Abstract
Background:
Long-gap peripheral nerve defects arising from tumor, trauma, or birth-related injuries requiring nerve reconstruction are currently treated using nerve autografts and nerve allografts. Autografts are associated with limited supply and donor-site morbidity. Allografts require administration of transient immunosuppressants, which has substantial associated risks. To overcome these limitations, we investigated the use of detergent-free decellularized nerve grafts to reconstruct long-gap nerve defects in a rodent model and compared it with existing detergent processing techniques.
Methods:
Nerve grafts were harvested from the sciatic nerves of 9 donor rats. Twenty-four recipient rats were divided into 4 groups (6 animals per group): (1) nerve grafts (NG, positive control), (2) detergent-free decellularized (DFD) grafts, (3) detergent decellularized grafts, and (4) silicone tube conduits (negative control). Each recipient rat had a 3.5-cm graft or conduit sutured across a sciatic nerve transection injury. All animals were harvested at 12 weeks postimplantation for functional muscle analysis and nerve histomorphometry.
Results:
Histomorphometry results indicated maximum growth in NG when compared with other groups. DFD and detergent decellularized groups showed comparable regeneration at 12 weeks. Silicone tube group showed no regeneration as expected. Muscle force data indicated functional recovery in NG and DFD groups only.
Conclusions:
This study describes a detergent-free nerve decellularization technique for reconstruction of long-gap nerve injuries. We compared DFD grafts with an established detergent processing technique and found that DFD nerve grafts are successful in promoting regeneration across long-gap peripheral nerve defects as an alternative to existing strategies.
Peripheral nervous system injuries are very common, accounting for approximately 2.8% of all traumatic injuries leading to lifelong disabilities.1 Upon injury, the peripheral nervous system has an inherent capacity to regenerate to a certain extent. In cases of severe injury, such as long-gap defects measuring ≥3 cm, the outcome of treatment is often unsatisfactory.2,3 The “gold standard” for treating long-gap injuries uses autologous nerve grafts (autografts) obtained from the patient’s own body. Although there are many benefits of using autografts, limitations such as donor-site morbidity and inadequate supply have led to the search for alternative treatment strategies.4–6
Allogeneic nerve grafts (allografts) are a proven clinical substitute for autografts. They are readily available and contain cellular and structural components similar to autografts that support nerve regeneration.7 Even though allografts support nerve regeneration, their major limiting factor is the requirement for systemic immunosuppression. This exposes patients to risks of infection, toxicity, malignancy, and other complications.8,9
There is an increasing interest in decellularized nerve grafts for peripheral nerve repair.7,8,10 Decellularized nerve grafts have intact basal lamina and extracellular matrix proteins to support nerve regeneration. The advantage of decellularized nerve grafts is that the cellular components from the nerve tissues are eliminated, overcoming the issue of antigenicity.11,12 Some of the existing nerve decellularization techniques include (a) cold preservation; (b) freezing and freeze-thaw; (c) chemical detergent clearing; and (d) irradiation technique.10,11 Although initial results have been promising, decellularized nerve grafts are still limited by disrupted endoneurial tubes, damaged basal lamina, poor axonal regeneration, and reduced distance of regeneration, which hinder their use for long-gap nerve repair.9,10
In this work, we sought to develop a detergent-free nerve decellularization technique to obtain functional regeneration across a 35-mm long nerve gap in a rat model of peripheral nerve injury. We also compared our detergent-free technique with established detergent-processed nerve grafts for reconstructing a long-gap nerve injury.
MATERIALS AND METHODS
All animal procedures were performed as per approved Institutional Animal Care and Use Committee protocols of the University of Texas Southwestern Medical Center at Dallas, Texas. Rats were anesthetized using intraperitoneal injection of ketamine hydrochloride (75 mg/kg)/dexmeditomidine hydrochloride (0.5 mg/kg) cocktail. Animals were euthanized with intraperitoneal injection of sodium pentobarbital (120 mg/kg).
Sciatic Nerve Harvest
Sciatic nerves were harvested from both hind limbs of 9 donor rats (male, Lewis, >350 g). Rats were anesthetized as described above and both hind limbs were shaved and sterilized using alcohol prep pads and povidone-iodine 3 times. Under aseptic conditions, the skin was incised using a scalpel, and the sciatic nerve was exposed by a thigh muscle-splitting procedure. The entire length of the sciatic nerve from the sciatic notch to the distal trifurcation was dissected and harvested, yielding nerves measuring approximately 4.2 cm in length. All nerve processing was performed under sterile conditions. After nerve harvest, donor animals were euthanized as noted above.
Detergent-free Decellularized Nerve Grafts
Detergent-free decellularized (DFD) nerve grafts were obtained using a protocol developed in our laboratory. Freshly harvested sciatic nerves were rinsed in a solution containing Dulbecco’s modified Eagle medium (DMEM, Gibco) with 10% fetal bovine serum (HyClone) and 4% penicillin/streptomycin/amphotericin B (Antibiotic-Antimycotic, Gibco). Nerves were secured to sterile rubber holders (5 × 1 × 1 cm) using 10-0 nylon suture (AROSurgical) for maintaining nerve length throughout graft processing.
Nerves were transferred into 15-ml conical tubes containing 7-ml DMEM with 10% fetal bovine serum and 2% penicillin/streptomycin/amphotericin (DMEM-10) and were cultured at 37°C with 5% CO2 for 2 weeks under constant agitation. During the culture period, 3 ml of medium was replaced with 3.5 ml of fresh DMEM-10 every 3 days to replenish nutrients in the medium. This process was performed to initiate Wallerian degeneration (WD) in vitro to clear axonal and myelin debris inside the nerves.
After the 2-week culture period, nerves were transferred to new 15-ml conical tubes containing phosphate-buffered saline (PBS) and kept for 1 week at 37°C with 5% CO2 under constant agitation. This process was used to decellularize the nerves by abruptly terminating nutrient supply.
Once the processing was complete, DFD grafts were stored at 4°C until implantation. At the time of implantation, DFD grafts were trimmed to 3.5 cm length and implanted in reversed orientation across transected right sciatic nerves as described below.
Sample DFD grafts were processed for histologic assessment. Processed grafts were immersion fixed in 4% paraformaldehyde overnight and washed in PBS. Nerves were embedded in optimal cutting temperature compound and snap frozen in liquid nitrogen for cryosectioning. Cross-sections were blocked with 4% goat serum (Life Technologies) and stained with mouse anti-laminin B2 gamma 1 (D18) primary antibody (Abcam, ab80581, 1:300) and goat anti-mouse Alexa Fluor 488 secondary antibody (Molecular Probes, A11017, 1:300). For imaging myelin and cellular components in DFD-processed grafts, samples were immersion fixed in 3% glutaraldehyde, postfixed in osmium tetroxide and embedded in Araldite 502. Ultrathin sections were stained using uranyl acetate-lead citrate solution for high-magnification imaging using transmission electron microscopy (TEM, FEI Tecnai G2 Spirit Biotwin).13
Detergent Decellularized Grafts
For processing detergent decellularized (DD) grafts, established protocols by Hudson et al14 and Neubauer et al15 were used to perform detergent processing and to eliminate chondroitin sulfate proteoglycans. All reagents used were purchased from Sigma-Aldrich Chemicals, unless specified. Freshly harvested sciatic nerves (4.2 cm) were placed in Roswell Park Memorial Institute medium to clear connective and fatty tissues. As described previously, nerves were secured to sterile rubber holders (5 × 1 × 1 cm) using 10-0 nylon suture for maintaining nerve length throughout the decellularization process.
Detergent processing was performed first to decellularize the nerves. Nerves were placed in 15-ml conical tubes containing deionized distilled water and agitated at 25°C for 7 hours. Table 1 describes the formulation of buffers and solutions used for DD graft processing. Following deionized water wash, the nerves were transferred to 15-ml conical tubes containing sulfobetaine-10 buffer and agitated at 25°C for 15 hours. Nerves were rinsed with washing buffer for 15 minutes. The washing buffer was replaced with SB-16 buffer, and the nerves were again agitated at 25°C for 24 hours. Nerves were rinsed in washing buffer 3 times for 5 minutes each. Nerves were then transferred into new 15-ml tubes containing SB-10 buffer and agitated at 25°C for 7 hours and rinsed with washing buffer for 15 minutes. The washing buffer was replaced with SB-16 buffer and agitated at 25°C for 15 hours, followed by 3 washes with 10 mM phosphate-50 mM sodium buffer for 15 minutes each.
Table 1.
Formulations Used for Preparing Buffers and Detergent Solutions

Following detergent processing, treatment with chondroitinase ABC was performed to eliminate inhibitory chondroitin sulfate proteoglycans.15 Detergent-processed nerves were incubated in PBS containing 2 U/ml chondroitinase ABC for 16 hours at 37°C and 5% CO2 in a cell culture incubator. To complete the process, the nerves were washed using cold Ringer’s solution 3 times for 15 minutes each and stored in Ringer’s solution at 4°C until implantation. At the time of implantation, DD grafts were trimmed to 3.5 cm length and implanted in reversed orientation across transected right sciatic nerves as described below.
Experimental Setup
Twenty-four rats (male, Lewis, 250–300 g) were randomly assigned to 4 groups: (1) nerve grafts (NG, unprocessed grafts used as positive control, n = 6), (2) DFD grafts (n = 6), (3) DD grafts (n = 6), and (4) silicone tube (ST) conduits (1.6 mm internal diameter, negative control, n = 6). All groups were assessed at 12 weeks postimplantation to measure gastrocnemius muscle tetanic tension and wet muscle mass and to obtain distal sciatic nerve samples for histomorphometry.
In recipient rats, the right sciatic nerve was exposed using a thigh-splitting approach under anesthesia. The sciatic nerve was transected at midthigh (1 cm proximal to trifurcation) and reconstructed using 3.5-cm-long reversed nerve graft (NG, DFD, and DD) or 3.5-cm-long hollow ST conduit. In the NG group, nerve grafts were implanted immediately after harvest from donor rats. For accommodating the long length of grafts/conduits, constructs were looped around the anterior head of biceps femoris muscle as described in our previous work.16 Nerve grafts were coapted to the proximal and distal stumps of the sciatic nerve using 10-0 nylon (AROSurgical) epineurial sutures under an operating microscope. In the ST group, proximal and distal stumps were placed inside the lumen of the tube and secured using horizontal mattress sutures of 7-0 polypropylene (Ethicon). Muscles were closed using 4-0 Vicryl sutures (Ethicon), and skin was closed using staples (Appose ULC, 35R). Animals were given buprenorphine, and carprofen tablets (Rimadyl, Pfizer) were placed in the cage for postoperative analgesia. All groups were harvested at 12 weeks postimplantation for analysis of recovery in the gastrocnemius muscle and regeneration in the distal sciatic nerve.
Gastrocnemius Tetanic-specific Tension and Wet Muscle Mass
At 12 weeks postimplantation, animals were anesthetized for evaluation of muscle functional recovery. Animals were immobilized in a rigid frame consisting of a stereotaxic head holder and clamps on the pelvis. The hind limb under study was further stabilized with a clamp on the hind foot. The gastrocnemius muscle in both experimental and contralateral sides was exposed and freed from the soleus and plantaris muscles that were excised. The Achilles tendon was isolated with its calcaneal insertion and detached from the remainder of the bone. A 4-0 nylon suture was tied to form a loop at the tendon insertion and attached to a strain gauge (Kulite BG1250) along the line of pull of the muscle for tension measurements. Stimulation of the sciatic nerve was performed using a bipolar hook electrode placed proximal to the nerve reconstruction. For tension measurements, the nerve was stimulated with 100 µs square pulses with voltage strength 3× above twitch threshold. Muscle length was then adjusted to produce peak twitch tension, and all remaining tension measurements were digitized and recorded at this length (CED 1401 Plus, Signal 3.0). To determine the peak tetanic tension that the muscle could produce, the sciatic nerve was stimulated at 100 pulses/s for 600 ms. The gastrocnemius muscles were then harvested to measure wet muscle mass. The ratio of experimental to contralateral muscle mass was used for comparison among groups. For comparison of tetanic tension between groups, ratio of experimental to contralateral muscle-specific tension (tension per gram of tissue) was calculated. Muscle-specific tension and wet mass ratios were compared between groups using Student’s t test.
Semiautomated Quantitative Histomorphometry
The distal sciatic nerve stumps were harvested for nerve histomorphometry. Nerves were prepared as per established protocols.17,18 Nerve tissues were immersion fixed in 3% glutaraldehyde at 4°C and postfixed using 1% osmium tetroxide. Serial dehydration was performed following fixation using ethanol, and specimens were embedded using Araldite 502 and cut into semithin sections followed by staining with 1% toluidine blue dye and mounting onto glass slides for imaging. Leco IA32 Image Analysis System was used for quantification of nerve samples. This setup was used to calculate the total fascicle area of the nerve specimen. To calculate the total axons, myelin width, percentage fibers, and axonal density, 5 randomly selected high-magnification images (1000×) per sample were used. Data comparison was performed using Newman-Keul’s post hoc test.
RESULTS
Characterization of DFD Grafts
Immunohistochemistry revealed intact endoneurial basal lamina, shown by the maintenance of laminin rings in the DFD nerve grafts as seen in Figure 1. TEM evaluation of the DFD grafts showed myelin reduction and degradation, clearance of axonal components, and elimination of cellular nuclei inside the grafts. Through these studies, we confirmed the degradation of myelin and the elimination of axonal and cellular material using our in vitro detergent-free processing technique.
Fig. 1.
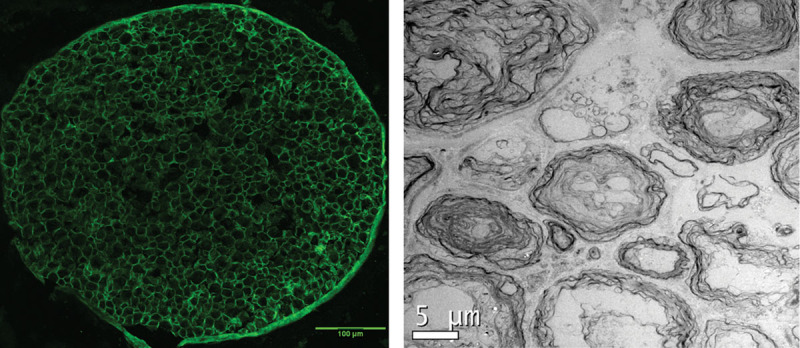
Anti-laminin staining of DFD nerve grafts showing intact endoneurial basal lamina. TEM image showing absence of nuclei and degraded myelin as an indication that decellularization has occurred.
Muscle Tension Recovery
Only NG (3 animals out of 6) and DFD (3 animals out of 5 that regenerated) groups demonstrated recovery of gastrocnemius function upon sciatic nerve stimulation. The DD group did not show any gastrocnemius contractile function. There was no significant difference in tetanic-specific tension between NG and DFD groups. However, wet muscle mass ratio in NG group was significantly greater than that of DFD group (Fig. 2).
Fig. 2.
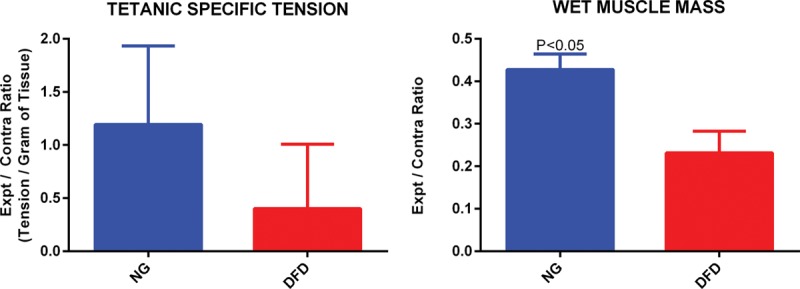
Muscle tetanic-specific tension ratio and wet muscle mass ratio comparison between NG and DFD groups. Student’s t test was used for statistical analysis.
Myelinated Axon Quantification Using Histomorphometry
Total axon count, percent myelinated fibers, and axon density were significantly higher in NG group; DFD and DD groups showed comparable nerve regeneration. The myelin width indicated no significant difference in maturity of axonal myelination among all groups (Fig. 3). There was no initiation of nerve regeneration in the ST group, as expected for the negative control (previously described “no regrowth” model).16
Fig. 3.
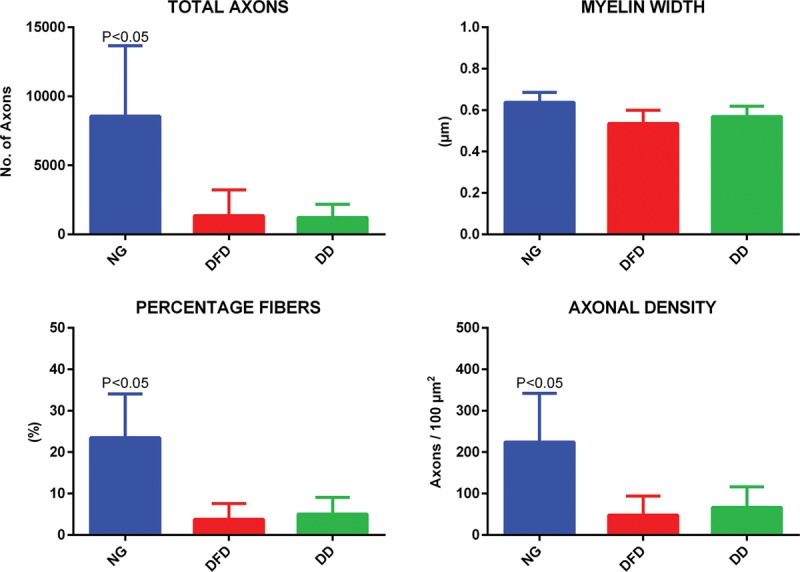
Quantitative histomorphometry comparison among NG, DFD, and DD groups. Total axons, myelin width, percentage fibers, and axonal density were used for comparison. Newman-Keul’s post hoc test was used for statistical analysis.
DISCUSSION
Regeneration across long-gap peripheral nerve injuries has been a frequently investigated research topic due to incomplete or absent functional recovery using existing modalities.19 Although autografts provide partial restoration of function, reconstruction is obtained by sacrificing function at the donor site. Other concerns of using autografts include inadequate supply and risk of pain or infection at the donor site.5 Allografts, on the other hand, are plentiful and are supplied with the native cellular and structural components that promote nerve regeneration. The use of allografts is limited by the strong immune reaction induced upon implantation into the recipient. To overcome this, systemic immunosuppression is required, which places the patient at risk of infection, malignancy, toxicity, and other complications.10 Synthetic nerve conduits have been used to overcome the disadvantages associated with autografts and allografts but are limited to noncritical defects (<3 cm) and are susceptible to misdirected target reinnervation.20 Because the above-mentioned strategies for nerve repair have substantial associated drawbacks, decellularized nerve grafts have been increasingly favored by peripheral nerve surgeons.
Decellularized nerve grafts possess structural components for supporting nerve growth, minimal immunogenicity, and ready availability, which make them a suitable alternative for nerve reconstruction.12 Clinical use of decellularized nerve grafts for extremely large critical defects (~7 cm) is being performed without sufficient experimental evidence,21 and to date, there have been no animal studies showing functional nerve regeneration across critical defects (≥3 cm) using decellularized nerve grafts.
Currently available options that use chemical detergents for decellularization of tissues can have toxic side effects if the detergents are not completely eliminated after processing.22,23 Because lipids are the major constituents of peripheral nerve myelin,24 residual chemical detergents from decellularized nerve grafts theoretically could hinder myelin formation and impair long-term maintenance of the myelin sheath. On the other hand, freeze-thaw techniques can be employed to avoid the use of chemicals, but they have their own limitations. Freeze-thawing kills the cells but does not eliminate their residue. Freeze-thawed nerves possess critical length limitations (2–3 cm),10 and the rapid tissue expansion and contraction associated with the freeze-thaw process can damage the continuity of the basal lamina and thereby impede axonal regeneration across a long nerve gap defect.
To overcome the limitations associated with currently available processing techniques, we investigated a detergent-free-processed nerve graft for reconstructing a 3.5-cm long-gap nerve injury. Our processing technique is inspired by WD, which is widely known as a naturally occurring in vivo event after nerve injury. WD occurs in the distal nerve stump, where activated Schwann cells, along with recruited extraneural cells, clear axonal and myelin debris and local inhibitory factors. WD is imperative for providing a growth-permissive environment for regenerating axons.25 Our processing technique uses the Schwann cells contained within our nerve grafts to degrade the residual myelin and to condition the environment for regenerating axons.
DFD grafts were first placed in growth-supportive DMEM-10 to support Schwann cells that are located within the grafts,26 to initiate in vitro WD. To subsequently eliminate these cells, we terminated the nutrient supply by replacing DMEM-10 with PBS. This nerve processing protocol yielded grafts with degeneration of myelin and absence of axonal components as seen by TEM (Fig. 1). We were able to maintain intact basal lamina rings as seen in Figure 1. This preserved endoneurial environment could provide a natural pathway for regenerating axons.
After axons regenerate across a nerve graft, recovery of muscle function is obtained when the regenerated axons innervate the appropriate targets. We measured gastrocnemius muscle tetanic-specific tension and wet muscle mass to compare functional muscle recovery following nerve regeneration across NG, DFD, and DD groups. At 12 weeks, we observed muscle function across NG and DFD reconstructions. There was no significant difference between the 2 groups in terms of tetanic-specific tension, but the wet muscle mass in NG group was significantly higher than that of DFD group (Fig. 2). One possible reason behind the greater muscle mass recovery in NG animals could be faster nerve regeneration leading to earlier muscle reinnervation.27 Unprocessed nerve grafts (NG group) contain all of the cellular and structural components required for nerve regeneration, which can optimize speed of nerve regeneration and thereby minimize time of muscle denervation, leading to reduced muscle atrophy and higher muscle mass compared with DFD group.
We examined the distal nerve stumps to compare nerve regeneration in NG, DFD, and DD groups using quantitative histomorphometry (Fig. 3). Parameters used for evaluation were total axons, myelin width, percentage fibers, and axonal density within the nerve. Axonal regeneration into the distal nerve stump was observed in all 3 groups. NG group had superior regeneration and was significantly higher when compared with DFD and DD groups in terms of total axons, percentage of myelinated fibers, and axon density. This also could account for the improved wet muscle mass in the NG group compared with the DFD group as mentioned earlier. Myelin width was comparable among all 3 groups, indicating that the maturity of myelination is similar among regenerated axons. It is notable that the DFD and DD groups had comparable nerve regeneration in terms of total axons, percentage myelinated fibers, and axon density. Even though nerve regeneration was similar in the 2 groups (DFD and DD), muscle functional recovery was observed only in the DFD group. This outcome indicates that our processing technique is more favorable for obtaining functional nerve regeneration across a long-gap nerve defect.
CONCLUSIONS
In this work, we have demonstrated a detergent-free decellularization technique for long-gap peripheral nerve reconstruction. By initiating WD inside the nerve grafts in vitro, a growth-permissive environment for regenerating axons was established. Nerve regeneration was similar to that of detergent-processed grafts, but our detergent-free-processed grafts yielded functional nerve regeneration that was not present in detergent-processed grafts.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was conducted with support from UT Southwestern Clinical and Translational Alliance for Research and National Institutes of Health/National Center for Advancing Translational Sciences (grant number UL1TR000451). The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 2.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 3.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Battiston B, Geuna S, Ferrero M, et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 5.Ehretsman RL, Novak CB, Mackinnon SE. Subjective recovery of nerve graft donor site. Ann Plast Surg. 1999;43:606–612. doi: 10.1097/00000637-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Santosa KB, Jesuraj NJ, Viader A, et al. Nerve allografts supplemented with schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve. 2013;47:213–223. doi: 10.1002/mus.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivlin M, Sheikh E, Isaac R, et al. The role of nerve allografts and conduits for nerve injuries. Hand Clin. 2010;26:435–446, viii. doi: 10.1016/j.hcl.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clin Plast Surg. 2011;38:643–660. doi: 10.1016/j.cps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 10.Szynkaruk M, Kemp SW, Wood MD, et al. Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng Part B Rev. 2013;19:83–96. doi: 10.1089/ten.TEB.2012.0275. [DOI] [PubMed] [Google Scholar]
- 11.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 12.Nagao RJ, Lundy S, Khaing ZZ, et al. Functional characterization of optimized acellular peripheral nerve graft in a rat sciatic nerve injury model. Neurol Res. 2011;33:600–608. doi: 10.1179/1743132810Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 13.Mackinnon S, Hudson A, Falk R, et al. Nerve allograft response: a quantitative immunological study. Neurosurgery. 1982;10:61–69. doi: 10.1227/00006123-198201000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 15.Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207:163–170. doi: 10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasudevan S, Yan JG, Zhang LL, et al. A rat model for long-gap peripheral nerve reconstruction. Plast Reconstr Surg. 2013;132:871–876. doi: 10.1097/PRS.0b013e31829fe515. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DA, Moradzadeh A, Whitlock EL, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox IK, Brenner MJ, Johnson PJ, et al. Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery. 2012;32:552–562. doi: 10.1002/micr.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes O, Sosa I, Kuffler DP. Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J. 2005;24:215–223. [PubMed] [Google Scholar]
- 20.Pfister BJ, Gordon T, Loverde JR, et al. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 21.Moore AM, MacEwan M, Santosa KB, et al. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44:221–234. doi: 10.1002/mus.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cebotari S, Tudorache I, Jaekel T, et al. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs. 2010;34:206–210. doi: 10.1111/j.1525-1594.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 23.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia RH, Yosef N, Burns AR, et al. Isolation, purification and verification of peripheral nerve myelin derived from bovine cauda equina. J Neurol Neurophysiol. 2012 S7. doi.org/10.4172/2155-9562.S7-002. [Google Scholar]
- 25.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Mauritz C, Grothe C, Haastert K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J Neurosci Res. 2004;77:453–461. doi: 10.1002/jnr.20166. [DOI] [PubMed] [Google Scholar]
- 27.Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


