Abstract
Background:
As experience with vascularized lymph node (VLN) transfer has grown, new VLN sources have become apparent. Descriptive studies have elucidated variable lymph node presence in these donor basins. Yet, no study has evaluated preoperative imaging evaluation between donor sites in patients undergoing VLN transfer. This study was to compare the findings on duplex ultrasonography of the submental, groin, and supraclavicular lymph node basins in patients undergoing VLN transfer.
Methods:
A review of a prospective database was performed for patients who had undergone preoperative planning for VLN transfer with duplex ultrasonography to provide objective donor-site characteristics. Multiple regression analysis was used to identify factors that correlated with specific flap characteristics. A P value less than 0.05 was considered statistically significant.
Results:
Sixty-eight patients (28 upper extremities and 40 lower extremities) were identified as undergoing preoperative duplex ultrasonography for VLN transfer. Little variation was seen when evaluating donor sites for laterality in patients. Groin and submental VLN sites had 3.1 and 3.3 lymph nodes, respectively, compared with 0.9 lymph nodes in the supraclavicular donor site (p < 0.01). Increasing age had an inverse relationship with estimated flap volume, whereas higher body mass index correlated with increasing flap thickness.
Conclusions:
Preoperative imaging with duplex ultrasonography before VLN transfer may allow for accurate identification of specific VLN donor-site characteristics. When considering lymph node–specific characteristics, higher quantity of lymph nodes were found on the groin and submental flap axis compared with the transverse cervical artery axis.
Lymphedema represents a debilitating chronic condition, affecting patients following oncologist lymph node excision. In extremity lymphedema, symptoms can lead to significant decreases in patient-reported quality-of-life outcomes.1,2 Recently, increased interest in the management of lymphedema has resulted in a rise in surgical options to treat this chronic condition. Two common surgical treatment options include lymphovenous anastomosis and vascularized lymph node (VLN) transfer. Each treatment option has the potential to provide venous shunting of lymphatic fluid, therefore reducing interstitial fluid accumulation in the affected extremity.3–7
The popularity in VLN transfer has been mirrored by increased descriptions of new donor sites for lymph node harvest. The groin region has remained the most popular due to its reliability and proven success. But, in instances of lower extremity lymphedema, alternative flaps are needed to avoid the possibility of inducing iatrogenic lower extremity lymphedema.8 As a result, the submental axis6 and supraclavicular/transverse cervical artery axis9,10 have been recently described as alternative sources of VLNs.
With increasing options related to VLN transfer, decision making regarding flap choice may influence outcomes. Until now, choice of VLN donor site has been surgeon-dependent with few exploring unfamiliar VLN sources. As a result, little is known about patient-specific variations in donor sites for VLN harvest. Therefore, the aim of this study was to compare findings of duplex ultrasonography within patients presenting for treatment of lymphedema to investigate specific VLN flap characteristics that may aid in transfer.
PATIENTS AND METHODS
Study Population and Design
An institutional review board–approved review of a prospectively maintained database was performed at Chang Gung Memorial Hospital. Duplex ultrasonography was performed on all patients who underwent surgical treatment for lymphedema from May 2012 to August 2013 for evaluation of lymph node basins. In patients with upper extremity lymphedema, 6 lymph node basins were evaluated as potential sources of VLN flaps: bilateral transverse cervical, submental, and groin areas. In patients with lower extremity lymphedema, 4 of the basins were evaluated: bilateral submental and transverse cervical regions.
Duplex Ultrasonography
A single radiologist (S.-Y.C.), who has 13 years of experience with duplex ultrasonography and who specializes in soft-tissue ultrasound, performed all imaging evaluations to ensure comparability of results. All patients were examined in supine positioning. Xario XG (Toshiba, Tokyo, Japan) ultrasound machine (12 MHz central frequency) was used for all evaluations. A lymph node greater than 5 mm in diameter can be identified by the duplex ultrasonography.
Demographics and Data Collection
Patient charts were reviewed for collection of demographic data. Duplex ultrasonography findings were documented to provide objective data regarding number of lymph nodes, flap thickness, venous and arterial diameter, and an estimated flap volume and lymph node density from each patient in either 4 or 6 sites, depending on whether 2 or 3 lymph node basins were evaluated bilaterally. Within each patient donor-site basin, characteristics were evaluated to determine if differences were seen between left and right side (laterality).
Lymph Node Density Estimation
Duplex ultrasonography allowed for accurate measurement of donor-site thickness. Coupled with data related to the quantity of lymph nodes in the area, common dimensions of potential flap harvest are used to estimate density. For groin-based flaps, 10 × 5 cm is the standard dimensions used during flap harvest. Accordingly, 10 × 5 cm and 10 × 5 cm were used for transverse cervical artery and submental flaps, respectively.
Statistical Analysis
Demographic data were evaluated using standard nonparametric tests for significance. To consider the missing or nonavailable data of measurements, a statistical method, multiple regression analysis, was used to determine patient factors related to positive/negative flap characteristics. A 0.05 criterion of statistical significance was used. Statistical analysis was performed using SPSS version 17.0 (Statistical Product and Service Solutions, IBM, Armonk, New York, USA).
RESULTS
Study Demographics
A total of 68 patients were identified as undergoing preoperative evaluation for VLN transfer. Twenty-eight patients had undergone evaluation for upper extremity lymphedema, whereas 40 patients underwent evaluation for lower extremity lymphedema. Patient demographics are shown in Table 1. Overall, average patient age was 56.1 years with a body mass index of 26.3 kg/m2. Overall, similar characteristics are seen between patients with upper and lower extremity lymphedema.
Table 1.
Patient Information and Demographics
Groin VLN Basin
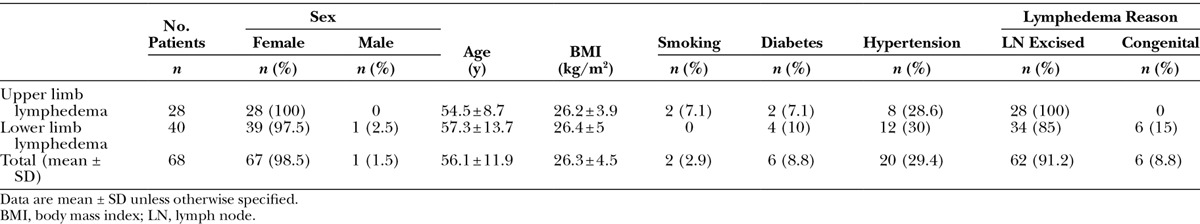
Site-specific characteristics are shown in Table 2. When evaluating pedicle characteristics, routine superficial circumflex iliac artery visualization was difficult, therefore arterial diameter of the pedicle artery was often not possible to measure. Instead, arterial diameter of the source artery (common femoral artery) was evaluated. On evaluation of other flap characteristics, similarities are seen between sides. Average flap volume and lymph node quantity present was approximately 600 mm3 and 3 nodes, respectively, independent of laterality. Flap thickness from this region was approximately 15–18 mm in thickness.
Table 2.
Groin Lymph Node Basin Characteristics
Submental VLN Basin
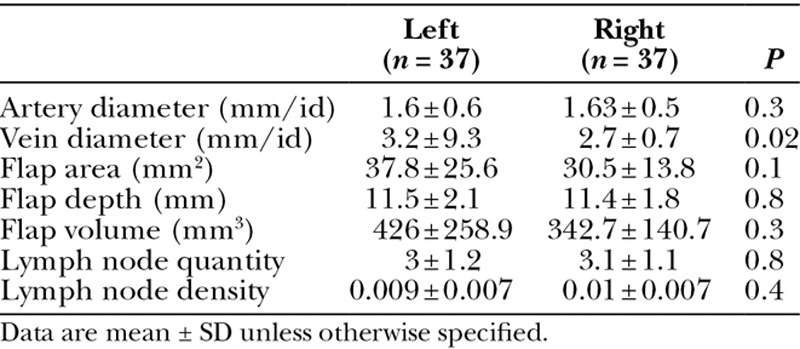
Characteristics of the submental flap are shown in Table 3. When focusing on the vascular pedicle, artery internal diameter was similar between the left and right side (1.6 vs 1.63 mm; P = 0.3). Average vein internal diameter was found to be larger on the left side (3.2 mm) compared with the right side (2.7 mm; P = 0.02). The flap thickness in this region was 11.5 mm on the left side and 11.4 mm on the right side (P = 0.8). Specific evaluation of the lymph nodes within this region revealed an average of approximately 3 lymph nodes on each side (Fig. 1) (P = 0.8).
Table 3.
Submental Lymph Node Basin Characteristics
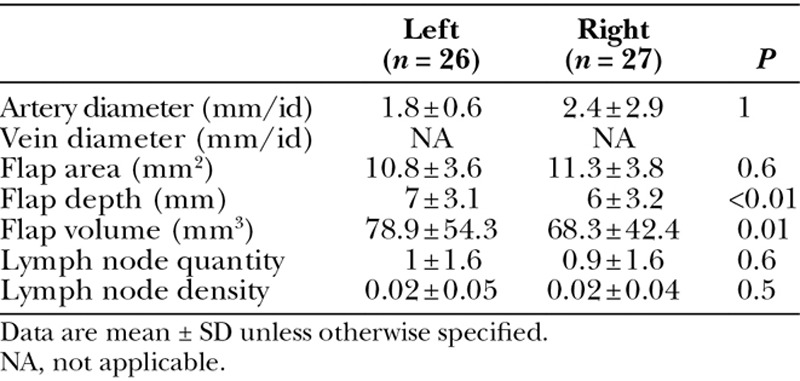
Fig. 1.
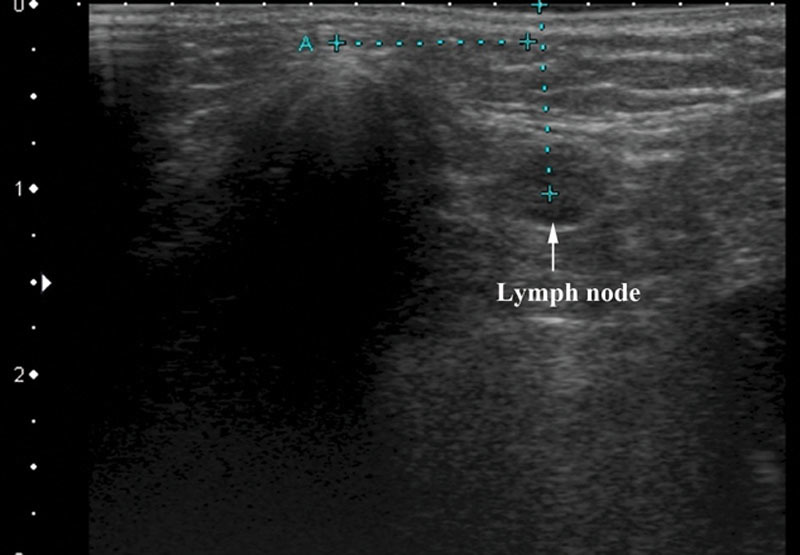
A level I lymph node is visualized for preplanning of a vascularized submental lymph node flap. Point A represents the chin point. Precise measurements may be obtained to ensure inclusion into the flap.
Transverse Cervical VLN Basin
On evaluation of the transverse cervical VLN donor basin, arterial internal diameter ranged from 1.8 to 2.4 mm (Table 4 and Figs. 2A, B). Predictable vein visualization was not possible with duplex ultrasonography, and therefore, measurements were not available for evaluation. In comparison of laterality, significant differences were seen with flap depth (L: 7 vs R: 6 mm; P < 0.01) and volume (L: 78.9 vs R: 68.3 mm3; P = 0.01). In addition, lymph node quantity was similar with approximately 1 lymph node identified on each side (P = 0.06).
Table 4.
Supraclavicular Lymph Node Basin Characteristics
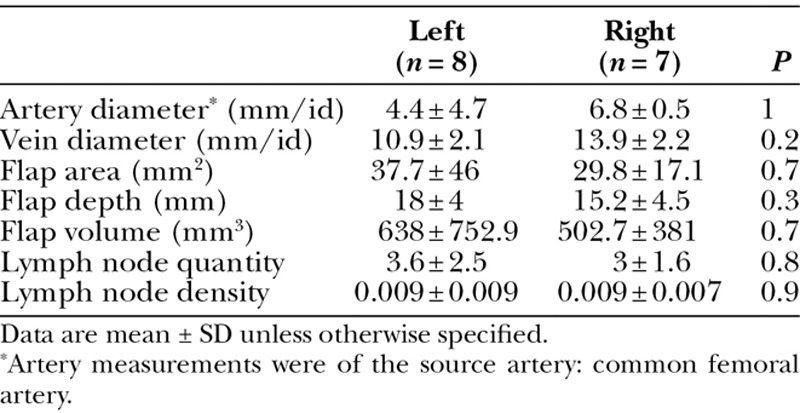
Fig. 2.
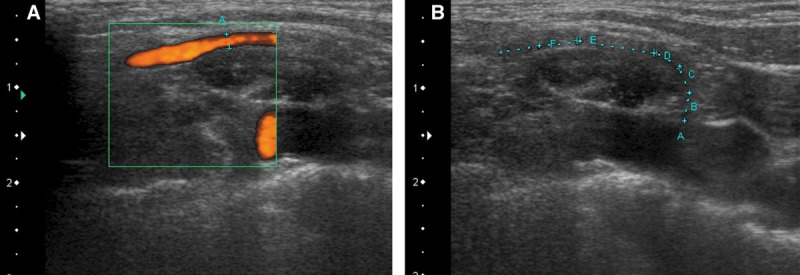
A, Color flow duplex imaging showing blood flow in the transverse cervical artery. B, The course of the transverse cervical artery can be determined to aid in flap harvest.
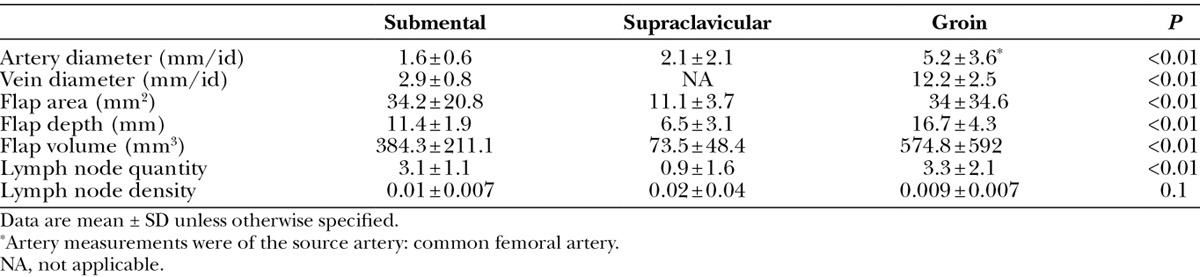
Comparison of VLN Basins
Findings on comparison of the 3 evaluated donor basins are shown in Table 5. When looking at flap characteristics, significant differences were seen between all groups when estimated flap size and volume. Smallest overall flaps were estimated with the supraclavicular donor site compared with the groin and submental regions (P < 0.01). Similarly, estimated lymph node quantity was least with the supraclavicular flap compared with the groin and submental flaps (P < 0.01).
Table 5.
Comparative Evaluation between Donor Lymph Node Basins
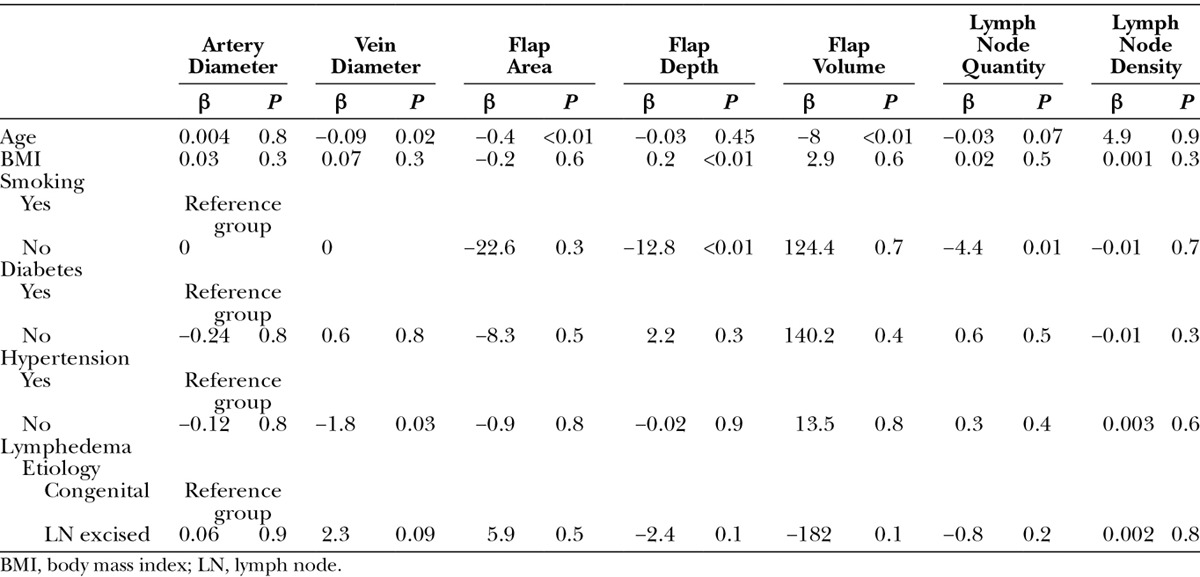
Influence of Patient Factors on Flap Characteristics
The impact of patient factors on flap characteristics is shown in Table 6. Age seems to influence overall VLN flaps in flap size characteristics. Increased patient age tends to inversely influence flap size, whereas increasing body mass index correlated with larger flap thickness. These findings can be attributed to age and obesity-related changes to subcutaneous fat stores in various sites in the body. Other findings included the correlation of smoking status on lymph node quantity, with smokers having higher number of visualized lymph nodes.
Table 6.
Multiple Regression Analysis
DISCUSSION
The treatment of extremity lymphedema has gained in popularity in recent years. Rightfully so, disease processes resulting in extremity lymphedema have continued to plague patients with this debilitating condition. Following breast cancer treatment, studies suggest that as many as 50% of surviving patients may have symptoms consistent with upper extremity lymphedema in their lifetime.11,12 Similarly, lower extremity lymphedema following gynecologic cancer excision and lymphadenectomy can occur in up to 18% of patients.13,14 Given the high incidence and the negative impact on quality of life in these affected patients,1 optimal patient-directed treatment options are necessary to ensure a successful outcome.
Previous studies have evaluated the positive effects of VLN transfer in the setting of lymphedema. Becker C et al15 evaluated 24 patients who had groin VLN transfer with a minimum of 5 years of follow-up. The study found significantly improved results in 92% of patients with a majority of patients having normal return of arm circumference.15 Althubaiti et al9 described the use of the supraclavicular VLN flap for treatment of lower extremity lymphedema. In their representative case, they found a 23% reduction in volume differential in a patient affected with significant lower extremity lymphedema.9
Our center has previously published on the use of the submental and groin flaps for extremity lymphedema.5–7 After critically evaluating these studies, the senior author (M.-H.C.) began using duplex ultrasonography to better evaluate the 3 common donor areas for VLN transfer. When contemplating VLN transfer, certain factors may aid in flap transfer. First, specific knowledge of the donor VLN flap vascular anatomy as related to vessel diameter and location will confirm size adequacy and appropriate placement in proximity to recipient vessels. Second, due to variations in body habitus, flaps harvested from areas that are thicker may result in dissatisfaction as related to contour irregularities at the recipient site leading to revisional surgery. Last, unpublished basic science data from our center indicate that inclusion of greater number of lymph nodes and higher lymph node densities likely result in improved lymphatic clearance in the lymphedematous extremity. With these parameters in mind, the results of this study highlight common findings in characteristics and laterality of donor site for VLN sources.
The submental flap and groin flap characteristics seem to be the most favorable donor sites given the higher lymph node quantity and density. Use of the submental flap must be weighed against donor-site morbidity related to the upper neck scar and risk of injury of marginal mandibular nerve. As our experience has grown with these flaps, the submental flap donor site seems to be tolerated well among patients. The groin flap seems to have favorable characteristics related to lymph node quantity and reliable vascular anatomy. These characteristics reinforce its popularity among surgeons treating upper extremity lymphedema. Despite flap reliability and positive flap characteristics, the potential for iatrogenic lower extremity lymphedema needs special consideration. Vignes et al8 recently detailed donor-site complications following VLN transfer. They found that 38% of patients developed complications at the groin donor site with iatrogenic ipsilateral limb lymphedema occurring most frequently.8 Flaps obtained from the transverse cervical artery axis seem to have the lowest lymph node quantity, unreliable venous system, and smaller vascular pedicle. Although these characteristics may make this flap less appealing to surgeons, the location, small flap volume and size, and inconspicuous scar may make this option attractive from the patient’s perspective. Cumulatively, these factors need to be considered when choosing an optimal donor site in individual patients.
Some patient-specific factors seem to influence certain flap characteristics. Smoking contributed to an overall higher quantity of lymph nodes visualized in potential donor site. This finding may be related to the constant chronic inflammatory state leading to larger lymph nodes, which are easier to visualize. In addition, overall body mass changes seen with aging and obesity correlate with decrease or increase in potential flap volumes. These factors may relate to cosmetic considerations when planning VLN flap transfers.
In addition to exploring VLN flap characteristics, we were able to demonstrate the potential benefits and drawbacks of duplex ultrasonography in planning VLN transfer. Ultrasonography has been used extensively in the setting of flap planning for various other reconstructive procedures.16,17 In comparison to other common imaging modalities, duplex ultrasonography may represent a less expensive alternative and negate any harmful effects from ionizing radiation. Although many important parameters can be evaluated, difficulties in visualization of vascular structures may occur as witnessed in our evaluation. In addition, the similar appearance of lymph nodes and fat lobules may obscure visualization of very small nodes. A collaborative working relationship with the radiology department is critical to ensure success with this imaging modality.
CONCLUSIONS
As the field of lymphatic microsurgery continues to grow, understanding available VLN donor sites will improve the decision-making process related to surgical procedures. Preoperative imaging with duplex ultrasonography before VLN transfer may allow for accurate identification of specific VLN donor-site characteristics. When considering lymph node–specific characteristics, higher quantity of lymph nodes were found on the groin and submental flap axis compared with the transverse cervical artery axis.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26:5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunberger G, Lindquist H, Waldenström AC, et al. Lower limb lymphedema in gynecological cancer survivors—effect on daily life functioning. Support Care Cancer. 2013;21:3063–3070. doi: 10.1007/s00520-013-1879-3. [DOI] [PubMed] [Google Scholar]
- 3.Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery. 2006;26:65–69. doi: 10.1002/micr.20214. [DOI] [PubMed] [Google Scholar]
- 4.Chang DW. Lymphaticovenular bypass surgery for lymphedema management in breast cancer patients. Handchir Mikrochir Plast Chir. 2012;44:343–347. doi: 10.1055/s-0032-1323762. [DOI] [PubMed] [Google Scholar]
- 5.Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265–1275. doi: 10.1097/PRS.0b013e31819e6529. [DOI] [PubMed] [Google Scholar]
- 6.Cheng MH, Huang JJ, Nguyen DH, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126:93–98. doi: 10.1016/j.ygyno.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286–1298. doi: 10.1097/PRS.0b013e31828bd3b3. [DOI] [PubMed] [Google Scholar]
- 8.Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg. 2013;45:516–520. doi: 10.1016/j.ejvs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Althubaiti GA, Crosby MA, Chang DW. Vascularized supraclavicular lymph node transfer for lower extremity lymphedema treatment. Plast Reconstr Surg. 2013;131:133e–135e. doi: 10.1097/PRS.0b013e318272a1b4. [DOI] [PubMed] [Google Scholar]
- 10.Sapountzis S, Singhal D, Rashid A, et al. Lymph node flap based on the right transverse cervical artery as a donor site for lymph node transfer. Ann Plast Surg. 2013 Jun 12 doi: 10.1097/SAP.0b013e31827fb39e. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.DiSipio T, Rye S, Newman B, et al. Incidence of uni lateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 12.Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Choi JH, Ki EY, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. 2012;22:686–691. doi: 10.1097/IGC.0b013e3182466950. [DOI] [PubMed] [Google Scholar]
- 14.Ryan M, Stainton MC, Slaytor EK, et al. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust N Z J Obstet Gynaecol. 2003;43:148–151. doi: 10.1046/j.0004-8666.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 15.Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isken T, Alagoz MS, Onyedi M, et al. Preoperative color Doppler assessment in planning of gluteal perforator flaps. Ann Plast Surg. 2009;62:158–163. doi: 10.1097/SAP.0b013e31817e9cbd. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotopoulos K, Soucacos PN, Korres DS, et al. Anatomical study and colour Doppler assessment of the skin perforators of the anterior tibial artery and possible clinical applications. J Plast Reconstr Aesthet Surg. 2009;62:1524–1529. doi: 10.1016/j.bjps.2008.06.013. [DOI] [PubMed] [Google Scholar]


