in vivo supports direct glucocorticoid-mediated suppression of steroidogenesis in Leydig cells. J Androl. 2008;29:345–51. doi: 10.2164/jandrol.107.004242. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Wang Q, Wang FF, Gao HB, Zhang P. Stress induces glucocorticoid-mediated apoptosis of rat Leydig cells in vivo. Stress. 2012;15:74–84. doi: 10.3109/10253890.2011.585188. [DOI] [PubMed] [Google Scholar]
- 8.Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, et al. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143:130–8. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- 9.Wang FF, Wang Q, Chen Y, Lin Q, Gao HB, et al. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J Androl. 2012;14:643–8. doi: 10.1038/aja.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagini G, Pich EM. Corticosterone administration to rat pups, but not maternal separation, affects sexual maturation and glucocorticoid receptor immunoreactivity in the testis. Pharmacol Biochem Behav. 2002;73:95–103. doi: 10.1016/s0091-3057(02)00754-2. [DOI] [PubMed] [Google Scholar]
- 11.Almeida SA, Anselmo-Franci JA, Rosa e Silva AA, Carvalho TL. Chronic intermittent immobilization of male rats throughout sexual development: a stress protocol. Exp Physiol. 1998;83:701–4. doi: 10.1113/expphysiol.1998.sp004151. [DOI] [PubMed] [Google Scholar]
- 12.Weber MA, Groos S, Höpfl U, Spielmann M, Aumüller G, et al. Glucocorticoid receptor distribution in rat testis during postnatal development and effects of dexamethasone on immature peritubular cells in vitro. Andrologia. 2000;32:23–30. [PubMed] [Google Scholar]
- 13.Xiao YC, Huang YD, Hardy DO, Li XK, Ge RS. Glucocorticoid suppresses steroidogenesis in rat progenitor Leydig cells. J Androl. 2010;31:365–71. doi: 10.2164/jandrol.109.009019. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DM, Lakshmi V, Monder C. Corticosteroid 11 beta-dehydrogenase in rat testis. Endocrinology. 1989;125:209–16. doi: 10.1210/endo-125-1-209. [DOI] [PubMed] [Google Scholar]
- 15.Ge RS, Hardy DO, Catterall JF, Hardy MP. Developmental changes in glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase oxidative and reductive activities in rat Leydig cells. Endocrinology. 1997;138:5089–95. doi: 10.1210/endo.138.12.5614. [DOI] [PubMed] [Google Scholar]
- 16.Rusvai E, Náray-Fejes-Tóth A. A new isoform of 11 beta-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem. 1993;268:10717–20. [PubMed] [Google Scholar]
- 17.Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP. Identification of a kinetically distinct activity of 11beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology. 1997;138:2435–42. doi: 10.1210/endo.138.6.5165. [DOI] [PubMed] [Google Scholar]
- 18.Moore XL, Hoong I, Cole TJ. Expression of the 11beta-hydroxysteroid dehydrogenase 2 gene in the mouse. Kidney Int. 2000;57:1307–12. doi: 10.1046/j.1523-1755.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharp V, Thurston LM, Fowkes RC, Michael AE. 11beta-hydroxysteroid dehydrogenase enzymes in the testis and male reproductive tract of the boar (Sus scrofa domestica) indicate local roles for glucocorticoids in male reproductive physiology. Reproduction. 2007;134:473–82. doi: 10.1530/REP-07-0126. [DOI] [PubMed] [Google Scholar]
- 20.Ge RS, Dong Q, Niu EM, Sottas CM, Hardy DO, et al. 11{beta}-Hydroxysteroid dehydrogenase 2 in rat leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology. 2005;146:2657–64. doi: 10.1210/en.2005-0046. [DOI] [PubMed] [Google Scholar]
- 21.Hu GX, Lian QQ, Lin H, Latif SA, Morris DJ, et al. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids. 2008;73:1018–24. doi: 10.1016/j.steroids.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978;235:E451–6. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- 23.Ge RS, Dong Q, Sottas CM, Chen H, Zirkin BR, et al. Gene expression in rat leydig cells during development from the progenitor to adult stage: a cluster analysis. Biol Reprod. 2005;72:1405–15. doi: 10.1095/biolreprod.104.037499. [DOI] [PubMed] [Google Scholar]
- 24.Okimoto DK, Blaus A, Schmidt MV, Gordon MK, Dent GW, et al. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive period. Endocrinology. 2002;143:1717–25. doi: 10.1210/endo.143.5.8819. [DOI] [PubMed] [Google Scholar]
- 25.Kenigsberg D, Littman BA, Hodgen GD. Medical hypophysectomy: I. Dose-response using a gonadotropin-releasing hormone antagonist. Fertil Steril. 1984;42:112–5. doi: 10.1016/s0015-0282(16)47968-9. [DOI] [PubMed] [Google Scholar]
- 26.Hikim AP, Swerdloff RS. Time course of recovery of spermatogenesis and Leydig cell function after cessation of gonadotropin-releasing hormone antagonist treatment in the adult rat. Endocrinology. 1994;134:1627–34. doi: 10.1210/endo.134.4.8137724. [DOI] [PubMed] [Google Scholar]
- 27.Kumar N, Crozat A, Li F, Catterall JF, Bardin CW, et al. 7alpha-methyl-19-nortestosterone, a synthetic androgen with high potency: structure-activity comparisons with other androgens. J Steroid Biochem Mol Biol. 1999;71:213–22. doi: 10.1016/s0960-0760(99)00143-0. [DOI] [PubMed] [Google Scholar]
- 28.Ge RS, Hardy MP. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–95. doi: 10.1210/endo.139.9.6183. [DOI] [PubMed] [Google Scholar]
- 29.Moralí G, Lemus AE, Munguía R, Arteaga M, Pérez-Palacios G, et al. Induction of male sexual behavior in the rat by 7 alpha-methyl-19-nortestosterone, an androgen that does not undergo 5 alpha-reduction. Biol Reprod. 1993;49:577–81. doi: 10.1095/biolreprod49.3.577. [DOI] [PubMed] [Google Scholar]
- 30.Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–71. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- 31.Teerds KJ, Rijntjes E, Veldhuizen-Tsoerkan MB, Rommerts FF, de Boer-Brouwer M. The development of rat Leydig cell progenitors in vitro: how essential is luteinising hormone? J Endocrinol. 2007;194:579–93. doi: 10.1677/JOE-06-0084. [DOI] [PubMed] [Google Scholar]
- 32.Shan LX, Hardy MP. Developmental changes in levels of luteinizing hormone receptor and androgen receptor in rat Leydig cells. Endocrinology. 1992;131:1107–14. doi: 10.1210/endo.131.3.1505454. [DOI] [PubMed] [Google Scholar]
- 33.Guo JJ, Ma X, Wang CQ, Ge YF, Lian QQ, et al. Effects of luteinizing hormone and androgen on the development of rat progenitor Leydig cells in vitro and in vivo. Asian J Androl. 2013;15:685–91. doi: 10.1038/aja.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


