Abstract
The cation channel of sperm (CatSper) protein family plays important roles in male reproduction and infertility. The four members of this family are expressed exclusively in the testis and are localized differently in sperm. To investigate the effects of Panax ginseng treatment on the expression of CatSper genes and sperm hyperactivation in male mice, sperm motility and CatSper gene expression were assessed using a computer-assisted semen analysis system, a Fluoroskan Ascent microplate fluorometer to assess Ca2+ influx, real-time polymerase chain reaction, Western blotting and immunofluorescence. The results suggested that the Ca2+ levels of sperm cells treated with P. ginseng were increased significantly compared with the normal group. The P. ginseng-treated groups showed increased sperm motility parameters, such as the curvilinear velocity and amplitude of lateral head displacement. Taken together, the data suggest that CatSper messenger ribonucleic acid levels were increased significantly in mouse testes in the P. ginseng-treated group, as was the protein level, with the exception of CatSper2. In conclusion, P. ginseng plays an important role in improving sperm hyperactivation via CatSper gene expression.
Keywords: Ca2+, CatSper, hyperactivation, panax ginseng
INTRODUCTION
Fertilization is the process in which sperm and egg combine. The sperm penetrates the zona pellucida of the egg, initiating the development of a new organism.1 Hyperactivated motility assists in the process of fertilization in vivo by allowing sperm to reach the oocyte through mucus-filled passages, in addition to helping the sperm penetrate the zona pellucida.2 A computer-assisted semen analysis (CASA) system has been developed to detect hyperactivation and to confirm the percentage of hyperactivated sperm in a sample. It measures the following motion parameters: curvilinear velocity (VCL, μm s−1), average-path velocity, μm s−1, straight-line velocity, μm s−1, beat cross frequency, Hz, straightness, amplitude of lateral head displacement (ALH, μm) and linearity. An increased VCL and ALH are indicative of hyperactivation.3,4
Cations – such as Na+, K+ and Ca2+ – are involved in regulating sperm motility and fertility. A sperm-specific Na+/H+ exchanger located at the principal component of the flagellum is required for motility and fertility.5 A rapid change in sperm motility is accomplished by the rapid diffusion of K+ and Ca2+ and Ca2+ across the sperm plasma membrane through selective ion channels.6
Calcium ion signaling affects all aspects of cellular life and death. Ca2+ regulates mitochondrial function, innate immunity, motility, transcription, viability and apoptosis.7 Ca2+ is commonly required for motility in epididymal sperm samples and Ca2+ regulates the activated and hyperactivated motility of ejaculated sperm.8,9,10,11,12,13 Intracellular Ca2+ stores are the main concern, particularly in hyperactivated motility regulation. Flagellar wave symmetry in permeabilized sperm is increased by Ca2+, which, at sufficiently high levels, inhibits motility.14 Further, Ca2+ is required for hyperactivation.15,16
Members of the cation channel of sperm (CatSper) family are expressed solely in spermatozoa. CatSper1 is localized to the principal piece of sperm and is required for evoked Ca2+ entry and hyperactivation control in sperm.16 CatSper2 is located in the sperm tail and is essential for regulating hyperactivation.17 CatSper3 and 4 are localized in the testes and sperm and are required for the motility of hyperactivated sperm.18 Studies have localized CatSper messenger ribonucleic acids (mRNAs) exclusively to the testes, while CatSper proteins were expressed in the testes and sperm.15,19,20
Korean Panax ginseng C. A. Meyer is a traditional medicinal plant. In Asia, it is considered the most precious of all medicinal plants. Originally, the efficacy of P. ginseng was based on oriental medical science theory.21 We reported previously that Korean ginseng induces spermatogenesis in rats via the activation of the cAMP-responsive element modulator. Rats treated with ginseng had a significantly increased epididymal sperm count and sperm motility.22
However, there are few studies of the effects of P. ginseng on sperm hyperactivation in male mice. Therefore, this study investigated the effects of P. ginseng treatment on sperm motility and hyperactivation with reference to CatSper expression in male mice.
MATERIALS AND METHODS
Chemicals and medium
For the analysis of sperm parameter, the medium consisted of M199 medium (GIBCO, Big Cabin, OK, USA), 0.5% bovine serum albumin (Sigma-Aldrich Co., St. Louis, MO, USA) and 1 mmol l−1 pyruvic acid (Sigma-Aldrich Co., St. Louis, MO, USA). CASA (Hamilton Thorne, Beverly, MA, USA), 2X-CEL disposable sperm analysis chambers (in depths of 80 μm) (Hamilton Thorne, Beverly, MA, USA) were used for analysis of sperm motility, parameters of sperm quality. TaqMan® Gene expression master mix (Applied Biosystems, Inc., Woburn, MA, USA) was used for the quantitative polymerase chain reaction (PCR) (Applied Biosystems, Inc., Woburn, MA, USA). And CatSper antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used for Western blot analysis and the immunofluorescence with hematoxylin staining.
Preparation of P. ginseng extract
P. ginseng, the root of P. ginseng C. A. Meyer was purchased from Won Kwang Herbal Drug Co. Ltd. (Seoul, Korea). Three hundred grams of dried P. ginseng were boiled with six liter of water for 2 h at 100°C and then the suspension was filtered and concentrated under reduced pressure. The filtrate was lyophilized and yielded 76.5 g (25.5%) of powder, which was kept at 4°C. Before each experiment, dried extract was dissolved in distilled deionized water (Millipone, Billerica, MA, USA) and vortexed for 2 min at room temperature.
Animal experiment
Animals
Five-week-old male C57BL/6J mice were purchased from SLC Inc. (Hamamatsu, Japan). The animals were housed in a specific pathogen-free environment with a 12/12-h light/dark cycle. Animals had free access to standard rodent pellets (Purina, Bundang-gu, Gyeonggi-do, Korea) and water. Animal care and experimental procedures followed the requirements in the “Guide for the Care and Use of Laboratory Animals” (Department of Health, Education and Welfare, National Institutes of Health, 1996), which was approved by Institutional Review Board of College of Korean Medicine in Kyung Hee University.
Treatment of P. ginseng
After 7 days of adaptation to the environment, the mice were divided into two groups: normal group (vehicle-treated, n = 8) and P. ginseng group (PG) (100, 500, 1000 mg kg−1, n = 8). P. ginseng was treated for 5 days a week for 5 weeks. The animals were weighed weekly in order to adjust the gavages volume and to monitor their general health.
Sperm preparation
Mice were killed by CO2 asphyxiation and cervical dislocation. Sperm were collected as previously described.10 Briefly, epididymal caudal and ductus deferens sperm were punctured with a 30-gauge needle and incubated at 37°C to allow sperm to disperse into surrounding medium.
Sperm analysis
Epididymal motility was evaluated using the method described by Connolly et al.,23 with some modifications. For assessment of sperm motility, sperms were recovered from excised ductus deferens, caudal epididymides and allowed to capacitate for 90 min in media at 37°C. For the confirmation of P. ginseng effect on sperm motility, sperms were incubated in medium containing 10 mmol l−1 1, 2-bis-(o-aminophenoxy)-ethane-N, N, N’, N’-tetra-acetic acid (BAPTA) for 1 min. Sperms were scored as motile if any movement was detected and used to analyze the motility, VCL and ALH by CASA system.
Ca2+ flux assay
Epididymal caudal and ductus deferens sperm were used for intracellular Ca2+ levels measurement, as previously described.24 Epididymal caudal sperm from the mouse were minced in sperm washing media incubated for 90 min at 37°C. The Ca2+ levels outcomes produced by manual evaluation using the Fluoroskan Ascent Microplate Fluorometer (Thermo, Marietta, OH, USA). Epididymal caudal sperm suspensions were loaded with Fluo-4 NW Calcium assay kits. For Fluo-4 NW, emission intensity was monitored at 485 nm and 538 nm as the wavelength pair.
RNA isolation and real-time polymerase chain reaction
One milliliter of trizol was added to the testis tissue samples. RNA samples were analyzed by denaturing formaldehyde/agarose/ethidium bromide gel electrophoresis. The final amount of RNA was estimated by spectrophotometer (Molecular Devices, Downingtown, PA, USA) at 260 nm. First strand cDNA synthesis with 5 μg of total RNA was performed using Moloney Murine leukemia virus reverse transcriptase and oligo dT primer for 1 h at 42°C. Subsequently, the PCR-amplification was performed by a modified method originally described by Saiki et al.25
Real-time PCR was performed in a Step one plus System Thermal Cycler (Applied Biosystems, Inc., Woburn, MA, USA). Real-time PCR was performed on a volume of 20 μl containing 2 μl (200 ng) of cDNA and 10 μl of PCR master mix, 1 μl of each taqman probe and 7 μl of diethyl pyrocarbonate-treated water. Gene expression assay mixes for CatSper1-4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems [assay ID: Mm00460530_m1 (CatSper1), Mm00467632_m1 (CatSper2), Mm00712792_m1 (CatSper3), Mm01190761_m1 (CatSper4) and Mm99999915_g1 (GAPDH)]. The program was set at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 60 s. Samples were amplified with GAPDH primers for determination of the initial relative quantity (RQ) of cDNA in each sample and then all PCR products were normalized to that amount. Samples were amplified in triplicate, averages were calculated and differences in cycle threshold (Ct) data were evaluated by Sequence Detection Software V1.3.1 (Applied Biosystems, Inc., Woburn, MA, USA). For data analysis, we used the comparative Ct method with the following formula: ∆Ct = Ct (Target, TLR) − Ct (Endo, GAPDH). Data are expressed as RQ and differences are shown in the figures as the expression ratio of the normalized target gene, according to the software results.
Western blot analysis
Proteins from homogenized testes were separated using nuclear extract kit according to manufacturer's protocol with minor modifications (Active and Motif, Carlsbad, CA, USA). The protein concentrations were determined by Bradford method.26 The sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed as described previously.27 Equivalent amount (50 μg) of protein extracts were separated in 10% Tris-glycine gels by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes using 25 mmol l−1 Tris and 250 mmol l−1 glycine containing 20% methanol, pH 8.3. Transfer was performed at a constant voltage of 20 mA for 1 h. After transfer, membranes were blocked in phosphate buffered saline (PBS) containing 0.05% Tween PBS-T with 5% skim milk for 2 h at room temperature and incubated with the primary antibody for CatSper 1 (sc-21180, 1:1000), CatSper 2 (sc-98539, 1:1000), CatSper 3 (sc-98818, 1:500) and CatSper 4 (sc-83126, 1:500) in PBS-T overnight at 4°C. After incubation, the membranes were rinsed 3 times with 1 × PBS and incubated with conjugated donkey anti-goat IgG (CatSper1, 4) and conjugated anti-rabbit IgG (CatSper2, 3) for 1 h at room temperature followed by three rinses with 1 × PBS. CatSper antibodies were validated by immunofluorescence staining using mouse spermatozoa.
Immunofluorescence detection with hematoxylin staining
Immunofluorescence detection with hematoxylin staining was performed according to the procedure described previously.28 For immunofluorescence detection with hematoxylin staining studies, the testes were fixed overnight in Bouin's solution, dehydrated in 70%, 80%, 95%, 100% ethanol, xylene and embedded in paraffin, and 7 mm thick tissue sections. The sections were deparaffinized and rehydrated in xylene, 100%, 95%, 80%, 70% ethanol. The sections were then treated in a microwave oven in 10 mmol l−1 citrate buffer, pH 6.0, for 12 min. After three washes in PBS, endogenous peroxidase activity was quenched by 3% hydrogen peroxide in PBS for 20 min and again washed 3 times in PBS. Sections were then incubated in a blocking (saponin 0.5 mg in gelatin 2 mg ml−1) for 1 h in order to block nonspecific binding. Subsequently, sections were incubated for overnight at room temperature with CatSper 1 (sc-21180, 1:100), CatSper 2 (sc-98539, 1:100), CatSper 3 (sc-98818, 1:100) and CatSper 4 (sc-83126, 1:100) in a humidified chamber. Sections were washed 3 times in PBS before being incubated with the appropriate secondary antibody [Cy3-conjugated anti-rabbit 1:500 (CatSper2, 3), Cy3-conjugated anti-biotin 1:500 (CatSper1, 4)] for 1 h at room temperature. Samples were washed 3 times in PB and covered with microscopy coverslips on mounting. All samples were counterstained with hematoxylin stain (Sigma-Aldrich Co., St. Louis, MO, USA).
Statistical analysis
All quantitative data derived from this study were analyzed statistically. The results were expressed as the mean ± standard deviation. Differences between groups were assessed by one-way ANOVA using the SPSS software package for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance at P < 0.001, <0.01 and <0.05 has been given respective symbols in the tables or figures.
RESULTS
Effects of Panax ginseng on sperm motility parameters
The sperm motility values of normal and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups were 52.96% ± 4.09% versus 63.84% ± 5.33%, 64.51% ± 6.09%, and 66.23% ± 4.63%, respectively; all P < 0.05. The P. ginseng treatment increased sperm motility significantly compared with the normal group. The sperm motility in mice treated with 10 mmol l−1 BAPTA was decreased significantly compared to the normal group (10.12% ± 2.72% vs. 52.96% ± 4.09%, P < 0.001). The groups treated with P. ginseng (100, 500 and 1000 mg kg−1) showed increased sperm motility compared to the BAPTA (10 mmol l−1) control group (10.12% ± 2.72% vs. 30.38% ± 5.41%, 23.81% ± 4.12% and 27.48% ± 4.26%, P < 0.05, 0.05, 0.01, respectively; Figure 1a). The VCL of the normal and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups were 106.36 ± 5.08 versus 118.48 ± 11.15, 116.98 ± 11.10 and 114.89 ± 9.99 μm s−1, respectively; all P < 0.05. Sperm treated with BAPTA had a significantly decreased VCL compared with the normal group (106.36 ± 5.08 vs. 50.91 ± 6.01 μm s−1, P < 0.001). In addition, the BAPTA (10 mmol l−1) and P. ginseng (100, 500 and 1000 mg kg−1) groups had higher curvilinear velocities than the control group (50.91 ± 6.01 vs. 80.67 ± 7.72, 69.24 ± 3.24 and 78.16 ± 3.32 μm s−1, P < 0.01, 0.05, 0.01, respectively; Figure 1b). The ALH of the normal and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups were 8.12 ± 0.25 versus 8.35 ± 0.20, 8.56 ± 0.34 and 8.33 ± 0.28 μm, respectively; all P < 0.05. The P. ginseng treatment significantly increased the ALH compared to the normal group. Sperm treated with BAPTA had a significantly decreased ALH compared to the normal group (3.42 ± 0.25 vs. 8.12 ± 0.25 μm, P < 0.01). Furthermore, the BAPTA (10 mmol l−1) and P. ginseng (100, 500 and 1000 mg kg−1) groups had a higher ALH than did the control group (3.42 ± 0.25 vs. 6.81 ± 0.20, 5.93 ± 0.34 and 6.71 ± 0.28 μm, respectively; all P < 0.05, Figure 1c). In addition, P. ginseng treatment increased the intracellular Ca2+ levels compared to the normal. The sperm cell Ca2+ levels in the P. ginseng-treated (100 mg kg−1) group were increased significantly (about 20%) at 60 min and were maintained up to 180 min (Figure 1d).
Figure 1.
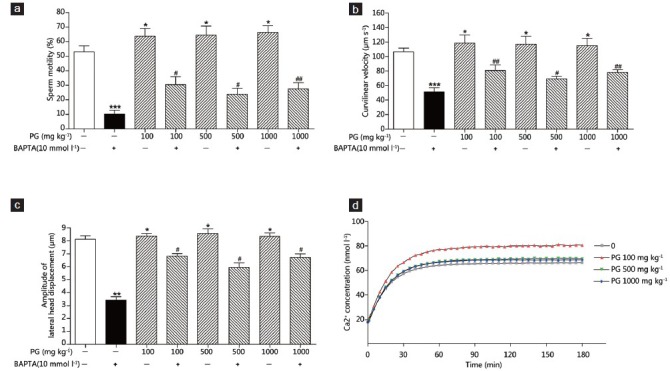
Effects of Panax ginseng on sperm motility parameters. Sperm motility are the results for the normal (N) and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups in the presence or absence of 10 mmol l–1 1,2-bis-(o-aminophenoxy)-ethane-N,N,N’,N’-tetra-acetic acid). (a) Sperm motility; (b) curvilinear velocity (μm s−1); (c) amplitude of lateral head displacement (μm) and (d) Ca2+ levels. *Significantly different from the normal value (*P < 0.05, **P < 0.01, ***P < 0.001). #The mean is significantly different from the control value (#P < 0.05, ##P < 0.01).
Effect of Panax ginseng on CatSper messenger ribonucleic acid levels by real-time polymerase chain reaction
To examine the effects of P. ginseng on CatSper mRNA levels in mouse testes, real-time PCR was performed. The total RNA of mouse testes in the 100, 500 and 1000 mg kg−1 P. ginseng-treated groups was examined. Real-time PCR showed that the CatSper1-4 mRNA levels were increased significantly in the P. ginseng-treated (100, 500 and 1000 mg kg−1) groups. The 100, 500 and 1000 mg kg−1 P. ginseng-treated groups had significantly increased CatSper1 mRNA levels compared with the normal group (1.00 ± 0.06 vs. 1.22 ± 0.13, 1.31 ± 017 and 1.35 ± 0.14, P < 0.05, P < 0.01, respectively; Figure 2a). The CatSper2 mRNA levels in the P. ginseng-treated (100, 500 and 1000 mg kg−1) groups were also increased significantly compared to that in the normal group (1.00 ± 0.03 vs. 1.39 ± 0.08, 1.23 ± 0.06 and 1.40 ± 0.02, P < 0.01, P < 0.001, respectively; Figure 2b). Likewise, the CatSper3 mRNA levels were higher in the P. ginseng-treated (100, 500 and 1000 mg kg−1) groups compared to the normal group (1.0 ± 0.05 vs. 1.19 ± 0.09, 1.38 ± 0.09 and 1.17 ± 0.04, P < 0.05, 0.01, 0.01, respectively; Figure 2c). In addition, the 100, 500 and 1000 mg kg−1 P. ginseng-treated groups showed enhanced CatSper4 mRNA levels (1.0 ± 0.10 vs. 1.73 ± 0.15, 1.52 ± 0.21 and 2.18 ± 0.08, P < 0.01, 0.05, P < 0.001, respectively; Figure 2d).
Figure 2.
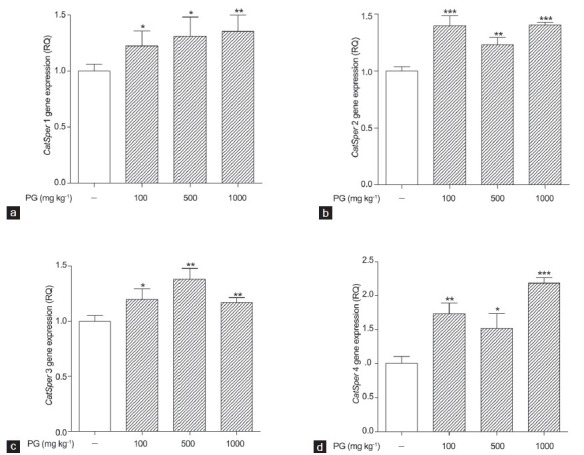
Effect of Panax ginseng treatment on CatSper messenger ribonucleic acids levels in mouse testes as determined by real-time polymerase chain reaction. Normal and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups. (a) CatSper1; (b) CatSper2; (c) CatSper3 and (d) CatSper4. Each column represents the mean ± standard deviation (n = 3). The normal group was used as the control (relative quantity, RQ = 1). *Significantly different from the vehicle-treated group (*P < 0.05, **P < 0.01 and ***P < 0.001).
Effects of Panax ginseng on CatSper protein levels in mouse testes by Western blotting
Western blotting was used to determine the effects of P. ginseng on CatSper1, 2, 3 and 4 protein levels in mouse testes. b-Tubulin was used as the internal control. There were dose-dependent increases in the CatSper1 protein levels in the P. ginseng-treated (100, 500 and 1000 mg kg−1) groups compared to the normal group (100% vs. 133.31%, 161.20% and 162.34%, respectively, all P < 0.01; Figure 3a). However, the CatSper2 protein levels in the P. ginseng-treated (100, 500 and 1000 mg kg−1) groups increased slightly (100% vs. 101.37%, 102.27% and 106.92%, respectively; Figure 3b), but the increase did not reach statistical significance. The CatSper3 protein levels in the P. ginseng-treated (500 and 1000 mg kg−1) groups increased in a dose-dependent manner (100% vs. 134.87% and 139.57%, respectively; both P < 0.05), as shown in Figure 3c. In addition, the CatSper4 protein level was increased significantly by P. ginseng treatment (100, 500 and 1000 mg kg−1) compared to the control (100% vs. 119.35%, 126.56% and 125.63%, respectively, all P < 0.05; Figure 3d).
Figure 3.
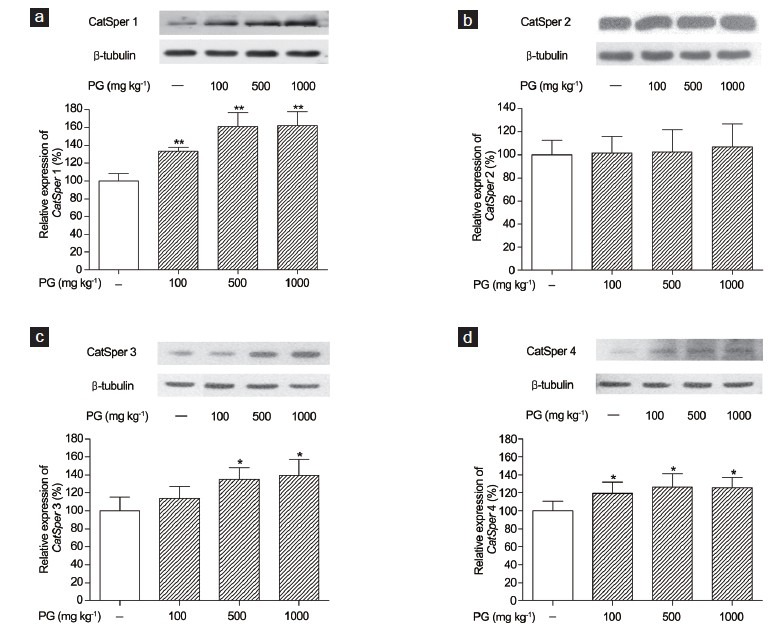
Effects of Panax ginseng treatment on CatSper protein levels in mouse testes by Western blotting using anti-CatSper and β-tubulin antibodies. Data are the results from the normal (N) and P. ginseng-treated (PG) (100, 500 and 1000 mg kg−1) groups. (a) CatSper1; (b) CatSper2; (c) CatSper3 and (d) CatSper4. Each column represents the mean ± standard deviation (n = 3). *Significantly different from the vehicle-treated group (*P < 0.05, **P < 0.01).
Effect of Panax ginseng on CatSper protein levels based on immunofluorescence with hematoxylin staining
Immunofluorescence detection of CatSper1, 2, 3 and 4 with hematoxylin staining was performed in mouse testes. The P. ginseng-treated groups (100, 500 and 1000 mg kg−1) had significantly increased dose-dependent levels of CatSper1, 3 and 4 proteins. In particular, P. ginseng treatment induced CatSper expression in mouse testes predominantly in spermatids and spermatozoa, as observed by fluorescence staining. By contrast, P. ginseng treatment did not increase CatSper2 levels, as determined by Western blotting (Figure 4). In the mouse testes, CatSper proteins were in a rounded, positive form (Figure 4a, 4d, 4g and 4j). All samples were counterstained with hematoxylin (Figure 4b, 4e, 4h and 4k). Overlaid CatSper immunofluorescence and hematoxylin-counterstained images suggested induction of CatSper proteins (Figure 4c, 4f, 4i and 4l).
Figure 4.
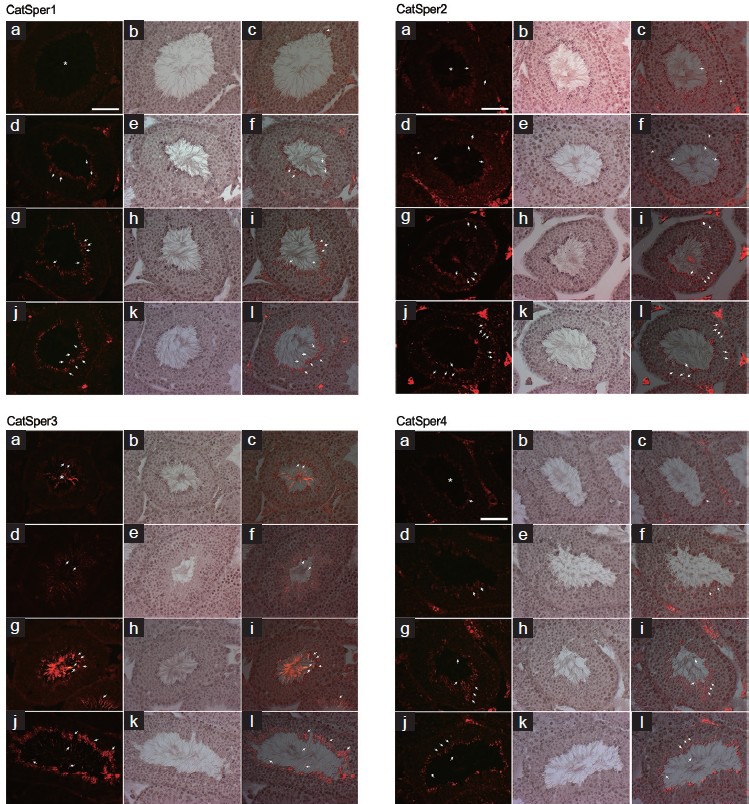
Effect of Panax ginseng on CatSper protein levels based on immunofluorescence with hematoxylin staining. CatSper1, 2, 3, and 4 levels are the results from normal (N) and P. ginseng-treated (100, 500 and 1000 mg kg−1) groups. CatSper proteins had a positive rounded form (a, d, g, j) in the mouse testes. All samples were counterstained with hematoxylin (b, e, h, k). Overlaid CatSper immunofluorescence and hematoxylin-counterstained images showing induction of CatSper proteins (c, f, i, l). In each column, a-c is the normal group and d-f, g-i and j-l are the P. ginseng-treated groups (100, 500 and 1000 mg kg−1), respectively. Images were obtained at a magnification of ×40. Scale bars = 50 μm.
DISCUSSION
During fertilization, sperm require high-amplitude flagellar bends associated with hyperactivation to penetrate the oocyte zona pellucida.29,30 Hyperactivated motility is characterized by asymmetrical flagellar bends of high amplitude and lower frequency, revealed as the swimming pattern shown by most spermatozoa.2,10,31,32 The initiation and maintenance of hyperactivated motility is related to an increase in Ca2+ concentration in the flagellum.8,33,34,35 Ca2+ signaling in sperm is important during fertilization. Ca2+ uptake is a process whereby mammalian sperm gain the capacity to undergo the acrosome reaction and fertilize oocytes.13,36 Motility activation occurs when sperm are released from the cauda epididymis. Flagellar Ca2+ levels during capacitation induce hyperactivation34,37 by increasing the amplitude of the principal flagellar bend, which produces asymmetrical beating.8 Intracellular Ca2+ levels were shown to regulate sperm motility and hyperactivation, capacitation and the acrosome reaction and were regulated by Ca2+ chelators, such as BAPTA.34,35,38,39,40,41 BAPTA reduced the elevation of Ca2+ and hyperactivation.42 Moreover, BAPTA-treated sperm had a lower VCL and smaller ALH compared with the normal group.43 In this study, in the presence of BAPTA, the sperm motility parameters were improved with P. ginseng treatment, as estimated using the CASA system. P. ginseng treatment induced sperm motility in male mice. The sperm motility and related parameters were increased significantly with P. ginseng treatment compared with the normal group. The epididymal sperm motility and subsequent parameters in mice treated with BAPTA decreased significantly compared to the normal group. Furthermore, mice treated with P. ginseng showed increased sperm motility parameters compared to the BAPTA (10 mmol l−1) control group. These results suggest that P. ginseng not only increased sperm motility but also promoted VCL and ALH hyperactivation (Figure 1a-c). In addition, intracellular Ca2+ levels were measured by a Fluoroskan Ascent Microplate Fluorometer after sperm were isolated from the ductus deferens and cauda epididymis. Sperm cell Ca2+ levels of the P. ginseng-treated groups were increased compared to the normal group (Figure 1d). Therefore, Ca2+ was important for sperm motility and hyperactivation. Moreover, calcium chelator treatment decreased the apparent sperm motility and hyperactivation. Therefore, increasing the concentration of calcium is critical for maintaining high sperm motility and hyperactivation. Furthermore, compared to the normal group, all P. ginseng concentrations significantly increased the calcium concentration, even in the presence of BAPTA.
CatSper is a cation-channel plasma membrane protein necessary for normal sperm motility, in addition to sperm penetration of the zona pellucida.15 CatSpers1–4 proteins are found only in the testes and are localized to the principal component of the flagellum.15,19,30,44 CatSper1, 3 and 4 are restricted to late-stage germ-line cells (spermatids) in the testes, while CatSper2 transcription begins during the early stages of spermatogenesis (pachytene spermatocytes).15,17,19,44 CatSper3 and 4 proteins are necessary for hyperactivated sperm motility during capacitation. Moreover, CatSpers1–4 form a tetramer cation channel, which is required for the development of hyperactivated motility during sperm capacitation in the female reproductive tract.45 CatSpers1–4-null mice have normal testicular histology, epididymal sperm counts and sperm morphology, indicating normal progression of spermatogenesis. By contrast, the phenotype of all CatSpers1–4-null mice is complete male infertility.46,47
The pharmacological effects of Korean P. ginseng, as demonstrated by modern science, include enhancement of immune system function, liver function and sexual function.48,49 To investigate the effects of P. ginseng on CatSper expression, we performed real-time PCR and Western blotting. CatSpers1–4 mRNA levels in the P. ginseng groups were increased significantly compared with the normal group (Figure 2). Western blot analysis was performed to determine the effect of P. ginseng on CatSpers1–4 protein levels in mouse testes. The CatSper 1, 3 and 4 protein levels in the P. ginseng groups were increased significantly in a dose-dependent manner (Figure 3). Furthermore, immunofluorescence detection with hematoxylin staining showed that the CatSper1, 3 and 4 protein levels were higher in the testes of P. ginseng-treated mice (Figure 4), whereas the CatSper2 mRNA level was increased, but the protein level remained unchanged. We believe that this is because CatSper1, 3 and 4 are expressed during the late stage of spermatogenesis (spermatids) in the testes, while CatSper2 transcription begins during the early stages of spermatogenesis. P. ginseng might affect the expression of the genes related to sperm motility at the late stage of spermatogenesis. These results suggest that P. ginseng treatment induces production of functional CatSper1, 3, and 4 mRNA and protein, which might be required to maintain and enhance sperm motility and hyperactivation via the VCL and ALH.
CONCLUSIONS
P. ginseng plays an important role in the improvement of sperm motility and hyperactivation via induction of CatSper expression. This suggests that P. ginseng could be used to treat reproductive dysfunction and male infertility.
AUTHOR CONTRIBUTIONS
EHP, SKP and MSC conceived of the study and participated in its design and wrote the manuscript. DRK, EHP and SKP carried out the animal studies, participated in the sperm analysis. HYK carried out the immunoassays and performed the statistical analysis. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea (No. 2010-0013296).
REFERENCES
- 1.Wassarman PM. Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis and fusion. Cell. 1999;96:175–83. doi: 10.1016/s0092-8674(00)80558-9. [DOI] [PubMed] [Google Scholar]
- 2.Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122:519–26. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- 3.Mortimer ST, Mortimer D. Kinematics of human spermatozoa incubated under capacitating conditions. J Androl. 1990;11:195–203. [PubMed] [Google Scholar]
- 4.Davis RO, Niswander PW, Katz DF. New measures of sperm motion. I. Adaptive smoothing and harmonic analysis. J Androl. 1992;13:139–52. [PubMed] [Google Scholar]
- 5.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–22. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 6.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, et al. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–75. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–17. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 9.Lindemann CB, Goltz JS. Calcium regulation of flagellar curvature and swimming pattern in triton X-100 – Extracted rat sperm. Cell Motil Cytoskeleton. 1988;10:420–31. doi: 10.1002/cm.970100309. [DOI] [PubMed] [Google Scholar]
- 10.Suárez SS, Osman RA. Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol Reprod. 1987;36:1191–8. doi: 10.1095/biolreprod36.5.1191. [DOI] [PubMed] [Google Scholar]
- 11.Tash JS, Means AR. Ca2+ regulation of sperm axonemal motility. Methods Enzymol. 1987;139:808–23. doi: 10.1016/0076-6879(87)39128-1. [DOI] [PubMed] [Google Scholar]
- 12.White DR, Aitken RJ. Relationship between calcium, cyclic AMP, ATP and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res. 1989;22:163–77. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- 13.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- 14.Tash JS, Means AR. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod. 1982;26:745–63. doi: 10.1095/biolreprod26.4.745. [DOI] [PubMed] [Google Scholar]
- 15.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–9. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, et al. CatSper1 required for evoked Ca2+entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. 2003;100:14864–8. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci U S A. 2001;98:12527–31. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol. 2003;1:53. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin JL, O’Doherty AM, Wang S, Zheng H, Sanders KM, et al. Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol Reprod. 2005;73:1235–42. doi: 10.1095/biolreprod.105.045468. [DOI] [PubMed] [Google Scholar]
- 20.Li HG, Ding XF, Liao AH, Kong XB, Xiong CL. Expression of CatSper family transcripts in the mouse testis during post-natal development and human ejaculated spermatozoa: relationship to sperm motility. Mol Hum Reprod. 2007;13:299–306. doi: 10.1093/molehr/gam009. [DOI] [PubMed] [Google Scholar]
- 21.Brekhman II. Leningrad: gosudaarst Isdat et Lit Med; 1957. Panax ginseng. [Google Scholar]
- 22.Park WS, Shin DY, Kim do R, Yang WM, Chang MS, et al. Korean ginseng induces spermatogenesis in rats through the activation of cAMP-responsive element modulator (CREM) Fertil Steril. 2007;88:1000–2. doi: 10.1016/j.fertnstert.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Connolly CM, Dearth AT, Braun RE. Disruption of murine Tenr results in teratospermia and male infertility. Dev Biol. 2005;278:13–21. doi: 10.1016/j.ydbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Hatakeyama S, Sugihara K, Lee SH, Nadano D, Nakayama J, et al. Enhancement of human sperm motility by trophinin binding peptide. J Urol. 2008;180:767–71. doi: 10.1016/j.juro.2008.03.185. [DOI] [PubMed] [Google Scholar]
- 25.Saiki RK, Bugawan TL, Horn GT, Mullis KB, Erlich HA. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163–6. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Florin A, Maire M, Bozec A, Hellani A, Chater S, et al. Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology. 2005;146:1532–40. doi: 10.1210/en.2004-0834. [DOI] [PubMed] [Google Scholar]
- 28.Sati L, Seval-Celik Y, Demir R. Lung surfactant proteins in the early human placenta. Histochem Cell Biol. 2010;133:85–93. doi: 10.1007/s00418-009-0642-9. [DOI] [PubMed] [Google Scholar]
- 29.Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod. 1995;53:1280–5. doi: 10.1095/biolreprod53.6.1280. [DOI] [PubMed] [Google Scholar]
- 30.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A. 2003;100:14869–74. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demott RP, Suarez SS. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992;46:779–85. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- 32.Katz DF, Vanagimachi R. Movement characteristics of hamster spermatozoa within the oviduct. Biol Reprod. 1980;22:759–64. doi: 10.1095/biolreprod22.4.759. [DOI] [PubMed] [Google Scholar]
- 33.Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci U S A. 1993;90:4660–4. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–15. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 35.Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem. 2005;280:32238–44. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- 36.Handrow RR, First NL, Parrish JJ. Calcium requirement and increased association with bovine sperm during capacitation by heparin. J Exp Zool. 1989;252:174–82. doi: 10.1002/jez.1402520209. [DOI] [PubMed] [Google Scholar]
- 37.Suarez SS, Dai X. Intracellular calcium reaches different levels of elevation in hyperactivated and acrosome-reacted hamster sperm. Mol Reprod Dev. 1995;42:325–33. doi: 10.1002/mrd.1080420310. [DOI] [PubMed] [Google Scholar]
- 38.Kirkman-Brown JC, Barratt CL, Publicover SJ. Nifedipine reveals the existence of two discrete components of the progesterone-induced [Ca2+]i transient in human spermatozoa. Dev Biol. 2003;259:71–82. doi: 10.1016/s0012-1606(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 39.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–57. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 40.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–8. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 42.Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod. 2007;76:660–5. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- 43.Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol. 2007;303:214–21. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin J, Jin N, Zheng H, Ro S, Tafolla D, et al. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod. 2007;77:37–44. doi: 10.1095/biolreprod.107.060186. [DOI] [PubMed] [Google Scholar]
- 46.Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- 47.Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol. 2008;52:607–13. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bittles AH, Fulder SJ, Grant EC, Nicholls MR. The effect of ginseng on lifespan and stress responses in mice. Gerontology. 1979;25:125–31. doi: 10.1159/000212330. [DOI] [PubMed] [Google Scholar]
- 49.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–18. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]


