Abstract
Adiponectin secreted by adipose tissue has been implicated in prostate carcinogenesis. Genetic variations in ADIPOQ are thought to influence the activity of adiponectin, thus relating to cancer occurrence. In this hospital-based case-control study of 917 prostate cancer (PCa) cases and 1036 cancer-free controls, we evaluated the association of single nucleotide polymorphisms in ADIPOQ with risk of PCa and adiponectin levels in Chinese Han men. Variants of ADIPOQ were genotyped by Taqman polymerase chain reaction method. The plasma adiponectin concentrations were measured by enzyme-linked immunosorbent assay (ELISA) in a subset of cases and controls. We found that the ADIPOQ rs3774262 variant AA genotype was associated with both decreased PCa risk [adjusted odds ratio (OR): 0.66, 95% confidence interval (CI) =0.48–0.92] and increased plasma adiponectin levels (P = 0.036 and 0.043), with significant difference by tumor grade, clinical stage, and aggressiveness. A significant interaction between ADIPOQ rs3774262 and body mass index was observed in modifying the risk of PCa (P = 6.7 × 10−3). ADIPOQ rs266729 and rs182052 were not related to PCa risk or plasma adiponectin levels. Our data support that ADIPOQ rs3774262 may affect PCa risk in combination with plasma adiponectin levels in Chinese Han men. It may contribute to the molecular basis for the association between obesity and PCa.
Keywords: adiponectin, ADIPOQ, polymorphism, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the second most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality among men worldwide.1 Large geographical variations in PCa risk suggest that lifestyle and environmental factors may also contribute to its etiology. In particular, it has been reported that overweight and obese individuals in populations across the Asia-Pacific region had a significantly increased risk of mortality from PCa.2
Many studies have investigated the roles of adipose tissue-derived factors (adipokines) as putative molecular mediators between obesity and PCa. As the most abundantly circulating adipokine, levels of circulating adiponectin are negatively correlated with central obesity, body mass index (BMI), visceral fat accumulation, and insulin resistance.3 Because adiponectin levels are inversely correlated with adiposity, it has been suggested that increased levels of adiponectin may explain the decreased risk of PCa,4,5 lower Gleason score,4,5,6,7 and early tumor stage.4,5,6 Several single nucleotide polymorphisms (SNPs) in ADIPOQ encoding adiponectin have been shown to be associated with PCa risk in Caucasians,8 of which rs266729 had been previously shown to be associated with risk of multiple cancers.9,10,11,12 A nested case-control study further confirmed that rs266729 and rs182052 were associated with not only PCa risk, but also plasma adiponectin levels in Caucasians.13 Recently, another SNP (rs3774262) in ADIPOQ was reported to be associated with both reduced cancer risk and obesity measurements in Chinese populations.14
In the current case–control study, we investigated the associations between three SNPs in the ADIPOQ gene and PCa risk in Chinese Han men. We also assessed whether plasma adiponectin levels were correlated with these genotypes as potential risk mediators between ADIPOQ genetic variations and PCa risk in two subgroups of subjects.
MATERIALS AND METHODS
Study population
We identified 1000 Chinese Han eligible patients with newly diagnosed and histopathologically confirmed primary PCa from Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China) between January 2007 and June 2011; 917 of them (92%) agreed to participate in this study. All patients came from Eastern China. The tumors were histopathologically confirmed as primary PCa assessed independently by two pathologists as routine diagnosis at FUSCC. All pathologic diagnoses were performed according to the World Health Organization (WHO) criteria of PCa, and any histologic diagnosis other than adenocarcinoma subtype of PCa was excluded. As previously described, exclusion criteria also included those cases who suffered from malignancies other than PCa, with a family history of PCa and those had radiotherapy or chemotherapy before recruitment.15 In the present study, PCa were divided into two categories of highly aggressive and less aggressive as defined by the prostate-specific antigen (PSA) levels, pathological staging, and Gleason score. Tumors with PSA serum level >20 ng ml−1, Gleason score of 7 (4 + 3), pathological stages T3 or higher were defined as highly aggressive disease, and the remainder were defined as less aggressive disease.16 The clinical information, including Gleason score, PSA level at diagnosis, staging and use of medications was determined by reviewing the medical records. During an in-person survey, all cases were interviewed with a questionnaire that collected information about demographic data and potential PCa risk factors including smoking status, occupational exposure, and family history of cancer. Nine hundred and seventeen anthropometric measurements, including weight and height were taken after the interview according to a standardized protocol.
A total of 1151 cancer-free ethnic Han Chinese control subjects were randomly selected from the Taizhou Longitudinal Study during a similar time period17 and those without response were excluded (n = 104). Frequency matched to cases by age (±5 years) and residence (urban or rural areas). The interviewer-administered questionnaire covered demographic characteristics and environmental exposure factors (e.g. smoking status and use of medications).17 The Institutional Review Board of FUSCC approved this study, and a written informed consent was obtained from all recruited individuals.
Adiponectin measurement
For laboratory tests, overnight fasting blood samples were provided by the Institutional Tissue Bank at FUSCC (for cases). After the questionnaire interview and physical measurements, 10 ml of overnight fasting blood samples were collected for long-term storage in Taizhou Longitudinal Study (for controls).17 For a subset of 305 cases and 330 matched controls who had genotype data during a similar time period, plasma adiponectin levels were measured with commercial enzyme-linked immunosorbent assay (ELISA) kit (Abcam Adiponectin Human ELISA Kit, USA) according to the manufacturer's manuals. The minimum detectable dose was 0.7 ng ml−1. The intra- and inter-assay coefficients of variation were 4.5% and 8.7%, respectively.
Single nucleotide polymorphism selection and genotyping
Three SNPs in the ADIPOQ gene (rs266729, rs182052, and rs3774262) were selected in an effort to compare results in this investigation with the findings from the published studies of prostate,8,13 colorectal,10 and endometrial cancers.14 These SNPs were originally selected based on their ability to tag the major haplotype blocks in ADIPOQ gene, a minor allele frequency of >5% among samples in their study. We chose these significant cancer risk associated SNPs with preferential selection given to SNPs with functional relevance (e.g. plasma adiponectin levels or obesity indicators).
Methods used for DNA isolation and genotyping have been described previously.18 Briefly, DNA isolation was performed by using the Qiagen Blood DNA Mini KIT (Qiagen Inc., Valencia, CA, USA) with the buffy-coat fraction of the blood samples donated by the participants. The quantification of DNA was determined by a Hybrid Reader (Synergy H4, BioTek Instruments, Inc., USA), and the final concentration of DNA used for genotyping was 2.5 ng ml−1. The TaqMan real-time polymerase chain reaction (PCR) amplification was run with a 7900 HT sequence detector system (Applied Biosystems, Foster City, CA, USA), and the genotypes were determined with the Sequence Detection Software (SDS2.4). The TaqMan genotyping master mix and predesigned primers and probes for each SNP were purchased from ABI (Applied Biosystems). To ensure the accuracy of genotyping results, each 384-well plate included four negative controls (no DNA), and four random-duplicated samples with genotype call rates >98%. Genotyping was randomly repeated in 10% of samples to check for the typing reliability, and the results were 100% in agreement.
Statistical methods
In the present study, we used the WHO cut points for overweight (BMI ≥25 kg m−2) in Asian populations.19 BMI was calculated as weight (kg) divided by the square of the height (m). Smoking status was divided into smokers and never smokers by whether or not they had smoked for more than 100 cigarettes in their lifetime. Regular use frequency of aspirin or aspirin-containing products was defined as ≥1 pill daily.20 Men were categorized as statin users if they were taking statins before diagnosis and those who did not have statins use information were considered nonusers.21
Pearson's x2 test was used to evaluate differences in the distributions of the selected demographic characteristics and the ADIPOQ genotype frequencies between cases and controls. Hardy-Weinberg equilibrium (HWE) for evaluation of genotype distribution of control subjects was performed by the goodness-of fit x2 test and the linkage disequilibrium (LD) coefficient r2 was assessed. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by using univariate and multivariate logistic regression models, respectively. Associations between the genotypes and risk of PCa among subgroups of age, BMI, smoking status, Gleason score, tumor staging, and aggressive grade were further evaluated by stratification analysis. The homogeneity of associations between subgroups was tested by using the Chi-square-based Q test. Haplotype frequencies were estimated using SAS PROC HAPLOTYPE process based on the observed genotypes. P values from the analysis of variance (ANOVA) were used to compare the plasma adiponectin levels by different genotypes.
Two-factor interaction analyses were also carried out by unconditional logistical regression to assess the interactions between the SNPs and environmental factors. The false-positive report probability (FPRP) was calculated based on the previously calculated probability, the power of the current study, and the observed P value, with an effort to detect the false-positive association findings.22 All statistical tests were two-sided, and a P value of 0.05 was considered significant. All analyses were performed using the SAS Software, version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of the study subjects
Among all the subjects, 11 controls failed to be genotyped after repeated assays, likely due to poor quality of DNA. Thus, the final analysis included a total of 917 cases and 1036 controls. The distributions of selected demographic variables between cases and controls were shown in Table 1. Approximately 40% of participants were considered overweight, with a significant difference in the BMI between cases and controls (P < 0.001). The two groups were similar with respect to aspirin use and statin use.
Table 1.
Distribution of selected characteristics in PCa cases and controls in Chinese men
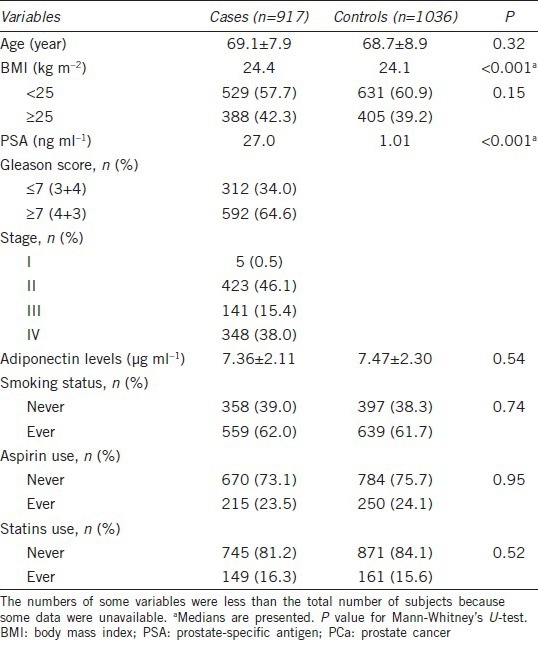
Associations between ADIPOQ single nucleotide polymorphisms and prostate cancer risk
The genotype frequencies and their associations with the risk of PCa were summarized in Table 2. The genotype frequencies of the three SNPs among the controls were all in agreement with HWE (P = 0.29 for rs266729, 0.83 for rs182052 and 0.09 for rs3774262). In multivariate logistic regression analysis, the rs3774262 was associated with PCa risk in the recessive (P = 0.01) models with adjustment for age, smoking status, and BMI. Compared with GG genotype, the rs3774262 variant AA genotype was associated with a decreased risk of PCa (OR: 0.66, 95% CI = 0.48–0.92). However, no associations with risk of PCa were observed for other two SNPs. Eight possible haplotypes based on the observed genotypes frequencies were estimated (Supplementary Table 1 (828.5KB, tif) ), there were no significant risk associations for these ADIPOQ haplotypes.
Table 2.
Associations between ADIPOQ SNPs and PCa risk in Chinese men
The frequency of haplotypes of the ADIPOQ gene and their associations with the risk of Pca in Chinese men
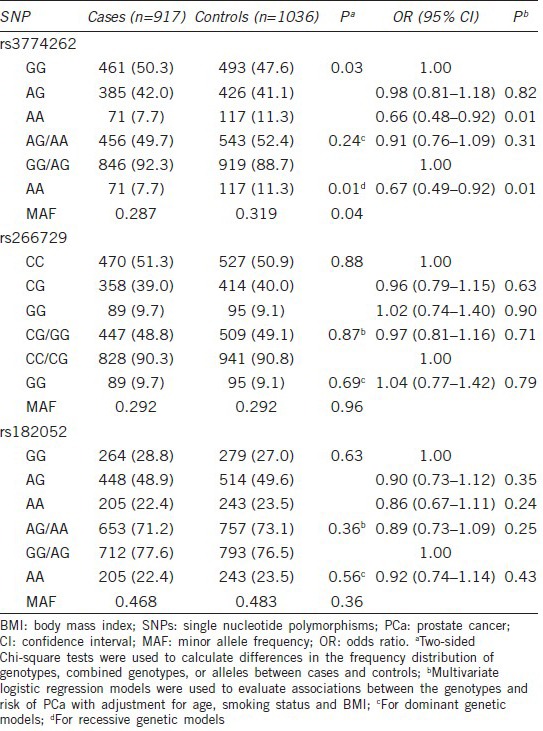
Associations between ADIPOQ single nucleotide polymorphisms and plasma adiponectin levels
We evaluated differences in adiponectin levels by genotypes in a subset of controls and cases (Table 3). rs3774262 was significantly associated with plasma adiponectin levels in ANOVA analysis (P = 0.036 and 0.043). Individuals with rs3774262 variant AA genotype had higher levels of plasma adiponectin than those with the AG or GG genotype. Considering that rs3774262 variant AA genotype was inversely associated with PCa risk, these genotype–phenotype and genotype-risk associations were in the expected inverse directions to each other, suggesting a biological causal relationship. There were no significant associations between the other two SNPs and plasma adiponectin levels.
Table 3.
Associations between ADIPOQ SNPs and adiponectin levels in Chinese men
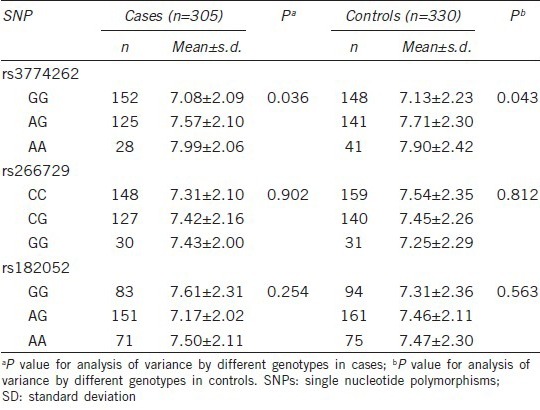
Stratification analysis
We further evaluated the associations between three SNPs and PCa risk by subgroups, assuming a recessive genetic model (Table 4). Those who carried rs3774262 variant AA genotype had a significant decreased risk, and this protective effect was more evident in normal weight subjects (OR: 0.47, 95% CI = 0.31–0.71, P = 3 × 10−4), supported by homogeneity test (P = 9 × 10−3). The decreased risk was also observed in men who were younger, never smokers, Gleason score ≥7 (4 + 3), stage III/IV, and high aggressive cancer. Further homogeneity tests suggested, however, that there were no differences in the risk estimates between these strata except for Gleason score, clinical stage, and aggressiveness.
Table 4.
Stratification analysis for associations between ADIPOQ SNPs and PCa risk by recessive genetic model in Chinese men
Gene-environment interaction analysis
To explore potential interactions of ADIPOQ rs3774262 with age, smoking status, and BMI, we performed gene-environment interaction analyses (Supplementary Table 2 (366.3KB, tif) ). Normal weight Individuals harboring the ADIPOQ rs3774262 AA genotype had a reduced cancer risk compared with overweight individuals carrying the GG genotype (P = 6.7 × 10−3), which suggesting an interaction effect between BMI and rs3774262. We did not observe any significant interaction of the genotypes with smoking status or age. To account for chance associations from multiple comparisons, the FPRP values for significant findings at different prior probability levels were shown in Supplementary Table 3 (1.1MB, tif) .
Risk of Pca associated with rs3774262 genotyps by BMI in Chinese men
False-positive report probability values for the observed associations
DISCUSSION
In this hospital-based case–control study of 917 PCa cases and 1036 cancer-free controls, we evaluated the associations between ADIPOQ SNPs (rs3774262, rs266729, and rs182052) with PCa risk and adiponectin levels in Chinese men. We found that ADIPOQ rs3774262 variant AA genotype was associated with both decreased PCa risk compared with GG or GG/AG genotypes and increased adiponectin levels in a subset of cases and controls. In addition, we observed a significant interaction between rs3774262 and BMI in modifying the risk of PCa.
There is an increasing interest in the correlation between genetic variations in the ADIPOQ gene and carcinogenesis. Kaklamani et al.8 evaluated the associations between five potential functional SNPs in ADIPOQ and PCa risk in 465 PCa patients and 411 healthy controls in a Caucasian population and reported that four of the five were risk-associated SNPs, which includes rs266729 that was evaluated in the present study. A nested case–control study from the Physicians’ Health Study found two risk-associated SNPs (rs266729 and rs182052) that were also overlapped with plasma adiponectin levels among US male physicians.13 However, we did not have the statistical evidence to support the associations of these two SNPs with PCa risk or adiponectin levels in the current study. It seems the inconsistent findings for the ADIPOQ rs266729 and rs182052 SNPs associated with PCa risk between previous and ours studies may be caused by considerably genetic differences in ethnicities.
In the Physicians’ Health Study of Caucasians origin,13 the minor allele frequency of ADIPOQ rs182052 in controls was 0.32, which is different from the observed in ours (0.47). The modest number of advanced cases in their study may also limit the statistical power to examine the genetic variants in relation to advanced PCa. In addition, the smaller study size and younger participants in Kaklamani's study may contribute to the observed differences,8 compared to ours. In other two studies, five SNPs in ADIPOQ including rs266729 were evaluated in 131 African American PCa cases,23 and four potential functional ADIPOQ SNPs including rs182052 in a cohort of Finnish men,24 but neither study yielded any associations between these two SNPs and PCa risk, a finding similar to our results. Moreover, a genome-wide association study25 and subsequent validation study11 demonstrated that ADIPOQ rs266729 and rs182052 SNPs were not associated with colorectal cancer in patients of Ashkenazi Jewish descent or other ethnic groups in Israel, which were consistent with our findings.
The results of the association between ADIPOQ rs3774262 and cancer susceptibility have not been consistent for multiple cancers nor for ethnic groups. A case–control study of 648 cases and 659 controls reported no association of ADIPOQ rs3774262 with postmenopausal breast cancer risk, BMI, adult weight gain, location of weight gain, or physical activity among US women.26 While in a study of 1028 endometrial cancer cases and 1003 controls in a Chinese population, Chen et al.14 identified three risk-associated SNPs, of which rs3774262 was in the same reported direction as in our findings, and the other two SNPs (rs1063539 and rs12629945) were in high and moderate LD (r2 = 0.84 and 0.60, respectively) with rs3774262.
ADIPOQ polymorphisms may affect PCa risk through various mechanisms involved in adiponectin. The opposing properties of adiponectin to the majority of other adipokines have resulted in its proposal as an “anticancer” adipokine with respect to PCa.3 In support of this hypothesis are the findings from case–control studies that found plasma adiponectin levels to be significantly lower in subjects with PCa compared to subjects with benign prostatic hyperplasia or healthy controls.5,6 For rs3774262, the rare A allele was associated with circulating adiponectin levels and overlapped with PCa risk in the expected opposite direction suggesting potential biological consequences. Genetic studies have previously implicated the ADIPOQ locus for a role in influencing variations in adiponectin levels. ADIPOQ rs3774261, which is in close proximity of rs3774262 (255 base pair apart) was identified as one of the top hits27 in a genome-wide association study of plasma adiponectin and replicated in several study populations.13,28,29 Our results confirmed and extended the evidence implicating adiponectin as a physiological modulator of PCa and point to genetic variations in the ADIPOQ gene as a key regulator of this effect.
Besides the main effect of ADIPOQ rs3774262, the present study also provided evidence of gene-environment interactions that were more significant than the modest effect of the single variant with a greater predictive power. In gene-environment interaction analyses and stratified analyses, we found that individuals carrying the ADIPOQ rs3774262 AA genotype presented different PCa risk in the normal weight and overweight subgroup. The interaction may have some biological plausibility. Large studies on PCa and overweight, most often measured as high BMI, have generally demonstrated a positive risk association.30 In addition, obesity could potentially influence the activation of ADIPOQ and its receptor genes and subsequent PCa risk.31,32 A positive association of ADIPOQ variants with cancer risk may be limited to persons who are overweight.33 Our observed interactions further suggest that this SNP may be associated with PCa through their complex association with obesity and might reveal a biological mediation of these factors. Lower levels of adiponectin in obese individuals may result in higher levels of prostatic oxidative stress which may explain the clinical association between obesity, hypoadiponectinemia and PCa.34
Previous studies show that low prediagnostic serum adiponectin levels were associated with metastatic and fatal PCa.3,4,5,6,7,31 Consistently, we found that the protective effect of rs3774262 AA genotype was more obvious in subgroups of Gleason score ≥7, stage III/IV and high aggressive cancer in the stratified analysis. FPRP analyses also supported that all these significant findings were noteworthy. Though some other significant findings in the stratified analysis may be by chance, because of the limited sample size in these subgroups, our data did add further evidence that ADIPOQ SNPs may play a role in high-grade or advanced stage PCa.
Our study has some inherent limitations. The present study included limited number of SNPs of the ADIPOQ gene and lack of additional rigorous replications. On the other hand, the finding that the risk-associated SNP was also linked with adiponectin levels among cases and controls argues against a chance finding. This hospital-based case-control study may have some selection and information biases, which might be minimized by frequency-matching between cases and controls as well as the adjustment for potential confounding factors in the statistical analyses. We realize that information on dietary factors was not ascertained thus limiting our ability to account for dietary and nutritional differences between two groups. There is emerging evidence that certain nutrients may affect PCa incidence and progression.35 As diets are made of multiple macro- and micro-nutrients, further prospective studies are warranted, particularly those investigating the relationship between whole foods instead of a single nutritional component.35 Another potential limitation of this study is that the assessment of body weight status was performed only by BMI. Considering that the relation between BMI and percentage body fat is influenced by ethnicity36 and previous study suggested that both BMI and waist circumference, also its related waist hip ratio might identically predict the presence of multiple metabolic risk factors in Chinese population.37 Thus, combining of anthropometric measurements and BMI may be superior to using only one of these parameters. Because chance findings cannot be ruled out due to limited sample sizes, particularly in the subgroup analyses, the interactions we have observed warrant further validation in larger studies.
CONCLUSION
We found that among the three selected SNPs, ADIPOQ rs3774262 SNP was associated with risk of PCa and circulating adiponectin levels with suggested biologically plausible directions. Our findings also suggest that a positive association of ADIPOQ gene variants with PCa may be more pronounced among persons who are overweight. With these implications, further investigation of SNP-cancer risk associations in a large cohort, in which clinical data will be prospectively collected, as well as more detailed in vitro and in vivo biological functional studies will be helpful to elucidate how exactly the ADIPOQ genetic variations influenced PCa development.
AUTHOR CONTRIBUTIONS
CYG and QXL carried out the laboratory experiments and wrote the manuscript. YZ performed the statistical analysis. DWY and QYW conceived of the study, participated in its design and helped to draft the manuscript. MYW and TYS participated in the statistical analysis. YJY, JCW, and LJ contributed reagents/materials/analysis tools. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 81272837), Shanghai municipal hospital emerging advanced technology joint research project (Grant No. SHDC12013122), “China's Thousand Talents Program” Recruitment at Fudan University, the Science and Technology Committee of Shanghai Municipality (Grant No. 12DZ2260100), the Ministry of Science and Technology (Grant No. 2011BAI09B00) and Ministry of Health (Grant No. 201002007).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, et al. Body-mass index and cancer mortality in the Asia-Pacific cohort studies collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11:741–52. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, et al. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–13. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 7.Sher DJ, Oh WK, Jacobus S, Regan MM, Lee GS, et al. Relationship between serum adiponectin and prostate cancer grade. Prostate. 2008;68:1592–8. doi: 10.1002/pros.20823. [DOI] [PubMed] [Google Scholar]
- 8.Kaklamani V, Yi N, Zhang K, Sadim M, Offit K, et al. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism. 2011;60:1234–43. doi: 10.1016/j.metabol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui E, Deng A, Wang X, Wang B, Mao W, et al. The role of adiponectin (ADIPOQ) gene polymorphisms in the susceptibility and prognosis of non-small cell lung cancer. Biochem Cell Biol. 2011;89:308–13. doi: 10.1139/o11-005. [DOI] [PubMed] [Google Scholar]
- 10.Kaklamani VG, Wisinski KB, Sadim M, Gulden C, Do A, et al. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA. 2008;300:1523–31. doi: 10.1001/jama.300.13.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gornick MC, Rennert G, Moreno V, Gruber SB. Adiponectin gene and risk of colorectal cancer. Br J Cancer. 2011;105:562–4. doi: 10.1038/bjc.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaklamani VG, Sadim M, Hsi A, Offit K, Oddoux C, et al. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008;68:3178–84. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon PK, Penney KL, Schumacher F, Rider JR, Sesso HD, et al. Common polymorphisms in the adiponectin and its receptor genes, adiponectin levels and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2618–27. doi: 10.1158/1055-9965.EPI-11-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Xiang YB, Long JR, Cai H, Cai Q, et al. Genetic polymorphisms in obesity-related genes and endometrial cancer risk. Cancer. 2012;118:3356–64. doi: 10.1002/cncr.26552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Gu C, Zhu Y, Wang M, Yang Y, et al. Polymorphisms in the mTOR gene and risk of sporadic prostate cancer in an Eastern Chinese population. PLoS One. 2013;8:e71968. doi: 10.1371/journal.pone.0071968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder JC, Bensen JT, Su LJ, Mishel M, Ivanova A, et al. The North Carolina-Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population-based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66:1162–76. doi: 10.1002/pros.20449. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Lu M, Qian J, Yang Y, Li S, et al. Rationales, design and recruitment of the Taizhou Longitudinal Study. BMC Public Health. 2009;9:223. doi: 10.1186/1471-2458-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Qiu LX, Wang MY, Hua RX, Zhang RX, et al. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum Genet. 2012;131:1235–44. doi: 10.1007/s00439-012-1152-8. [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer. 2012;107:207–14. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan N, Klein EA, Li J, Moussa AS, Jones JS. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J Urol. 2011;186:86–90. doi: 10.1016/j.juro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–42. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beebe-Dimmer JL, Zuhlke KA, Ray AM, Lange EM, Cooney KA. Genetic variation in adiponectin (ADIPOQ) and the type 1 receptor (ADIPOR1), obesity and prostate cancer in African Americans. Prostate Cancer Prostatic Dis. 2010;13:362–8. doi: 10.1038/pcan.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore SC, Leitzmann MF, Albanes D, Weinstein SJ, Snyder K, et al. Adipokine genes and prostate cancer risk. Int J Cancer. 2009;124:869–76. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber SB, Moreno V, Rozek LS, Rennerts HS, Lejbkowicz F, et al. Genetic variation in 8q24 associated with risk of colorectal cancer. Cancer Biol Ther. 2007;6:1143–7. doi: 10.4161/cbt.6.7.4704. [DOI] [PubMed] [Google Scholar]
- 26.Teras LR, Goodman M, Patel AV, Bouzyk M, Tang W, et al. No association between polymorphisms in LEP, LEPR, ADIPOQ, ADIPOR1, or ADIPOR2 and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:2553–7. doi: 10.1158/1055-9965.EPI-09-0542. [DOI] [PubMed] [Google Scholar]
- 27.Ling H, Waterworth DM, Stirnadel HA, Pollin TI, Barter PJ, et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–44. doi: 10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henneman P, Aulchenko YS, Frants RR, Zorkoltseva IV, Zillikens MC, et al. Genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome-related traits. Diabetes Care. 2010;33:908–13. doi: 10.2337/dc09-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hivert MF, Manning AK, McAteer JB, Florez JC, Dupuis J, et al. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57:3353–9. doi: 10.2337/db08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedland SJ, Sokoll LJ, Platz EA, Mangold LA, Bruzek DJ, et al. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174:1266–70. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 33.Tang H, Dong X, Hassan M, Abbruzzese JL, Li D. Body mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:779–92. doi: 10.1158/1055-9965.EPI-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu JP, Hou ZF, Duivenvoorden WC, Whelan K, Honig A, et al. Adiponectin inhibits oxidative stress in human prostate carcinoma cells. Prostate Cancer Prostatic Dis. 2012;15:28–35. doi: 10.1038/pcan.2011.53. [DOI] [PubMed] [Google Scholar]
- 35.Masko EM, Allott EH, Freedland SJ. The relationship between nutrition and prostate cancer: is more always better? Eur Urol. 2013;63:810–20. doi: 10.1016/j.eururo.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deurenberg-Yap M, Deurenberg P. Is a re-evaluation of WHO body mass index cut-off values needed? The case of Asians in Singapore. Nutr Rev. 2003;61:S80–7. doi: 10.1301/nr.2003.may.S80-S87. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Tong G, Tong W, Lu L, Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health. 2011;11:35. doi: 10.1186/1471-2458-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The frequency of haplotypes of the ADIPOQ gene and their associations with the risk of Pca in Chinese men
Risk of Pca associated with rs3774262 genotyps by BMI in Chinese men
False-positive report probability values for the observed associations


