Abstract
The aim of this study was to evaluate the outcomes of multiple advanced surgical treatments (i.e. microsurgery, laparoscopic surgery and endoscopic surgery) for acquired obstructive azoospermia. We analyzed the surgical outcomes of 51 patients with suspected acquired obstructive azoospermia consecutively who enrolled at our center between January 2009 and May 2013. Modified vasoepididymostomy, laparoscopically assisted vasovasostomy and transurethral incision of the ejaculatory duct with holmium laser were chosen and performed based on the different obstruction sites. The mean postoperative follow-up time was 22 months (range: 9 months to 52 months). Semen analyses were initiated at four postoperative weeks, followed by trimonthly (months 3, 6, 9 and 12) semen analyses, until no sperm was found at 12 months or until pregnancy was achieved. Patency was defined as >10,000 sperm ml−1 of semen. The obstruction sites, postoperative patency and natural pregnancy rate were recorded. Of 51 patients, 47 underwent bilateral or unilateral surgical reconstruction; the other four patients were unable to be treated with surgical reconstruction because of pelvic vas or intratesticular tubules obstruction. The reconstruction rate was 92.2% (47/51), and the patency rate and natural pregnancy rate were 89.4% (42/47) and 38.1% (16/42), respectively. No severe complications were observed. Using multiple advanced surgical techniques, more extensive range of seminal duct obstruction was accessible and correctable; thus, a favorable patency and pregnancy rate can be achieved.
Keywords: azoospermia, endoscopy, laparoscopy, male infertility, microsurgery
INTRODUCTION
Acquired seminal duct obstruction is a potentially correctable cause of male infertility. In the intracytoplasmic sperm injection (ICSI) era, a number of patients with obstructive azoospermia considered ICSI to be the best choice and a number of physicians also recommend ICSI because surgical repairs are complicated and technically challenging. To the best of our knowledge, multiple surgical techniques, such as transurethral resection of the ejaculatory duct (TURED), vasovasostomy (VV) and vasoepididymostomy (VE), have continuously improved over time,1,2,3 but there are still challenges that must be faced. How can we further improve the surgical techniques for acquired seminal duct obstruction? In an attempt to achieve better overall outcomes, we modified and performed the surgical techniques that are currently used to treat seminal duct obstruction.
MATERIALS AND METHODS
We prospectively enrolled patients between January 2009 and May 2013. All patients were informed the risks and benefits of surgery and ICSI in detail and voluntarily signed consent forms approved by the Ethics Committee at our hospital. For eligible patients with acquired obstructive azoospermia who submitted to surgical reconstruction, the following inclusion and exclusion criteria were applied.
Inclusion criteria
(1) Patients who planned to conceive children naturally, with no sperm found in at least three semen analyses and one centrifuged (1500 g, 15 min) semen sample. (2) Patients in whom at least one side of the seminal duct was normally developed. (3) Patients in whom serum follicle-stimulating hormone, luteinizing hormone, prolactin, estradiol, progesterone, and testosterone levels were within the normal limits. (4) Patients with evidence of normal spermatogenesis (by a testicular biopsy) in cases of suspected hypospermatogenesis. (5) Patients with semen neutral alpha-glucosidase <20 mU per ejaculate.
Exclusion criteria
Patients with azoospermia caused by an earlier vasectomy were excluded in our study because vasectomy reversal is relatively simple, and the patency rate is high.
Transurethral incision of the ejaculatory duct with holmium laser
Patients with low semen volume (<1.5 ml), low semen fructose (<13 umol per ejaculate) and seminal vesicle dilation (at least one side ≥15mm by transrectal ultrasonography) were diagnosed as having ejaculatory duct obstruction (EDO). With the patient placed in the dorsal lithotomy position and under epidural anesthesia, a Storz rigid ureteroscope (8F tip) was inserted into the urethra. After a routine examination, the ureteroscope (guided by a 4F ureteral catheter) was passed through the orifice of verumontanum and entered the prostatic utricle under direct vision. Because the orifices of the ejaculatory duct were occluded or too small to be found, the best method of entering the seminal vesicle is through a dilated ejaculatory duct. Under direct vision, a 4F ureteral catheter tentatively penetrated the two sides of the bottom of the prostatic utricle where the ejaculatory duct is located (Figure 1). If the ureteral catheter went into the ejaculatory duct, the rigid ureteroscope followed the catheter to enter the ejaculatory duct. In a few cases, it was too difficult to find the ejaculatory duct. Attempts to enter the ejaculatory duct should depend on the experience and skill of the operator, or be guided by transrectal ultrasonography; we could use ultrasonogram as a guide to indicate the dilated ejaculatory duct intraoperatively. After the ureteroscope enters the ejaculatory duct and seminal vesicle, liquid (for the semen examination) was extracted through an F4 ureteral catheter, and then calculi, jelly-like substances, or infectious liquid were removed or washed away. To increase the likelihood of keeping the ejaculatory duct open postoperatively, a holmium laser was used to incise and to enlarge the orifice penetrated by the ureteroscope. A urethral catheter was left for 24 h and then removed. Patients started to ejaculate 4 days post-surgery. Initial hemospermia spontaneously disappeared within 7–10 days.
Figure 1.
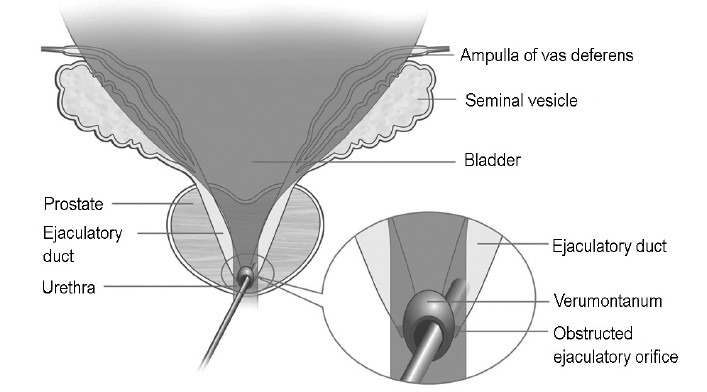
Transurethral incision of the ejaculatory duct. The ureteroscope enters the ejaculatory duct through the prostatic utricle.
Laparoscopy assisted microscopic vasovasostomy
Patients with iatrogenic injuries to the bilateral vas (inguinal portions were resected) from childhood surgeries (Figure 2) were placed in a supine position. With the patient under general anesthesia, 3–4 cm incision was made on the previous inguinal surgery scar, and the scar was resected. The proximal (testicular) end of the vas was easily identified near the external ring. The proximal vas was dissected free, and the scarred portion was removed, a drop of vasal fluid was examined for the presence of sperm. All patients were found to have sperm in the proximal vas bilaterally. Assisted laparoscopy was needed if the distal vas end was retreated into the pelvis and inaccessible at the inguinal area or if the distal (pelvic) vas end was found and dissected near the internal ring, but the defective vas was too long to anastomose with the proximal end of the vas. This procedure is similar to Shaeer pelviscrotal VV.4 A three-port transperitoneal approach is placed after artificial pneumoperitoneum. The initial 10 mm port was placed at the inferior umbilical crease and housed the laparoscope. Ports number 2 (5 mm) and number 3 (5 mm) were placed one fingerbreadth outside the lateral border of the rectus muscle and two fingerbreadths below the umbilicus. After an incision of the peritoneum on the internal ring, the distal end of the vas was easily identified and dissected distally, and 7–8 cm length of distal vas was dissected free. Another 5 mm trocar was placed on the external ring and created a new canal by penetrating the abdominal wall into the peritoneal cavity adjacent to the outside of the obliterated umbilical artery fold. This canal was a shortcut for a tension-free VV (Figure 3). The distal vas were delivered intact and then trimmed under direct vision of the microscope. The scarred end of the vas was cut-off until the vasal lumen appeared healthy. The distal vas was near the proximal vas, and VV was performed (the procedure used was similar to the previously reported technique5). The patients started to ejaculate 3 weeks after surgery.
Figure 2.
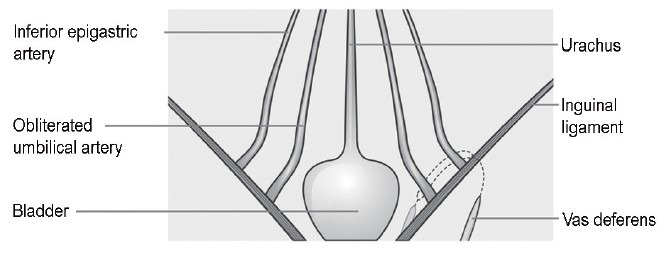
Inguinal vas was resected from childhood surgery and the pelvic vas end retreat into the pelvic cavity while the body grows and develops.
Figure 3.
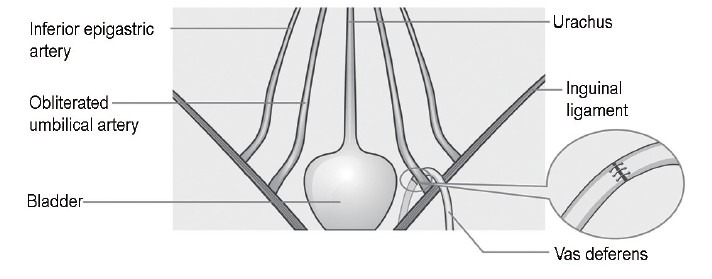
A laparoscopic-assisted vasovasostomy (VV). A tension-free VV with a shortcut.
Microscopic vasoepididymostomy
Patients who were presumptively diagnosed with vasal or epididymal obstruction underwent scrotum exploration under spinal anesthesia. The vas was transected at the site near the epididymis. The patency of the distal vas lumen was checked by injecting 10 ml of saline into the cannulated vas deferens. If saline could not pass through the vas lumen, a 0.032-inch Zebra guidewire was inserted into the lumen until it reached the blockage. The length of guidewire that was inserted indicated the obstruction site.
If the obstruction site was in the pelvis or inguina, reconstructive surgery was impossible because of uncertainty regarding the length of obstructed segment and the procedure was ended immediately. If the obstruction was in the scrotum and sperm were found in the fluid of the proximal vas intraoperatively, the obstructed segment was cut and discarded until a healthy lumen was found, and then microscopic VE was considered.
If the patency of the vas lumen was confirmed and no sperm was found in the fluid of the proximal vas intraoperatively, the epididymal tubule was dissected free, and the fluid of the epididymis was checked under high-power magnification for the presence of sperm. If sperm were found, transverse two suture intussusception VE was performed. The procedure used is similar to the previously reported technique6 but with modifications. First, instead of a transversely linear incision in the loop of the epididymal tubule, a round tubulotomy between two transverse sutures was cut using a microsurgical curved scissors. The diameter of the round tubulotomy was matched to the diameter of the vasal lumen. Second, sutures place of the inner layer in the vas deferens were full thickness, which allow a deeper invagination of the epididymal tubule into the vasal lumen (Figure 4). Patients started to ejaculate 3 weeks after the reconstructive surgery.
Figure 4.
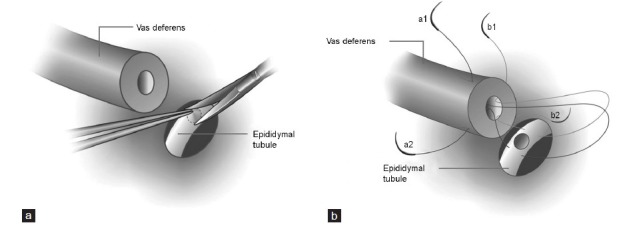
A modified vasoepididymostomy. (a) A round window-shape tubulotomy was created using a microsurgical curved scissors. (b) The sutures placed in the vas deferens were full thickness to make a deeper invagination of the epididymal tubule into vasal lumen and to avoid fluid leakage.
Postoperation follow-up
Semen analyses were initiated at 4 weeks, followed by trimonthly (months 3, 6, 9, and 12 months) semen analyses until no sperm was found at 12 months or until pregnancy was achieved. The mean follow-up time was 22 months (range: 9 months to 52 months). Patency was defined as >10 000 sperm ml−1 of semen.
RESULTS
A total of 51 patients (age: 22–45 years old; mean = 31.6 years old) were consecutively enrolled in the study. Their partners ranged in age from 20 to 42 years old (mean age: 28.5 years old). In this group, there were 39 cases of primary infertility, including nine with a history of urethral or genital duct infection, three with iatrogenic injuries to the bilateral vas (inguinal portion) from childhood bilateral spermatic cord hydrocele surgeries and 27 with unknown causes for their obstructions; two patients suffered from previous ICSI failures. Among the 12 patients with secondary infertility, four had a history of urethral or genital duct infection and eight have unknown causes for their obstructions.
Eleven patients underwent transurethral incision of the ejaculatory duct (TUIED) with holmium laser; three underwent laparoscopically assisted microscopic VV; 33 underwent microscopic VE, and the other four patients were surgically explored and unable to be treated with surgical reconstruction because of pelvic vas or intratesticular tubules obstruction.
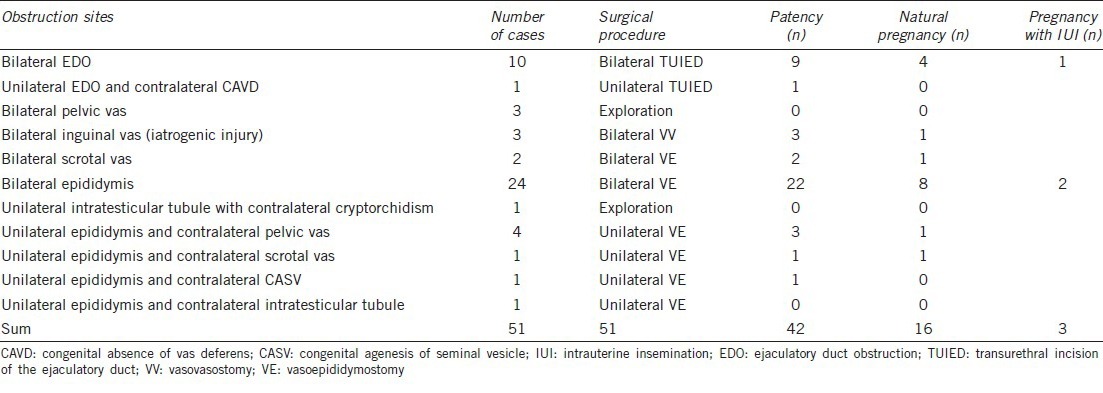
Forty-seven patients underwent bilateral or unilateral surgical reconstruction. The reconstruction rate was 92.2% (47/51); 42 patients had return of sperm to the ejaculate during follow-up, and patency rate was 89.4% (42/47). Among the 42 patients, 16 naturally achieved pregnancy. The natural paternity rate was 38.1% (16/42), three of the nine patients with successful operations achieved pregnancy after their partners underwent intrauterine insemination (IUI) (Table 1). No severe complications were observed. There was only one case of hemospermia, which lasted 2 weeks after TUIED and was cured by a 7 days course of antibiotics.
Table 1.
Obstruction sites and postoperative outcomes
DISCUSSION
With the aim of improving and evaluating the overall outcomes of surgical treatment of acquired obstructive azoospermia, we used and modified surgical reconstruction techniques that have been previously reported. The seminal duct is a long and circuitous course through which sperm passes. Acquired obstruction can occur in any part of the duct. The reconstructive techniques were chosen to treat obstruction of the duct based on the obstruction site.
The common therapy for EDO is TURED. However, TURED is possibly associated with a series of complications, including retrograde ejaculation and infection of the seminal duct.7 Recently, some studies have reportedly examined transurethral 6F seminal vesiculoscopy.8 In our study, an 8F ureteroscope with a holmium laser has been used as a minimally invasive alternative technique to treat EDO. The 8F ureteroscope is more frequently available. During the ureteroscopic procedure, the ureteroscope penetrated into the ejaculatory duct and seminal vesicle, and holmium laser was used to incise and enlarge the pass way penetrated by the ureteroscope to keep the ejaculatory duct open. The patency rate was 90.9% (10/11). Only one case of >2 weeks hemospermia occurred, and no other complication was observed. Radiography showed that no reflux occurred during voiding.
Compared to previously published reports of iatrogenic injury to the vas during inguinal hernia repair and vasectomy at the time of hernia repair,4,9,10 in our study, three of the iatrogenic injuries to the bilateral vas (inguinal portion) were caused by childhood bilateral spermatic cord hydrocele surgeries. The whole inguinal vas segment was inadvertently removed during childhood surgery; furthermore, the pelvic vas end will likely retreat into the pelvic cavity, while the body grows and develops. With laparoscopic surgery, it is easy to find and dissect not only the distal vas free, but also to deliver the pelvic vas end outside through the tunnel directly linking the external ring to the inner pelvis without passing through the inguinal canal. Then, restoring the continuity with a laparoscopic-assisted microscopic VV could be an easy and effective method of bridging the long vasal gap tension-free.
Although VE has evolved and advanced in recent years, the technique remains one of the most technically challenging microsurgical procedures. The best patency rate reported in recent years is 73%–92%.1,11,12,13 The common anastomosis is longitudinal or transverse two suture intussusception VE. In our study, the procedure is similar to transverse two suture intussusception previously reported,6 but we used microsurgical curved scissors to create a round tubulotomy between two transverse sutures. The sutures placed in the vas deferens were full thickness, which allow a deeper invagination of the epididymal tubule into vasal lumen. Studies have shown that the patency rate of bilateral VE was up to 92.3% (24/26), and the unilateral patency rate is 71.4% (5/7). We believe that the round tubulotomy and deeper invagination have three advantages: (1) round tubulotomy match to the round lumen of the vas much better; thus, deeper invagination could avoid fluid leakage. (2) Round tubulotomy is not prone to occlusion. (3) If the diameter of the round-window tubulotomy is equal to the length of the linear incision, the round anastomotic cross-section area is larger than that of the lineal incision.
Fifty-one patients with suspected acquired seminal duct obstruction were involved in this study. Although the inclusion and exclusion criteria could not completely rule out the possible existence of congenital defects of the seminal duct, these criteria could be used to screen the patients and to reduce the possibility of failure of seminal duct reconstruction during surgical exploration. Note that neutral alpha-glucosidase is exclusively produced by the epididymis.14 Normal activity (≥20 mU per ejaculate)15 indicate that obstruction could be at higher level of epididymis or rete testis, which it is difficult (or even impossible) to repair. Among the 51 patients, 47 underwent reconstructive procedures; the other four cases could not be repaired because of bilateral pelvic vasal obstruction or intratesticular tubular obstruction with contralateral cryptorchidism. The reconstruction rate was up to 92.2% (47/51).
Of all 51 patients, 42 achieved successful patency, and the overall patency rate was 89.4% (42/47); 16 patients naturally achieved pregnancy and three achieved pregnancy with IUI. The natural pregnancy rate was 38.1% (16/42), and the overall pregnancy rate was 45.2% (19/42) after an average 22 months follow-up. It has been reported that for all ages and with all different sperm types used, clinical pregnancy with ICSI is approximately 45%.16 Although it is difficult to compare the natural pregnancy rate of surgical reconstruction with that of ICSI because of multiple factors, such as the ages of the couple and maternal factors, the outcomes of surgical reconstruction are optimistic. After all, conception through intercourse conforms to physiologic mechanisms and avoids the high cost, potential complications and other risks associated with ICSI. Even if some patients fail to conceive naturally after successful reconstructive surgery, they have further sequential options, such as IUI and in vitro fertilization (IVF), based on semen analysis and maternal factors. In our study, nine patients received IUI 1 year after the return of sperm to the ejaculate, and three patients achieved pregnancy. ICSI might be the last option for patients with acquired obstructive azoospermia. Furthermore, patients who underwent successful surgery and failed to achieve pregnancy through IUI and IVF could use fresh ejaculated sperm for ICSI and avoid sperm retrieval from the epididymis or testis.
CONCLUSION
Using multiple advanced surgical techniques, more extensive range of seminal duct obstruction was accessible and correctable; a favorable patency and pregnancy rate can be achieved for properly selected patients with acquired obstructive azoospermia.
AUTHOR CONTRIBUTIONS
HTJ and JGY conceived of the study, participated in its design, drafted, revised the manuscript and performed the statistical analysis. HTJ and JGY coordinated the study and helped to draft the manuscript. HTJ, YL, QY, ZQL, ZYZ, and KFX participated in the surgical procedures and data acquisition. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Chan PT. The evolution and refinement of vasoepididymostomy techniques. Asian J Androl. 2013;15:49–55. doi: 10.1038/aja.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolettis PN. Restructuring reconstructive techniques – advances in reconstructive techniques. Urol Clin North Am. 2008;35:229–34. doi: 10.1016/j.ucl.2008.01.016. viii. [DOI] [PubMed] [Google Scholar]
- 3.Parekattil SJ, Gudeloglu A, Brahmbhatt J, Wharton J, Priola KB. Robotic assisted versus pure microsurgical vasectomy reversal: technique and prospective database control trial. J Reconstr Microsurg. 2012;28:435–44. doi: 10.1055/s-0032-1315788. [DOI] [PubMed] [Google Scholar]
- 4.Shaeer OK, Shaeer KZ. Pelviscrotal vasovasostomy: refining and troubleshooting. J Urol. 2005;174:1935–7. doi: 10.1097/01.ju.0000176738.55343.75. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159:188–90. doi: 10.1016/s0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 6.Chan PT, Li PS, Goldstein M. Microsurgical vasoepididymostomy: a prospective randomized study of 3 intussusception techniques in rats. J Urol. 2003;169:1924–9. doi: 10.1097/01.ju.0000059360.97108.c4. [DOI] [PubMed] [Google Scholar]
- 7.Heshmat S, Lo KC. Evaluation and treatment of ejaculatory duct obstruction in infertile men. Can J Urol. 2006;13(Suppl 1):18–21. [PubMed] [Google Scholar]
- 8.Wang H, Ye H, Xu C, Liu Z, Gao X, et al. Transurethral seminal vesiculoscopy using a 6F vesiculoscope for ejaculatory duct obstruction: initial experience. J Androl. 2012;33:637–43. doi: 10.2164/jandrol.111.013912. [DOI] [PubMed] [Google Scholar]
- 9.Kramer WC, Meacham RB. Vasal reconstruction above the internal inguinal ring: what are the options? J Androl. 2006;27:481–2. doi: 10.2164/jandrol.06031. [DOI] [PubMed] [Google Scholar]
- 10.Kim A, Shin D, Martin TV, Honig SC. Laparoscopic mobilization of the retroperitoneal vas deferens for microscopic inguinal vasovasostomy. J Urol. 2004;172:1948–9. doi: 10.1097/01.ju.0000140449.39217.b0. [DOI] [PubMed] [Google Scholar]
- 11.Marmar JL. Modified vasoepididymostomy with simultaneous double needle placement, tubulotomy and tubular invagination. J Urol. 2000;163:483–6. [PubMed] [Google Scholar]
- 12.Schiff J, Chan P, Li PS, Finkelberg S, Goldstein M. Outcome and late failures compared in 4 techniques of microsurgical vasoepididymostomy in 153 consecutive men. J Urol. 2005;174:651–5. doi: 10.1097/01.ju.0000165573.53109.92. [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Yuan Y, Zhang Z, Gao B, Song W, et al. Patency rates of microsurgical vasoepididymostomy for patients with idiopathic obstructive azoospermia: a prospective analysis of factors associated with patency – single-center experience. Urology. 2012;79:119–22. doi: 10.1016/j.urology.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Peña P, Risopatrón J, Villegas J, Miska W, Schill WB, et al. Alpha-glucosidase in the human epididymis: topographic distribution and clinical application. Andrologia. 2004;36:315–20. doi: 10.1111/j.1439-0272.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooper TG. Measurement of neutral α-glucosidase in seminal plasma. In: Cooper TG, editor. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. pp. 134–6. [Google Scholar]
- 16.Sabanegh E, Agarwal A. Male infertility. In: Wein AJ, Kavoussi LR, Partin AW, Novick AC, Peters CA, editors. Campbell-Walsh Urology. 10th ed. Philadephia: Elsevier Saunders; 2012. pp. 616–47. [Google Scholar]


