Abstract
Summary:
As nipple-sparing mastectomy with implant-based reconstruction has increased, attention must be paid to the viability of the nipple-areolar complex. This article describes the use of preoperative Doppler ultrasound to identify the internal mammary artery perforators. Preserving the internal mammary artery improves vascular supply to the nipple-areolar complex.
Nipple-sparing mastectomy (NSM) with implant-based reconstruction (IBR) has gained in popularity.1,2 Greater than 15% of nipple-areolar complex (NAC) loss is attributed to vascular compromise.3 Moreover, in patients who subsequently undergo IBR, NAC necrosis can lead to chronic open wounds, infection, implant exposure, and need for explantation.4–8
Blood supply of the breast stems from a deep and a superficial arterial system. The superficial system is composed of perforators from both lateral thoracic and internal mammary arteries.9 According to Palmer and Taylor,10 the internal mammary artery (IMA) contributes significant blood supply to the NAC. IMA perforators are superficial and can be identified using a handheld Doppler probe.9
Previous investigations have used Doppler ultrasound to identify major perforators to the NAC to increase nipple viability in reduction mammoplasty for gigantomastia.11 However, the application of Doppler ultrasound has not been applied to NSM with IBR.
In this study, we introduce a novel, easy, and inexpensive technique for improving NAC viability in NSM with IBR. Specifically, we employ preoperative Doppler ultrasound to identify IMA perforators to augment NAC perfusion.
PATIENTS AND METHODS
Patient Selection
With institutional review board approval, we retrospectively studied outcomes of a prospectively enrolled database of consecutive patients who received NSM with IBR in 2010–2012. Group A did not receive Doppler ultrasound and group B did. One oncologic surgeon (A.S.) and 1 plastic surgeon (M.T.) performed all procedures at Weill Cornell Medical Center. NSM was not offered if tumor size was greater than 2.5 cm or if tumor-to-nipple distance was less than 4 cm.12 NSM was not offered to patients with grade III ptosis or cup size greater than C. Outcomes were reviewed. Nipple ischemia ranged from epidermolysis to full-thickness necrosis; we applied the same grading system from our earlier works.13,14
Ultrasound Analysis
Patients were marked in a supine position with a handheld 8-MHz linear probe Doppler ultrasound (Siemens, Erlangen, Germany) by the oncologic surgeon. The probe was placed on the breast just lateral to the sternum and directed cranially to caudally, from the clavicle to the inferior costal margin. IMA perforators were identified on the skin surface (Fig. 1).
Fig 1.
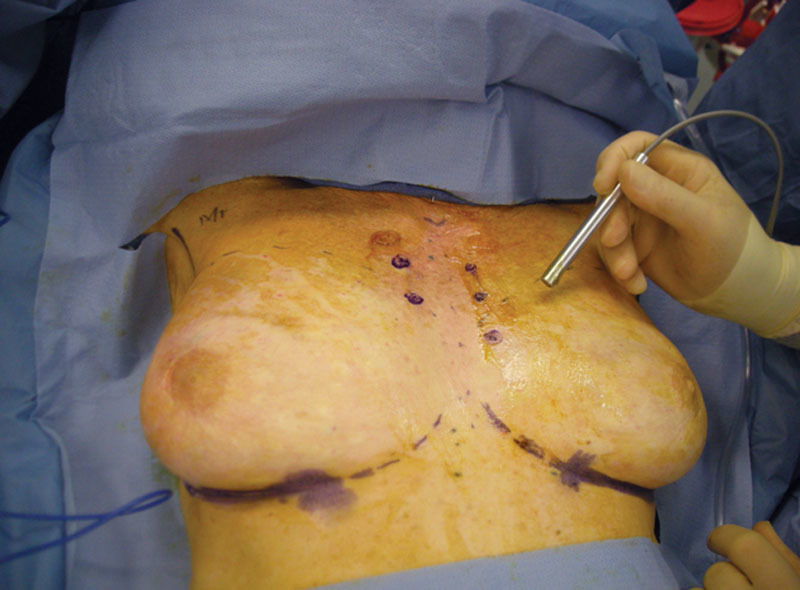
Doppler technique.
Surgical Technique
NSM was performed using a subdermal technique, as described in earlier works.13,14 IMA perforators corresponding to the Doppler mapping were identified and spared (Fig. 2). IBR was then performed, in 1-stage or 2-stage procedures, depending on patient and surgeon preference, as described in earlier works.13,14
Fig 2.
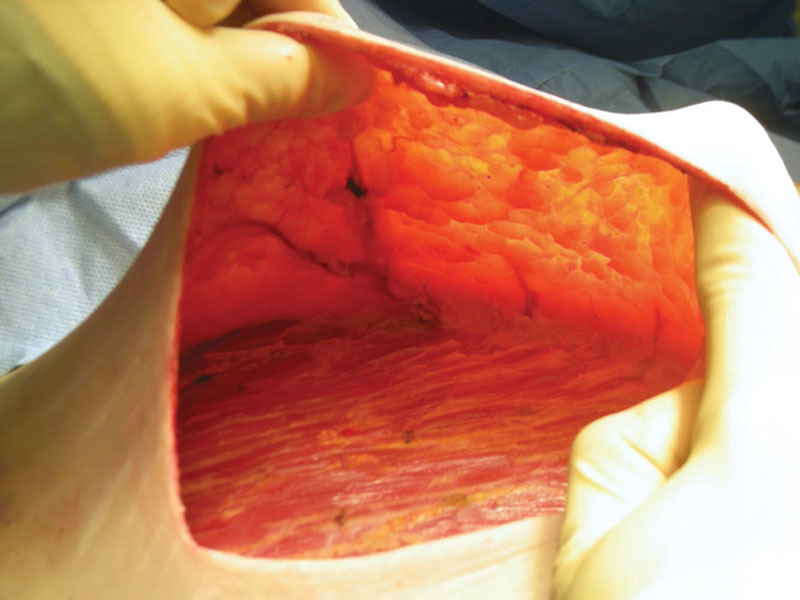
Intraoperative preservation of IMA perforators.
This article was composed with the highest ethical standards and that the Institutional Review Board of Weill Medical College (New York, N.Y.) approved all study procedures in accordance with state and federal guidelines.
RESULTS
On hundred ninety-four NSM with IBR (117 patients) were reviewed in this series: 97 breasts (56 patients) did not receive Doppler ultrasound (group A) and 97 breasts (61 patients) did (group B). No patients were excluded from the database because of demographic factors, risk factors, oncologic burden, or postoperative results. When the ultrasound Doppler was used, all patients had identifiable IMA perforators, and the corresponding vasculature was visualized in flap dissection. There were no adverse events related to ultrasound. This clinical application added approximately 4 minutes to the surgical procedure. The results are summarized in Table 1.
Table 1.
Summary of Patient Demographics, Surgical Indications, Operative Technique, and Postoperative Complications
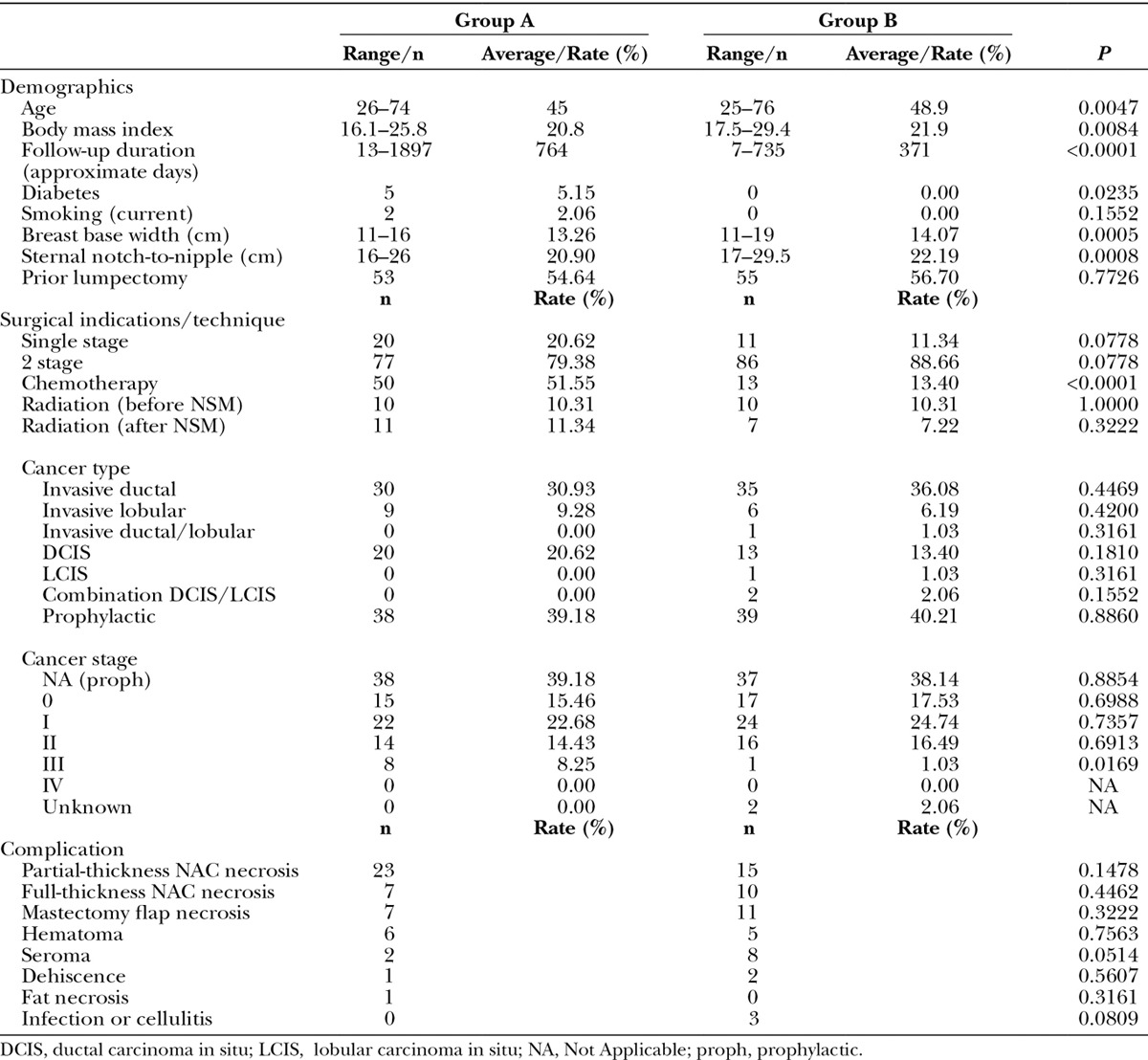
This series demonstrated the use of Doppler ultrasound to define the vascular anatomy of mastectomy skin flaps; this study was not powered to correlate NAC ischemia with prespecified demographic criteria, comorbid conditions, or operative details. As such, no statistically significant relationship could be found between NAC ischemia and these endpoints. For example, for a 2-tailed Fisher’s exact test with n=97 in each group, and full-thickness NAC ischemia of 7.2% for group A and 10.3% for group B, and type I error of 0.05, the statistical power is low, 7.5%.
DISCUSSION
NAC ischemia after NSM occurs in 2.5%–60% of patients; rates vary significantly between institutions with respect to patient selection criteria, operative technique, and other factors.4–8 Previous investigators have reported surgical techniques to reduce the rate of NAC ischemia in NSM. In his series of NSM, Stolier et al15 discusses the importance of the incision to preserve sufficient inflow to the NAC. The most commonly employed incisions in NSM are inframammary, radial, and lateral.16–18 Colwell et al19 suggest that an inferior radial incision optimizes IMA exposure and nipple blood supply. In our experience, inframammary incisions provide superior cosmetic results and maintain adequate perfusion of the NAC.
Strategies for NSM preservation have been reported. Mastectomy flap thickness and sharp dissection with minimal use of electrocautery have been described.20 For high-risk nipple necrosis, surgeons have surgically delayed the NAC to maximize the viability of the nipple for a future NSM.1,21 Furthermore, preoperative patient selection of women with small, nonptotic breasts with limited comorbidities improves surgical aesthetic outcome for NSM.4–8 Also, adjunctive postoperative measures such as topical nitroglycerin paste have been useful.22
More advanced technologies that aid in the objective diagnosis of ischemia are currently in development, such as the SPY Elite System (LifeCell, Bridgewater and Branchburg, N.J.). For example, a study by Komorowska-Timek and Gurtner23 showed a significant decrease in ischemic complications from 15.1% to 4% (P < 0.01) after laser-assisted indocyanine green perfusion mapping was performed. Given the limited reports of SPY and the cost ($1000.00 with each screening and the fixed cost of the imaging device), we opted not to use this technique in our study.
Although Doppler ultrasound has been used to identify the vascular supply to the NAC in breast surgery,11 our investigation uniquely reports its use with NSM and IBR; however, there are several limitations of this article. This investigation is a small case series designed to highlight a novel technique; this article is not powered to draw correlative conclusions about comorbid conditions or operative details, which may be expected to play a role in NAC ischemia.
CONCLUSIONS
Preoperative Doppler ultrasound of IMA perforators in NSM with IBR is a clinically useful adjunct to visualize perfusion of mastectomy skin flap to maximize nipple viability. In addition, this technique is easy, inexpensive, and rationally based.
ACKNOWLEDGMENTS
We would like to thank Dr. Andrew Weinstein of New York Presbyterian Hospital Plastic Surgery Division for statistical analysis.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238:120–127. doi: 10.1097/01.SLA.0000077922.38307.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rusby JE, Kirstein LJ, Brachtel EF, et al. Nipple-sparing mastectomy: lessons from ex vivo procedures. Breast J. 2008;14:464–470. doi: 10.1111/j.1524-4741.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 3.Shestak KC, Gabriel A, Landecker A, et al. Assessment of long-term nipple projection: a comparison of three techniques. Plast Reconstr Surg. 2002;110:780–786. doi: 10.1097/00006534-200209010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Radovanovic Z, Radovanovic D, Golubovic A, et al. Early complications after nipple-sparing mastectomy and immediate breast reconstruction with silicone prosthesis: results of 214 procedures. Scand J Surg. 2010;99:115–118. doi: 10.1177/145749691009900302. [DOI] [PubMed] [Google Scholar]
- 5.Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol. 2012;38:125–129. doi: 10.1016/j.ejso.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 7.Komorowski AL, Zanini V, Regolo L, et al. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg. 2006;30:1410–1413. doi: 10.1007/s00268-005-0650-4. [DOI] [PubMed] [Google Scholar]
- 8.Regolo L, Ballardini B, Gallarotti E, et al. Nipple sparing mastectomy: an innovative skin incision for an alternative approach. Breast. 2008;17:8–11. doi: 10.1016/j.breast.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Findlay EJ. Aesthetic Breast Surgery: Concepts and Techniques. St. Louis:: Quality Medical; 2011. Applied anatomy: key concepts for modern breast surgery. pp. 67–69. [Google Scholar]
- 10.Palmer JH, Taylor GI. The vascular territories of the anterior chest wall. Br J Plast Surg. 1986;39:287–299. doi: 10.1016/0007-1226(86)90037-8. [DOI] [PubMed] [Google Scholar]
- 11.Basaran K, Ucar A, Guven E, et al. Ultrasonographically determined pedicled breast reduction in severe gigantomastia. Plast Reconstr Surg. 2011;128:252e–259e. doi: 10.1097/PRS.0b013e3182268bb1. [DOI] [PubMed] [Google Scholar]
- 12.Vlajcic Z, Zic R, Stanec S, et al. Nipple-areola complex preservation: predictive factors of neoplastic nipple-areola complex invasion. Ann Plast Surg. 2005;55:240–244. doi: 10.1097/01.sap.0000171680.49971.85. [DOI] [PubMed] [Google Scholar]
- 13.Dent BL, Small K, Swistel A, et al. Nipple-areolar complex ischemia after nipple-sparing mastectomy with immediate implant-based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J. 2014;34:560–570. doi: 10.1177/1090820X14528352. [DOI] [PubMed] [Google Scholar]
- 14.Huston TL, Small K, Swistel AJ, et al. Nipple-sparing mastectomy via an inframammary fold incision for patients with scarring from prior lumpectomy. Annals. 2014 doi: 10.1097/SAP.0000000000000004. In press. [DOI] [PubMed] [Google Scholar]
- 15.Stolier AJ, Sullivan SK, Dellacroce FJ. Technical considerations in nipple-sparing mastectomy: 82 consecutive cases without necrosis. Ann Surg Oncol. 2008;15:1341–1347. doi: 10.1245/s10434-007-9753-5. [DOI] [PubMed] [Google Scholar]
- 16.Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203:704–714. doi: 10.1016/j.jamcollsurg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Stanec Z, Zic R, Stanec S, et al. Skin-sparing mastectomy with nipple-areola conservation. Plast Reconstr Surg. 2003;111:496–498. doi: 10.1097/00006534-200301000-00099. [DOI] [PubMed] [Google Scholar]
- 18.Woods JE. Subcutaneous mastectomy: current state of the art. Ann Plast Surg. 1983;11:541–550. doi: 10.1097/00000637-198312000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg. 2010;65:140–143. doi: 10.1097/SAP.0b013e3181c1fe77. [DOI] [PubMed] [Google Scholar]
- 20.Stolier AJ, Levine EA. Reducing the risk of nipple necrosis: technical observations in 340 nipple-sparing mastectomies. Breast J. 2013;19:173–179. doi: 10.1111/tbj.12078. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol. 2012;19:3171–3176. doi: 10.1245/s10434-012-2528-7. [DOI] [PubMed] [Google Scholar]
- 22.Kutun S, Ay AA, Ulucanlar H, et al. Is transdermal nitroglycerin application effective in preventing and healing flap ischaemia after modified radical mastectomy? S Afr J Surg. 2010;48:119–121. [PubMed] [Google Scholar]
- 23.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1073. doi: 10.1097/PRS.0b013e3181d17f80. [DOI] [PubMed] [Google Scholar]


