Abstract
We investigated serum folic acid (FA) levels in patients with erectile dysfunction (ED) and/or premature ejaculation (PE). Fasting serum samples were obtained from 42 patients with ED, 36 with PE, 25 ED patients with PE, and 30 healthy men; the mean intravaginal ejaculation latency time (IELT) was measured during a 4 weeks baseline period. Levels of sex hormones (follicle-stimulating hormone, luteinizing hormone, total testosterone), homocysteine (Hcys), and FA were measured using chemiluminescent immunoassays. The sexual functions of PE patients and normal control men were evaluated using the Chinese Index of Premature Ejaculation (CIPE). The abridged International Index of Erectile Function-5 (IIEF-5) questionnaire was used to gauge erectile quality for ED patients and for normal controls. Serum FA concentrations were lower in ED (7.61 ± 3.97 ng ml−1), PE (9.37 ± 3.40 ng ml−1), and ED/PE (8.84 ± 4.28 ng ml−1) patients than in healthy men (12.23 ± 5.76 ng ml−1, P < 0.05). No significant differences in sex hormone levels were found between patients with sexual dysfunction and healthy controls (P > 0.05). There were positive correlations between serum FA concentrations and CIPE scores (r = 0.530, P < 0.01), IIEF-5 scores (r = 0.589, P < 0.01), and IELT (r = 0.445, P < 0.01); negative correlations with Hcys concentrations (r = −0.487, P < 0.01) were found in all participants. These findings showed a strong relationship between serum FA levels and sexual dysfunction, possibly due to an effect of FA on the metabolism of nitric oxide, Hcys, and 5-hydroxytryptamine.
Keywords: 5-hydroxytryptamine, erectile dysfunction, folic acid, homocysteine, nitric oxide, premature ejaculation
INTRODUCTION
Erectile dysfunction (ED) and premature ejaculation (PE) are the major components of male sexual dysfunction, both of which negatively influence the quality of life for millions of men worldwide. The Massachusetts Male Aging Study organization reported that the overall prevalence of ED in the Boston area, among 40–70 years old men was 52% ± 1.3%, and Porst showed that the prevalence of PE was 20%–25%.1 Interestingly, ED is often associated with PE.
At present, the pathogenesis of ED and PE constitutes a focus area in andrology research. Although ED occurs as a result of multifaceted, complex mechanisms that can involve disruptions in neural, vascular, and hormonal signaling, the most extensive efforts in penile erection physiology have focused on elucidating the mechanisms that regulate the functions of the endothelium and vascular smooth muscle of the corpus cavernosum.2 Penile erection is a vascular event that requires an intact endothelium; thus, the pathogenesis of both endothelial dysfunction (EDys) and ED are intimately linked through decreased expression and activation of endothelial nitric oxide (NO) synthase (NOS).3 Hyperhomocysteinemia (HHcy), an important regulator of NOS, has a marked inhibitory effect on endothelium-dependent NO formation4 and has recently been shown to be a novel risk factor for ED.5
The control of male ejaculatory function is a multifunctional process involving sympathetic neuronal input, release of the ductus ejaculatory closure resistance, and coordinated contraction of the seminal vesicle and ductus deferens smooth muscle. Many neurotransmitters, including NO and 5-hydroxytryptamine (5-HT), are involved in the control of ejaculation.6,7 The NO/cyclic guanosine monophosphate (cGMP) pathway appears to play a central role in sexual behavior, as suggested by the activity of NOS and guanylate cyclase in areas of the central nervous system (CNS) that are responsible for sex drive and sexual behavior.8 Furthermore, this signaling pathway is involved in the relaxation of corporal smooth muscles, and might also affect the smooth muscle of the reproductive tract. Similarly, 5-HT is also an important neurotransmitter that is involved in regulating the ejaculation process; its overall effect appears to be inhibition of ejaculation.9
Interestingly, folic acid (FA) plays important roles in the metabolism of NO, homocysteine (Hcys), and 5-HT.10,11,12 These findings suggest that FA is closely related to the mechanisms of ED and PE. However, direct evidence regarding serum FA levels in ED and/or PE patients is lacking. Therefore, in this study, serum FA concentrations were measured, and the relationship between serum sex hormones, Hcys concentrations, Chinese Index of Premature Ejaculation (CIPE) scores, International Index of Erectile Function-5 (IIEF-5) scores, and FA concentrations were investigated in men with and without sexual dysfunction.
MATERIALS AND METHODS
Participants
In total, 42 ED patients, 36 PE patients, and 25 ED patients with PE constituted the study groups, with 30 healthy men serving as a control group. The patients were recruited from our urology and reproductive medical center outpatient clinics; the control men were healthy hospital staff members who volunteered to participate.
The control men and the patients having a chief complaint of ED completed the IIEF-5 questionnaire,13 which permitted the classification of these individuals as either a control (score 21–25) or as an individual with ED (score ≤ 21). Patients with PE alone or in combination with ED, diagnosed according to the current criteria,14 and the control individuals completed the CIPE questionnaire, which provided a useful method for evaluating the sexual function.15 All participants were sexually active, did not have difficulty comprehending the IIEF-5 and/or CIPE questionnaires, and agreed to complete them based on their sexual activities within the previous 6 months. The following circumstances excluded potential participants from continuing in the study: (1) not living with their sexual partner; (2) history of diabetes, coronary artery disease, dyslipidemia, gastrointestinal disease, neurological disease, pelvic trauma, anemia, major psychiatric disorder, thyroid disease, acute, or chronic urinary tract inflammation; (3) end-stage renal disease; (4) on-going treatment for ED or PE; (5) use of drugs that affect sex hormone and/or vitamin metabolism within 3 months of the start of the study; and (6) drug abuse. The study protocol was approved by our local ethics committee.
Medical history and physical examination
All participants provided specific information, such as their age, duration of sexual dysfunction, smoking status, and past medical history. Their mean intravaginal ejaculation latency time (IELT) was measured during a 4 weeks baseline period, during which participants were required to experience coitus at least 4 times. The IELT was the time between the start of vaginal insertion and the start of intravaginal ejaculation.16 Physical examinations included measurements of height, weight, and blood pressure; body mass index (BMI) was calculated as weight divided by the square of the height (kg m−2).
Laboratory tests
After informed consent was obtained, fasting serum samples were collected from the patients and controls, and were stored at −20°C until assayed. The levels of serum sex hormones (follicle-stimulating hormone(FSH), luteinizing hormone (LH), and total testosterone (total T)), Hcys, Vitamin B12, and FA were measured using chemiluminescent immunoassays (ADVIA Centaur XP, Siemens Healthcare Diagnostics, Beijing, China).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), version 13.0 (SPSS, Chicago, IL, USA) for Windows XP. Data are shown as means ± standard deviation (s.d.). One-way analyses of variance with multiple comparisons (least significant difference) were used for the statistical analyses of measured data; chi-squared tests were used for categorical data. Correlation coefficients were assessed by using the Pearson method. All statistical analyses were two-sided, and P < 0.05 were considered as significant.
RESULTS
The clinical data and fasting endocrine values are listed in Table 1. There were no significant differences in mean age, BMI, years of marriage, years of education, prevalence of hypertension, smoking rates, divorce rate, or serum levels of FSH, LH, total T, and Vitamin B12 among the groups. The serum levels of Hcys were higher in the ED patients (11.40 ± 3.07 μmol l−1) and ED patients with PE (12.28 ± 2.36 μmol l−1) than in the PE (8.15 ± 2.68 μmol l−1) and control (7.78 ± 2.94 μmol l−1) groups (P < 0.01). The CIPE scores of PE patients and the IIEF-5 scores of ED patients were lower than those for the control group, especially in patients with both ED and PE, indicative of a reduced quality of sexual life.
Table 1.
Participant clinical data and fasting endocrine values
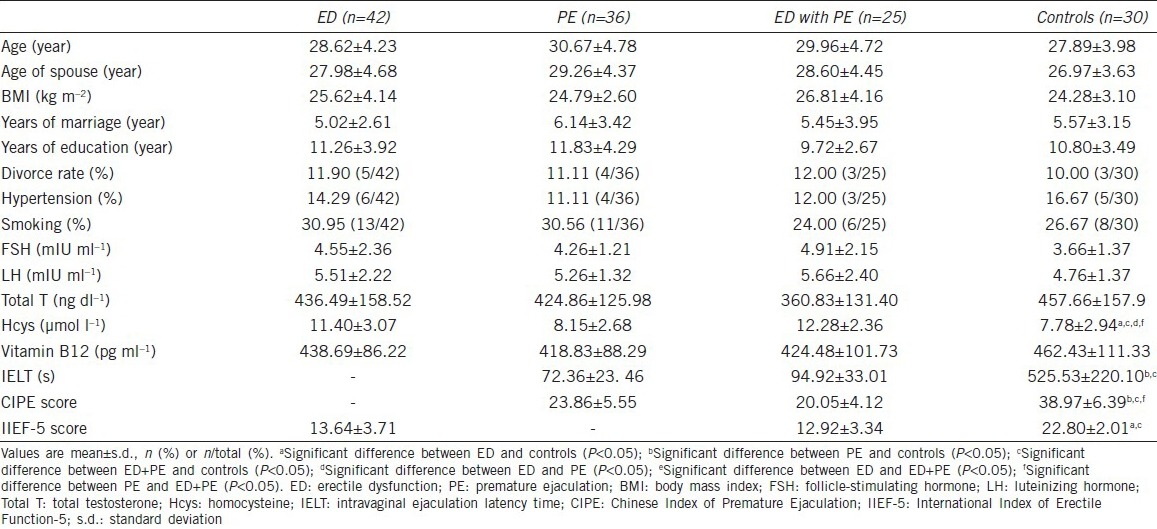
The serum FA concentrations were lower in patients with ED (7.61 ± 3.97 ng ml−1), those with PE (9.37 ± 3.40 ng ml−1), and in ED patients with PE (8.84 ± 4.28 ng ml−1) than in healthy men (12.23 ± 5.76 ng ml−, P < 0.05). No differences were found between men with ED and those with PE (Figure 1).
Figure 1.
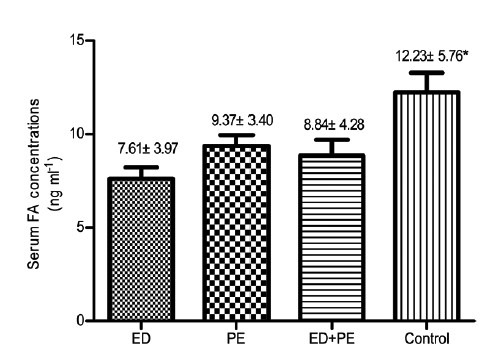
Serum folic acid (FA) concentrations in patients with sexual dysfunctions and in control participants. The bars show the average serum FA concentration in each group. *Significantly higher FA levels.
The serum FA levels were positively correlated with IIEF-5 scores (r = 0.589, P < 0.01; Table 2 and Figure 2) and CIPE scores (r = 0.530, P < 0.01; Table 2 and Figure 3).
Table 2.
Correlation coefficients of FA concentrations with clinical parameters
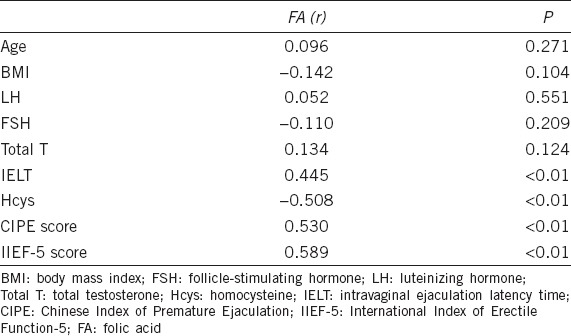
Figure 2.
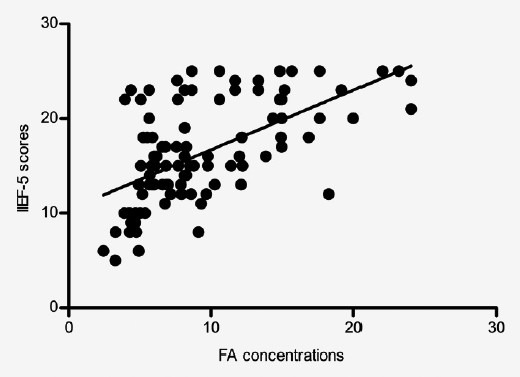
Correlation between International Index of Erectile Function-5 scores and folic acid concentrations. The scatter diagram shows that the correlation coefficient (r) is 0.589, P < 0.01.
Figure 3.
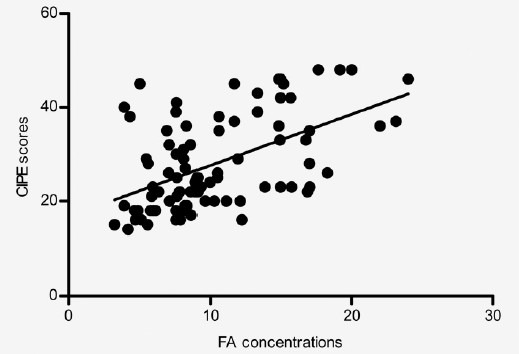
Correlation between Chinese Index of Premature Ejaculation scores and folic acid concentrations. The scatter diagram shows that the correlation coefficient (r) is 0.530, P < 0.01.
Table 2 shows the correlation coefficients of FA with age, BMI, sex hormone concentration, and Hcys concentration. The serum FA levels were negatively correlated with Hcys concentration and positively correlated with IELT (r = −0.508, 0.445, respectively; P < 0.01).
DISCUSSION
To the best of our knowledge, this is the first report of serum FA concentrations in men with sexual dysfunction, including ED and PE. Both ED and PE negatively influence the quality of life. ED is a well-known symptom of underlying disease rather than a disease itself. A study including 1464 male patients with clinical diagnoses of sexual dysfunction revealed that 92.6% of the patients had ED and that 50.8% had PE; 80% of those with ED demonstrated organic causes of the condition,17 consistent with our clinical observations. Penile erection is currently recognized as an event involving the vascular endothelium, bundles of smooth muscle cells, numerous autonomic nerves, and an extracellular matrix formed by collagen, elastin, and fibroblasts.18 Researchers have also found that ED is prevalent in patients with diabetes mellitus and those with disseminated vascular diseases, such as coronary and peripheral atherosclerosis; men with penile vascular damage may also have EDys in other vascular beds.19 Data suggest that ED is an early manifestation of EDys in the presence or absence of cardiovascular risk factors.20 Therefore, intact endothelial function is needed for erectile function. Previous studies have also proven that FA is related to EDys,21 and our research further shows that serum FA concentrations are associated with ED: patients with ED had FA deficiency, accompanied by HHcy. Therefore, we speculate that the mechanism underlying ED is connected with the metabolism of Hcys, which is influenced by FA.
The accumulation of Hcys has deleterious effects on cells, including induction of vascular diseases such as atherosclerosis. There are six main mechanisms of HHcy-induced EDys: (1) NO inhibition, (2) prostanoid regulation, (3) endothelium-derived hyperpolarizing factor suppression, (4) angiotensin II receptor-1 activation, (5) endothelin-1 induction, and (6) oxidative stress. Among these, the most important is the decreased expression and activation of endothelial NOS.4 HHcy inhibits NOS activity in endothelial cells via protein kinase C and threonine 495 phosphorylation pathways.22
Homocysteine results from the demethylation of methionine (Met), via S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH), and can be further metabolized via two alternative pathways: degradation, through transsulfuration, to cysteine or remethylation to Met;4,22 the latter pathway requires FA to provide a methyl group. FA deficiency prevents remethylation of Hcys to Met, and permits the accumulation of Hcys, which induces EDys. In addition, FA provides the methyl group for the conversion of Met to SAM, the methyl donor in over 115 different cellular methyltransferase reactions, including those involving DNA, RNA, proteins, and lipids.23 Thus, FA deficiency decreases DNA methylation and impairs DNA repair, which results in genetic mutations or the triggering of endothelial apoptosis.24
Currently, the causes and pathogenesis of PE are not very clear. Ejaculation is controlled by a complex reflex arc that includes nerve receptors, afferent pathways, cerebral sensory and motor areas, motor areas of the spinal cord, efferent pathways, effectors, and ejaculation organs;25 this process involves the central and peripheral nervous systems. Neurotransmitters are essential for nerve conduction, and 5-HT is an important neurotransmitter in the CNS. 5-HT neurons, which exist in the hypothalamus, brainstem, and spinal cord, are involved in the regulation of the entire ejaculation process. 5-HT can effect ejaculation by binding to its receptors, mainly 5-HT1A receptors and 5-HT2C receptors, an effect that is more clearly understood as a result of recent studies. IELT can be shortened using 5-HT1A agonists to activate 5-HT1A receptors, whereas use of 5-HT2C agonists can prolong IELT to delay ejaculation.9 The overall effect of 5-HT is inhibition of ejaculation.9 Figure 4 shows the 5-HT ejaculation regulation pathway. Selective 5-HT reuptake inhibitors (selective serotonin re-uptake inhibitors, (SSRIs)) have become important drugs for treating PE because that they can inhibit 5-HT reuptake by the central neurons, increase the CNS concentration of 5-HT, inhibit ejaculation, and prolong IELT.26
Figure 4.
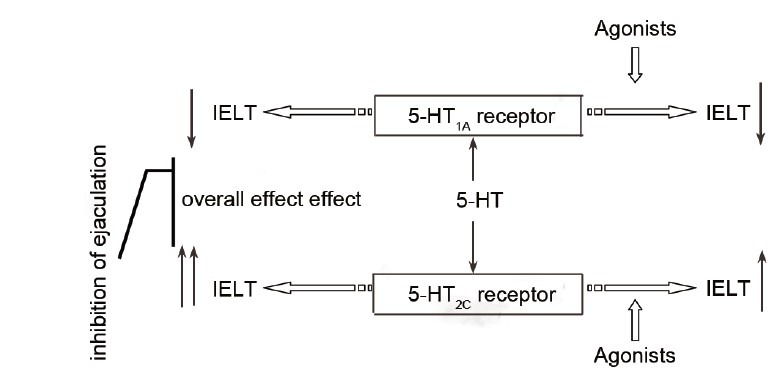
5-HT pathway regulating ejaculation. IELT: intravaginal ejaculation latency time; 5-HT: 5-hydroxytryptamine
Nitric oxide/cyclic guanosine monophosphate is a nonadrenergic, noncholinergic neurotransmitter involved in the control of ejaculation by the central and peripheral nervous systems.25 The main physiological functions of NO include vascular dilation, prevention of vascular smooth muscle proliferation, improvement of endothelial function, and smooth muscle relaxation (including genitourinary smooth muscle). Genitourinary smooth muscle relaxation, particularly that associated with the vas deferens delays ejaculation. In vitro experiments have proven that different NO donor drugs can significantly improve cGMP levels in human seminal vesicle smooth muscle tissue, thereby inhibiting seminal vesicle smooth muscle contraction.27 Clinical trials with phosphodiesterase type 5 inhibitors such as sildenafil and vardenafil demonstrated effective treatment of PE by prolonging IELT, thereby improving sexual life quality. Their mechanisms of action may be explained as a result of the effects on the peripheral nervous system and CNS.28
The CNS effect of phosphodiesterase type 5 inhibitors is through the cGMP pathway that reduces sympathetic nerve impulses transmitted to the peripheral nervous system, particularly through the regulation of NO activity and the reduction of sympathetic nervous tensity, to delay ejaculation. The peripheral nervous system effect involves heightened NO levels and increased cGMP, inhibiting smooth muscle contraction of the seminal vesicle, vas deferens, prostate, and urethra to inhibit ejaculation. The peripheral mechanism has been proven in animal experiments,29 wherein sildenafil relaxed rat vas deferens specimens in a concentration-dependent fashion, despite contraction induced by electrical stimulation or high concentrations of potassium chloride. 125I radioimmunoassays also showed that sildenafil increased cGMP levels, which can be blocked by a NOS inhibitor. These results indicated that sildenafil-induced vas deferens relaxation was mediated through the NO/cGMP pathway.
Folic acid is a water-soluble B vitamin that assists in human red blood cell maturation, and is the coenzyme that participates in the transfer and metabolism of a single carbon unit from Hcys to Met and is important for the synthesis of DNA, RNA, and neurotransmitters. 5-HT system abnormalities are recognized in the pathogenesis of depression. As a result, when hyposexuality and delayed orgasms and ejaculations were observed in SSRI-treated depression, SSRIs began being used for PE treatment. In addition, many scholars have found that depression is closely related to low serum FA levels, including a meta-analysis of 15 315 individuals that reported depression being significantly related to low serum FA levels.30 FA plays an important role in the synthesis of SAM, which influences the metabolism of 5-HT. Moreover, FA is important for the synthesis of tetrahydrobiopterin (BH4), which is essential for the hydroxylation of phenylalanine and tryptophan, the rate-limiting step in the synthesis of dopamine, noradrenalin, and 5-HT.10 An animal study confirmed that FA relieves depression, possibly through the 5-HT receptor (5-HT1A and 5-HT2A/2C) and NA receptor (α1 and α2).10 Thus, FA has an indirect, but important, effect on 5-HT metabolism.
Tetrahydrobiopterin, an important NOS co-factor, is essential in the synthesis of NO and influences NOS bioactivity. Additionally, BH4 provides electrons for the synthesis of NO. Thus, an appropriate amount of BH4 is needed to maintain normal NO levels.11 When adequate BH4 is available, L-arginine can be oxidized to L-guanidine, with the concurrent synthesis of NO; insufficient BH4 leads to decreased NO synthesis and vascular endothelium injury, as a result of NOS uncoupling.31 Therefore, FA plays important roles in the metabolism of NO, Hcys, and 5-HT (Figure 5).
Figure 5.
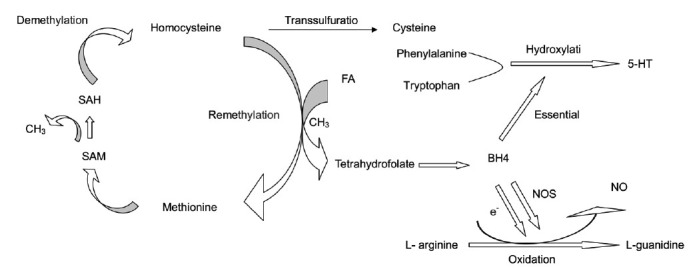
The metabolic pathways and relationships between FA and homocysteine, 5-HT, and NO. BH4: tetrahydrobiopterin; CH3: methyl; 5-HT: 5-hydroxytryptamine; FA: folic acid; NO: nitric oxide; NOS: NO synthase; SAH: S-adenosylhomocysteine; SAM: S-adenosylmethionine.
In summary, FA plays an important role in male sexual function; FA supplementation may provide potential therapeutic advantages for men with sexual dysfunction. However, additional experimental and clinical studies are needed to determine appropriate doses of FA.
AUTHOR CONTRIBUTIONS
WJY, NY, and JY conceived and designed the study. TLY collected the data. YJZ performed the statistical analyses. WJY and NY drafted and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interest.
ACKNOWLEDGMENTS
This study was partially supported by the key research project of Ministry of Public Security (No. 2010 ZDYJHBST007).
REFERENCES
- 1.Porst H. Premature ejaculation. Urologe A. 2009;48:663–74. doi: 10.1007/s00120-009-2019-z. [DOI] [PubMed] [Google Scholar]
- 2.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, et al. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010;17:38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 3.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–75. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z, Yang X, Wang H. Hyperhomocysteinemia and endothelial dysfunction. Curr Hypertens Rev. 2009;5:158–65. doi: 10.2174/157340209788166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir T, Comlekçi A, Demir O, Gülcü A, Calýpkan S, et al. Hyperhomocysteinemia: a novel risk factor for erectile dysfunction. Metabolism. 2006;55:1564–8. doi: 10.1016/j.metabol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Birowo P, Uckert S, Kedia GT, Sonnenberg JE, Thon WF, et al. Characterization of the effects of various drugs likely to affect smooth muscle tension on isolated human seminal vesicle tissue. Urology. 2010;75:974–8. doi: 10.1016/j.urology.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Janssen PK, Bakker SC, Réthelyi J, Zwinderman AH, Touw DJ, et al. Serotonin transporter promoter region (5-HTTLPR) polymorphism is associated with the intravaginal ejaculation latency time in Dutch men with lifelong premature ejaculation. J Sex Med. 2009;6:276–84. doi: 10.1111/j.1743-6109.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Christ GJ, Horita H, Adachi H, Suzuki N, et al. The effects of alterations in nitric oxide levels in the paraventricular nucleus on copulatory behavior and reflexive erections in male rats. J Urol. 1999;162:2182–5. doi: 10.1016/S0022-5347(05)68156-6. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano F, Clément P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol. 2006;50:454–66. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Brocardo PS, Budni J, Kaster MP, Santos AR, Rodrigues AL. Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology. 2008;54:464–73. doi: 10.1016/j.neuropharm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Stoll S, NejatyJahromy Y, Woodward JJ, Ozarowski A, Marletta MA, et al. Nitric oxide synthase stabilizes the tetrahydrobiopterin cofactor radical by controlling its protonation state. J Am Chem Soc. 2010;132:11812–23. doi: 10.1021/ja105372s. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–46. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 13.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic Criteria from DSM-IV-TR. [Google Scholar]
- 15.Yuan YM, Xin ZC, Jiang H, Guo YJ, Liu WJ, et al. Sexual function of premature ejaculation patients assayed with Chinese Index of Premature Ejaculation. Asian J Androl. 2004;6:121–6. [PubMed] [Google Scholar]
- 16.Waldinger MD, Hengeveld MW, Zwinderman AH. Paroxetine treatment of premature ejaculation: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 1994;151:1377–9. doi: 10.1176/ajp.151.9.1377. [DOI] [PubMed] [Google Scholar]
- 17.El-Sakka AI. Association of risk factors and medical comorbidities with male sexual dysfunctions. J Sex Med. 2007;4:1691–700. doi: 10.1111/j.1743-6109.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 18.Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med. 2009;6:2390–404. doi: 10.1111/j.1743-6109.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–84. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, et al. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. 2008;51:2040–4. doi: 10.1016/j.jacc.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 21.Hoch AZ, Lynch SL, Jurva JW, Schimke JE, Gutterman DD. Folic acid supplementation improves vascular function in amenorrheic runners. Clin J Sport Med. 2010;20:205–10. doi: 10.1097/JSM.0b013e3181df59f4. [DOI] [PubMed] [Google Scholar]
- 22.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55:131–8. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 23.Scott JM, Weir DG, Molloy A, McPartlin J, Daly L, et al. Folic acid metabolism and mechanisms of neural tube defects. Ciba Found Symp. 1994;181:180–7. doi: 10.1002/9780470514559.ch11. [DOI] [PubMed] [Google Scholar]
- 24.Pogribny IP, Basnakian AG, Miller BJ, Lopatina NG, Poirier LA, et al. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–901. [PubMed] [Google Scholar]
- 25.McMahon CG, McMahon CN, Leow LJ, Winestock CG. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: a systematic review. BJU Int. 2006;98:259–72. doi: 10.1111/j.1464-410X.2006.06290.x. [DOI] [PubMed] [Google Scholar]
- 26.Waldinger MD, Schweitzer DH, Olivier B. On-demand SSRI treatment of premature ejaculation: pharmacodynamic limitations for relevant ejaculation delay and consequent solutions. J Sex Med. 2005;2:121–31. doi: 10.1111/j.1743-6109.2005.20112.x. [DOI] [PubMed] [Google Scholar]
- 27.Machtens S, Ckert S, Stief CG, Tsikas D, Frlich Jr, et al. Effects of various nitric oxide-donating drugs on adrenergic tension of human seminal vesicles in vitro. Urology. 2003;61:479–83. doi: 10.1016/s0090-4295(02)02165-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Keren-Paz G, Bar-Yosef Y, Matzkin H. The role of phosphodiesterase type 5 inhibitors in the management of premature ejaculation: a critical analysis of basic science and clinical data. Eur Urol. 2007;52:1331–9. doi: 10.1016/j.eururo.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Tang Q, Hu BR, Fu Q, Ma R, et al. Relaxant effects and mechanism of sildenafil on the vas deferens of rats. Herald Med. 2008;27:150–3. [Google Scholar]
- 30.Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression. A meta-analysis and exploration of heterogeneity? J Epidemiol Community Health. 2007;61:631–7. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]


