Abstract
For more than 70 years, it has been believed that a severe reduction of serum androgen levels caused regression of prostate cancer (PCa) and that increasing androgen levels enhanced growth of PCa. However, numerous recent studies have questioned this traditional belief. In our study, LNCaP and MDA PCa 2b PCa cells were treated with various levels of androgens for 10 or 20 days, and the cell growth was measured with crystal violet mitogenic assay. The results indicated that the effect of androgens on the proliferation of PCa cells occurs in a biphasic pattern, with the androgen levels promoting optimal cell growth at approximately 0.23 ng ml−1 for LNCaP cells and between 1 and 2 ng ml−1 for MDA PCa 2b cells. Both of the optimal androgen levels are within the adult men's physiological low range (<2.4 ng ml−1). At lower concentrations than the optimal androgen level, increasing androgen concentration promoted the proliferation of PCa cells. However, at the higher concentrations, increasing androgen concentration resulted in a dose-dependent proliferative inhibition. We conclude that physiologically normal levels of androgen inhibit the proliferation of PCa cells in vitro. However, at very low levels androgens are essential for initial growth of PCa cells.
Keywords: androgen, proliferation, prostate cancer, testosterone
INTRODUCTION
In 1941, Huggins and Hodges1 published two landmark articles reporting the relationship of testosterone (T) and prostate cancer (PCa). Since then, it has been believed that severe reductions in serum T cause regression of PCa and increases in T levels enhance growth of PCa.
Several recent clinical studies examining the use of testosterone replacement therapy (TRT) in hypogonadal men with a history of PCa have shown an improvement in serum T levels without a corresponding increase in prostate specific antigen. To date, in these studies a total of 177 patients have been treated with TRT after radical prostatectomy (RP), and tumor has recurred in <1%.2,3,4 This recurrence rate is far less than the expected natural recurrence of the disease, which is roughly15%–40%.5,6 The recurrence rates in these series of men being treated with TRT after treatment of PCa are even lower than in patients with favorable pathology after RP and not treated with TRT.7 The significantly low rate of PCa recurrence and progression in those men being treated with TRT after RP leads to the question of whether T supplementation could offer a protective effect in men with a history of PCa.
In recent years, numerous studies have reported conclusions different from those originally presented by Huggins and Hodges. Current literature suggests that men with lower T levels not only are more likely to have PCa but also to have more aggressive PCa. There are also data to suggest that androgens may have a beneficial effect on PCa by promoting a less aggressive phenotype.8,9,10,11,12,13,14,15 A review of the original article by Huggins and Hodges16 shows that their conclusion regarding “enhanced growth” was based on a single patient and use of the acid phosphatase test which has since been abandoned because it provides erratic results. Today, the real effect of androgens on PCa remains debatable.
Our study assessed the effects of different concentrations of androgen on the proliferation of PCa cells in vitro, with designated experiments focusing on comparing the physiologically low and normal androgen levels in more physiological conditions. In this study, the experiments were done mainly with two original androgen receptor (AR) positive PCa cell lines, LNCaP17 and MDA PCa 2b18, along with AR negative cell lines, PC3 and DU145, as controls.
MATERIALS AND METHODS
Cell culture
LNCaP (ATCC, CRL-1740), PC3 (ATCC, CRL-1435) and Du145 (ATCC, HTB-81) cells were cultured in RPMI 1640 medium (Gibco, 61870036) supplemented with 10% fetal bovine serum (FBS) (Hyclone, SH30910.03HI) and 1× Antibiotics-Antimycotic (Gibco, 15240062) in incubator (37°C, 5% CO2 atmosphere). MDA PCa 2b (ATCC, CRL-2422) cells were cultured in F-12K medium (ATCC, 30-2004) supplemented with 20% FBS. Medium was changed every 2–3 days.
Elisa assay of sex hormone level in cell culture fetal bovine serum
The T, dihydrotestosterone (DHT), and estradiol (E2) concentrations in FBS, charcoal-dextran-treated FBS (CDT-FBS), and charcoal stripped FBS (CS-FBS) used in our lab for cell culture were tested with T, DHT, and E2 Elisa kits (MyBioSouce, MBS580035, MBS366006, and MBS366008).
Cell proliferation assay
LNCaP, MDA PCa 2b, PC3, and Du145 cells were seeded in 96 well plates (Falcon, 353072) in medium as above supplemented with 10% FBS. Respectively, 8000, 10 000, and 12 000 cells of LNCaP, 10 000 cells of MDA PCa 2b, 600 cells of PC3 and 300 cells of Du145 were seeded in each well in 200 μl medium. MDA PCa 2b cells were seeded in poly-lysine coated plates. After the cells were incubated for 24 h, the medium was changed to fresh one containing various concentrations of T (Sigma, T1500) (date was designated as day 0). The cells were kept incubated and media were changed every 48 h. Parallel experiments were also done in medium supplemented with 10% CDT-FBS in the plates coated with poly-lysine. The final concentrations of exogenous T in media are 0, 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 ng ml−1, respectively (The normal range of adult men's serum T level is about 2.4–9.5 ng ml−1 Mayo Clinic). Each concentration was assayed in triplicate. The cell growth was determined by the crystal violet mitogenic assay which measures the uptake and elution of crystal violet dye by cells.19 Briefly, the cell growth was terminated at day 10 (day 20 for MDA PCa 2b cells). After being fixed in 1% glutaraldehyde (in 1 × PBS) for 15 min, the cells were stained with 0.5% crystal violet for 15 min and washed with tap water for 30 min. Then, the culture plates were air dried and 200 μl of Sorenson's solution (0.03 mol l−1 Tris buffer-50% ethanol, pH 7.0) was added to each well. The plates were shaken for 10 min at 200 rpm and an aliquot of 50 μl of eluted dye from each well was measured at 560 nm.
Similar experiments were also done with DHT (Sigma, A8380). The concentrations of exogenous DHT in the medium were 0, 0.125, 0.25, 0.5, 1, 2, 4, and 8 ng ml−1, respectively.
For dynamic change observation, another group of plates were seeded with 10 000 LNCaP cells/well and treated with various concentrations of T. The cell growth was terminated at days 0, 2, 4, 6, 8, and 10, and the relative cell number was also determined by the crystal violet mitogenic assay.
Reagent preparation
The T and DHT powder was first dissolved in 100% ethanol at the concentration of 1 mg ml−1, and then diluted with RPMI-1640 medium to make 1000× stock of each concentration. The final volume of ethanol added into each well with 200 μl medium was the same, i.e. 0.0064 μl (0.0032%). The normal range of adult men's serum androgen level is about 2.4–9.5 ng ml−1 for T; 0.11–0.96 ng ml−1 for DHT (Mayo Clinic). The concentrations of both T and DHT tested in the experiments contain at least two points in the physiological normal range and all the concentrations increased by two-fold.
Poly-lysine coated plates were used in the experiments because MDA PCa 2b cells adhere very poorly, as do the LNCaP cells when cultured in 10% CDT-FBS supplemented medium. The plates were coated with 50 μl per well of 50 μg ml−1 poly-d-lysine (in PBS) for 30 min at 37°C, and washed twice with distilled water.
Cell growth characteristic observation
To characterize the proliferation and morphological changes of LNCaP, MDA PCa 2b, PC3, and Du145 cells that were treated with different levels of T, the cells were observed under a microscope every day during the whole experiment and pictures were taken at day 6 for LNCaP, PC3, and Du145 cells and at day 14 for MDA PCa 2b cells.
We also seeded 100 000 per well LNCaP cells in poly-lysine-coated 24-well plates in regular medium. After 24 h, the medium was changed to 10% CDT-FBS supplemented medium or serum-free medium for 30 days with medium changed every week. The cell status in these media was observed under microscope every day until day 30.
Statistical analysis
Statistical significance was determined on the basis of the one-way ANOVA test. All data are shown as mean ± standard deviation (s.d.).
RESULTS
Sex hormone concentrations in fetal bovine serum
In order to determine if androgens brought to the culture medium from regular FBS would artificially elevate the reported androgen concentrations significantly, the FBS was tested by Elisa kits for its concentrations of T, DHT, and E2, the main forms of sex hormones. Table 1 demonstrates that T and DHT levels in regular FBS were 1 ng ml−1 and 0.07 ng ml−1, respectively, indicating that when regular FBS was used at 10% in culture medium, the medium had 0.1 ng ml−1 of T and 0.007 ng ml−1 of DHT, which were brought from FBS. This level is very low, and thus will not artificially cause a significant change in the androgen concentration tested, especially at men's physiologically normal levels (T 2.4–9.5 ng ml−1 and DHT 0.11–0.96 ng ml−1). E2 concentration in medium with 10% FBS is 0.034 ng ml−1, a level which will not make a big difference in the total sex hormone concentration in the medium.
Table 1.
Sex Hormone concentrations in cell culture fatal bovine serum
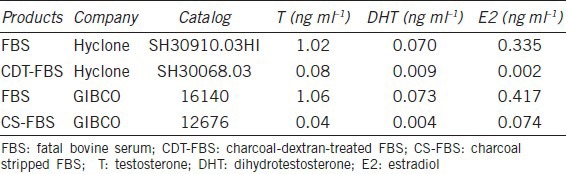
Cell proliferation assay and growth characteristic study
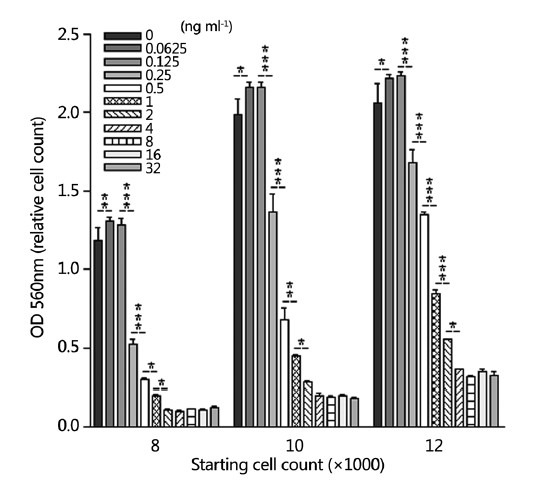
The cell culture experiment treating LNCaP cells with T for 10 days demonstrated that the effect of androgen on the proliferation of LNCaP cells has a biphasic pattern, with the optimal androgen levels for promoting cell growth at approximately 0.23 ng ml−1 (0.125 ng ml−1 of exogenous T plus 0.1 ng ml−1 of T and 7 pg ml−1 of DHT from FBS). At lower concentrations than the optimal androgen level, increasing T concentration promoted the proliferation of PCa cells. However, at the higher concentrations, increasing T concentration resulted in a dose-dependent inhibition of proliferation. LNCaP cells grew very well in the control condition (in RPMI-1640 medium supplemented with 10% regular FBS). When the exogenous T level was raised from 0 to 0.0625 and 0.125 ng ml−1, there was greater proliferation of cells than in the control group. However, when the T level was raised to 0.25 ng ml−1 and greater, T showed a very clear inhibitory effect on the proliferation of LNCaP cells, in all the experiments starting with 8000, 10 000, and 12 000 cells per well. The inhibition was in a dose-dependent fashion (Figure 1). We observed the cells from day 0 to day 10 and we found that when treated with T, in the first 2 days, the LNCaP cells continued to proliferate as untreated cells. After that, the growth slowed down, and after 4 days, the growth stopped in high T groups (>2 ng ml−1) (Figure 2).
Figure 1.
Effect of testosterone on the growth of LNCaP cells in regular medium. Eight thousand, ten thousand, or twelve thousand cells per well were seeded in medium supplemented with 10% regular fetal bovine serum and the cells were treated with various levels of testosterone for 10 days. The stars represent the statistic difference between each two groups. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
Growth curve of testosterone treated LNCaP cells in medium supplemented with 10% regular fetal bovine serum from day 0 to day 10. Start with 10 000 cells per well.
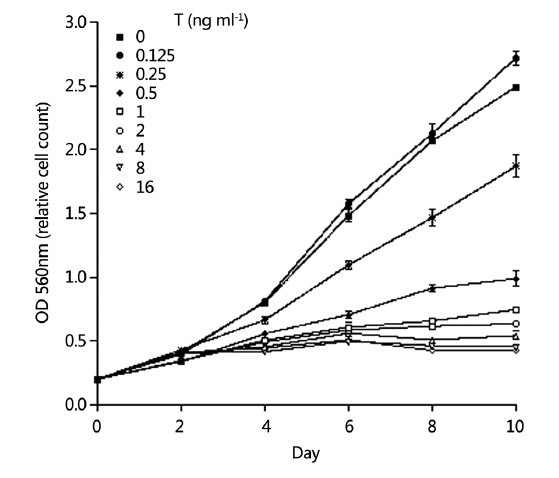
When parallel experiments were performed in the medium supplemented with 10% CDT-FBS, similar growth inhibitory effects of T on LNCaP cells were also demonstrated. However, the cells grew much more slowly in medium supplemented with CDT-FBS. It was also noted that in regular FBS-supplemented medium, the optimal growth condition was at 0.0625 and 0.125 ng ml−1 of T, while in medium supplemented with CDT-FBS, the optimal growth condition was shifted to 0.25 and 0.5 ng ml−1 of T (Figure 3a).
Figure 3.
Effect of testosterone (T) and dihydrotestosterone on the growth of LNCaP and MDA PCa 2b cells in different culture conditions. Ten thousand cells were seeded in each well. LNCaP cells were treated for 10 days and MDA PCa 2b cells were treated for 20 days. The stars represent the statistic difference between each two groups. *P < 0.05, **P < 0.01, ***P < 0.001.
DHT had an effect similar to that of T on the proliferation of LNCaP cells. The cells had optimal growth at 0.125 ng ml−1 of DHT in medium with regular FBS. When the DHT concentration was increased, there was a growth inhibitory effect which occurred also in a dose-dependent fashion. In CDT-FBS medium, cells grew much more slowly and the growth-inhibitory effect was shown at 0.5 ng ml−1 and higher concentrations (Figure 3b).
Similar effects of T and DHT were also shown on MDA PCa 2b cells after 20 days of treatment although these cells grew much more slowly than LNCaP cells. In regular medium, the cells demonstrated increased growth when exogenous T was raised from 0 to 1 ng ml−1, or exogenous DHT was raised from 0 to 0.25 ng ml−1. The growth-inhibitory effect was shown starting at 4 ng ml−1 T and 1 ng ml−1 DHT levels. In medium supplemented with CDT-FBS, similar effects were demonstrated although the effects were not as prominent as those in regular medium (Figure 3c and 3d).
The results of the experiments described above indicated that the optimal androgen levels for the growth of LNCaP cells are approximately 0.23 ng ml−1 (0.125 ng ml−1 of exogenous T plus 0.1 ng ml−1 of T and 7 pg ml−1 of DHT from FBS) and between 1 and 2 ng ml−1 for MDA PCa 2b cells. Although the optimal androgen level for growth for the two different PCa cell lines differ, both fall within the adult men's physiological low range (<2.4 ng ml−1).
Morphological changes were observed every day when LNCaP and MDA PCa 2b cells were treated with different levels of T. Compared with controls and in high T conditions, the cells showed signs of very active proliferation in their optimal growth condition, especially for MDA PCa 2b cells after 14 days of T treatment (Figure 4).
Figure 4.
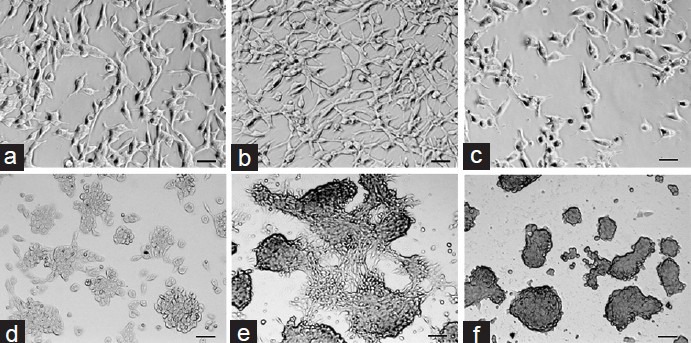
Photographs of LNCaP and MDA prostate cancer (PCa) 2b cells treated with testosterone (T) for 6 and 14 days respectively. The photos demonstrated that the PCa cells increased significantly in number and showed very active proliferation signs in their optimal growth condition (middle row, LNCaP cells in 0.1 ng ml−1 T and MDA PCa 2b in 1 ng ml−1 T). (a) LNCaP T 0 ng ml−1 day 6; (b) LNCaP T 0.1 ng ml−1 day 6; (c) LNCaP T 8 ng ml−1 day 6; (d) MDA PCa 2b T 0 ng ml−1 day 14; (e) MDA PCa 2b T 1 ng ml−1 day 14; (f) MDA PCa 2b T 8 ng ml−1 day 14. Scale bars=50 μm.
After LNCaP cells cultured in regular medium were shifted to 10% CDT-FBS-supplemented medium or serum free medium, which had an extremely low level or no androgen, the cell number did not change significantly until day 30. This demonstrated that the cells did not undergo apoptosis without androgen. These findings suggest that with enough nutrients other than androgen, PCa cells will continue to survive, though without growth.
Parallel experiments were also performed with AR negative PC3 and Du145 cells for all of the above assays and studies, but no changes were shown on the growth of PC3 and DU145 cells when concentrations of T in the culture media were changed from 0 to 32 ng ml−1, thus indicating that androgens have no effect on the proliferation of AR negative cells (Figure 5).
Figure 5.
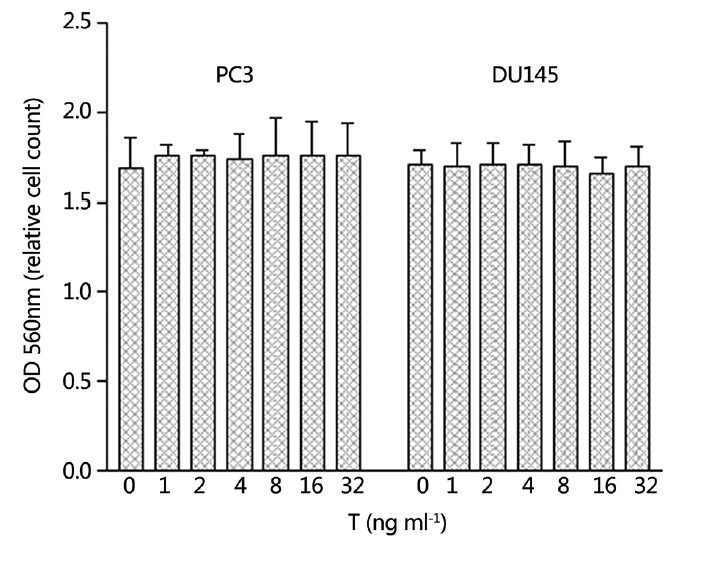
Six hundred PC3 and three hundred Du145 cells per well were seeded in medium supplemented with 10% regular fetal bovine serum and the cells were treated with various levels of testosterone (T) for 10 days. T had no effect on the growth of either PC3 or Du145 cells.
DISCUSSION
Most PCa originates from prostatic epithelial cells, and the cancer cells are AR positive. There have been only a few AR positive PCa cell lines available until now, with LNCaP being the most commonly used. Since the LNCaP cell line was established in 1980, it has been considered to represent a useful tool to explore the mechanism of sex hormone action on cell proliferation in an “in culture-in animal” model. The investigators in the lab where LNCaP cell line was established studied the effect of DHT on the growth of LNCaP cells in vitro. They reported the stimulatory effect of DHT on the growth of LNCaP cells in media supplemented with CS-FBS and they found that the presence of complete FBS prevented the demonstration of DHT growth-stimulatory effects.20
For several decades, CS-FBS or CDT-FBS has been required in the cell culture medium for studies related to hormones, growth factors, and steroids and their receptors, to reduce the concentration error of targeted factors. On the basis of our results, we suggest that CDT-FBS supplemented medium should be avoided if concentrations of targeted factors are not significantly elevated artificially by using regular medium. Charcoal and dextran absorb away not only the targeted factors, but also most hormones, growth factors, and other nutrients, causing the cell culture conditions to be far different from the physiological one and thus subject to production of erroneous results. Therefore, it is important to test the level of targeted factors in regular FBS to determine if it is necessary to use CDT-FBS in the experiments. PCa cells have a weakly adherent culture property, and we found that when they are cultured in medium supplemented with CDT-FBS, they adhere even more poorly to the culture plate. They are easily detached and many cells wash away during medium changes, also producing some errors. Moreover, all the cells grew very slowly in medium supplemented with CDT-FBS; a result which we believe is a suboptimal condition for studying the growth effect of chemicals. We seeded the PCa cells in poly-lysine coated culture plates to improve their adherence and were extremely careful not to wash away the cells when the experiments were done with CDT-FBS supplemented medium. Our results demonstrated similar growth-inhibitory effects of androgen in both regular FBS medium and CDT-FBS medium, although the effect in CDT-FBS condition is less apparent than in regular FBS medium.
Our data suggests that the optimal set of concentrations of androgens tested should be positioned within the physiological range and include at least two points within each physiological range, low, normal, and high. We found in almost all the articles reporting the effect of androgens on PCa cells, the tested androgen concentrations increased by tenfold, which resulted in tested concentrations having only one point inside the physiological normal range (2.4–9.5 ng ml−1 for T) and thus missed some information when the concentration changed inside the normal range.20,21,22 Our data showed a dose-dependent inhibition of proliferation when the androgen's concentration increased in the physiological normal range. We also suggest that to test the effects of androgens on the growth of PCa cells, the period of experiment should be longer than 8 days for LNCaP cells and 18 days for MDA PCa 2b cells. We observed not only that PCa cells grow slowly, especially the MDA PCa 2b cells, but also that when treated with various levels of androgen, in the first couple days, the cells in the high androgen groups grew very well as did those in the low androgen group. The difference became apparent only after certain days.
Our results indicated that physiologically low levels of androgen are essential for PCa cell's growth, but normal and high levels inhibit its proliferation. This conclusion may explain the minimal PCa recurrence rates in those men being treated with TRT after PCa. It also can help explain why androgen deprivation therapy (ADT) (castration or anti-androgen treatment) can cause PCa to regress but in many cases recur in just 12–33 months after ADT.6 When androgen levels rise from extremely low levels to low levels after certain period after ADT, the tumor will recur. That prolonged treatment with anti-androgen drugs following castration will delay the recurrence is a fact that supports our hypothesis, since the follow-up treatment keeps the serum androgen level at an extremely low level.23
Our experiments were done with original PCa cell lines in regular cell culture conditions, and the data clearly indicated the inhibitory effect of androgen on the proliferation of these natural PCa cell lines at the androgen's physiologically normal range of adult men, while the low level stimulated the growth. The optimal androgen level for PCa cells’ growth is relatively low but is inside the physiologically low range, although the optimal level may vary for different cancer cell lines or for different individuals. This study suggests that raising serum T level to a physiologically normal range or to certain levels above the optimal growth level may be a potential option for the treatment of PCa. Clearly further basic science and clinical studies are needed to validate these findings.
CONCLUSIONS
Data indicated that PCa cells will not grow without androgen and that PCa cells will grow very slowly when androgen levels are extremely low. PCa cells will proliferate at low androgen levels, but the growth will be inhibited at normal physiological androgen levels. It is concluded that normal physiological levels of androgens inhibit the proliferation of PCa cells in vitro, while low levels of androgens are essential for the growth.
AUTHOR CONTRIBUTIONS
Both authors were responsible for conception, experimental design, and drafting the manuscript and intellectual content. WS was in charge of performing experiments, data acquisition and analysis. Both authors read and approved the final manuscript.
COMPETING INTERESTS
Dr. Weitao Song has no conflicts of interest. Dr. Mohit Khera is consultant for Merck, Lilly, and Auxilium.
ACKNOWLEGMENTS
The authors thank for the help in editing of the manuscript by Dr. Carolyn Schum, senior editor of Scott Department of Urology, Baylor College of Medicine.
REFERENCES
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. [Google Scholar]
- 2.Khera M, Grober ED, Najari B, Colen JS, Mohamed O, et al. Testosterone replacement therapy following radical prostatectomy. J Sex Med. 2009;6:1165–70. doi: 10.1111/j.1743-6109.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 3.Morgentaler A. Testosterone therapy for men at risk for or with history of prostate cancer. Curr Treat Options Oncol. 2006;7:363–9. doi: 10.1007/s11864-006-0004-y. [DOI] [PubMed] [Google Scholar]
- 4.Rhoden EL, Averbeck MA. Testosterone therapy and prostate carcinoma. Curr Urol Rep. 2009;10:453–9. doi: 10.1007/s11934-009-0072-1. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 6.Chuu CP, Kokontis JM, Hiipakka RA, Fukuchi J, Lin HP, et al. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J Biomed Sci. 2011;18:63. doi: 10.1186/1423-0127-18-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Oort IM, Kok DE, Kiemeney LA, Hulsbergen-van de Kaa CA, Witjes JA. A single institution experience with biochemical recurrence after radical prostatectomy for tumors that on pathology are of small volume or “insignificant”. Urol Oncol. 2009;27:509–13. doi: 10.1016/j.urolonc.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol. 2000;163:824–7. [PubMed] [Google Scholar]
- 9.Lane BR, Stephenson AJ, Magi-Galluzzi C, Lakin MM, Klein EA. Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology. 2008;72:1240–5. doi: 10.1016/j.urology.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68:1263–7. doi: 10.1016/j.urology.2006.08.1058. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro M, Ruff P, Falkson G. Low serum testosterone and a younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol. 1997;20:605–8. doi: 10.1097/00000421-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Sofikerim M, Eskicorapci S, Oruç O, Ozen H. Hormonal predictors of prostate cancer. Urol Int. 2007;79:13–8. doi: 10.1159/000102906. [DOI] [PubMed] [Google Scholar]
- 14.Teloken C, Da Ros CT, Caraver F, Weber FA, Cavalheiro AP, et al. Low serum testosterone levels are associated with positive surgical margins in radical retropubic prostatectomy: hypogonadism represents bad prognosis in prostate cancer. J Urol. 2005;174:2178–80. doi: 10.1097/01.ju.0000181818.51977.29. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto S, Yonese J, Kawakami S, Ohkubo Y, Tatokoro M, et al. Preoperative serum testosterone level as an independent predictor of treatment failure following radical prostatectomy. Eur Urol. 2007;52:696–701. doi: 10.1016/j.eururo.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Morgentaler A. Destroying the myth about testosterone replacement and prostate cancer. Life Ext. 2008:12. [Google Scholar]
- 17.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, et al. The LNCaP cell line – A new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–32. [PubMed] [Google Scholar]
- 18.Navone NM, Olive M, Ozen M, Davis R, Troncoso P, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–500. [PubMed] [Google Scholar]
- 19.Gillies RJ, Didier N, Denton M. Determination of cell number in monolayer cultures. Anal Biochem. 1986;159:109–13. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 20.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–18. [PubMed] [Google Scholar]
- 21.Sonnenschein C, Olea N, Pasanen ME, Soto AM. Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res. 1989;49:3474–81. [PubMed] [Google Scholar]
- 22.Arnold JT, Le H, McFann KK, Blackman MR. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am J Physiol Endocrinol Metab. 2005;288:E573–84. doi: 10.1152/ajpendo.00454.2004. [DOI] [PubMed] [Google Scholar]
- 23.Seruga B, Tannock IF. Intermittent androgen blockade should be regarded as standard therapy in prostate cancer. Nat Clin Pract Oncol. 2008;5:574–6. doi: 10.1038/ncponc1180. [DOI] [PubMed] [Google Scholar]


