Abstract
Long-term production of murine hematopoietic cells in vitro is dependent on establishment of a complex microenvironment consisting of a variety of stromal cells and an extensive extracellular matrix which includes collagen, fibronectin, laminin, proteoglycans, and other undefined components adherent to the culture dishes. Cis-4-hydroxyproline (CHP), a relatively specific inhibitor of collagen secretion, was used to examine the role of extracellular collagen deposition in supporting hematopoiesis in long-term C57B1/6J mouse bone marrow cell cultures. Throughout the 10-wk culture period, all culture dishes contained either 0, 10, 25, or 50 micrograms/ml of CHP. All medium and nonadherent cells were removed at weekly intervals and replaced with fresh medium containing the previous concentrations of CHP. Nonadherent cells were assayed weekly for total cells and pluripotent, erythroid, megakaryocytic, and granulocytic-macrophage progenitor cells. Dishes were killed at selected intervals to assess protein and collagen synthesis in the adherent layer. Adherent cell numbers, as judged by microscopic examination and DNA assays, correlated inversely with CHP concentrations used and paralleled degree of collagen synthesis inhibition. The decreased hemopoietic progenitor cell production correlated closely with percent inhibition of collagen synthesis and stromal cellularity. The CHP concentrations tested were not directly toxic to hemopoietic progenitor cells. These studies demonstrate that collagen deposition in the extracellular matrix of murine bone marrow cell cultures is essential to the establishment of a functional stromal microenvironment that is supportive of long-term hematopoiesis.
Full text
PDF

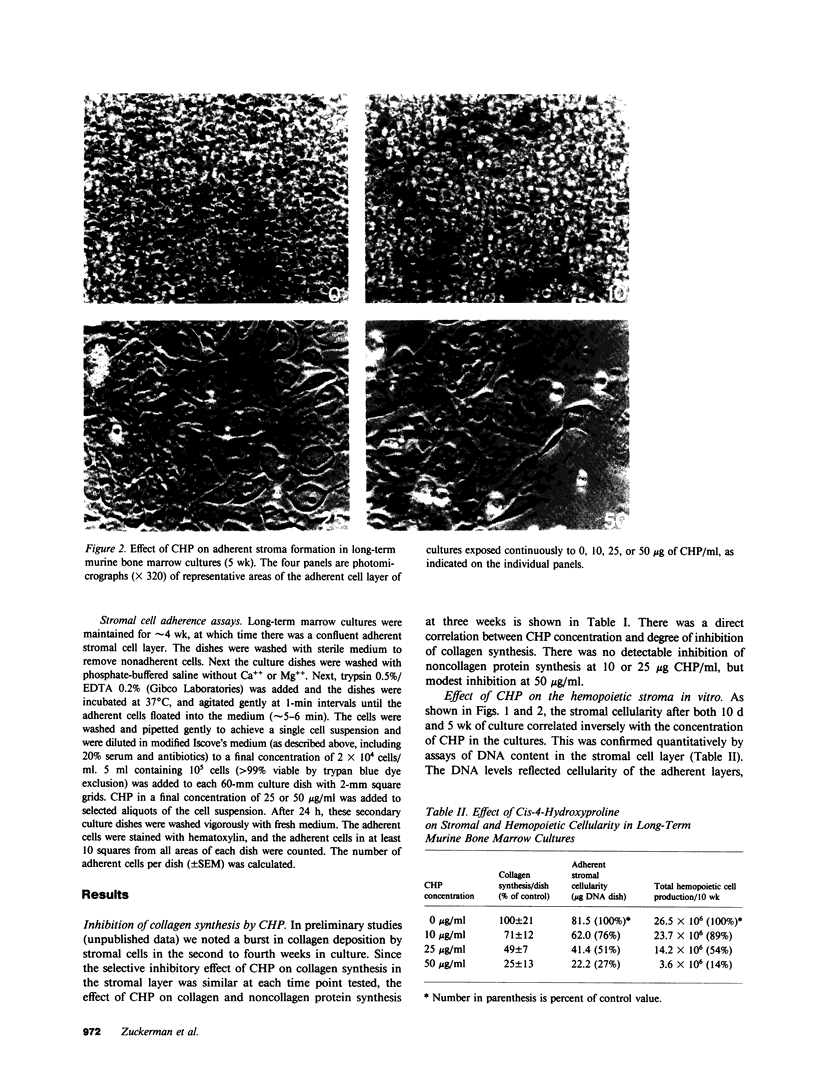

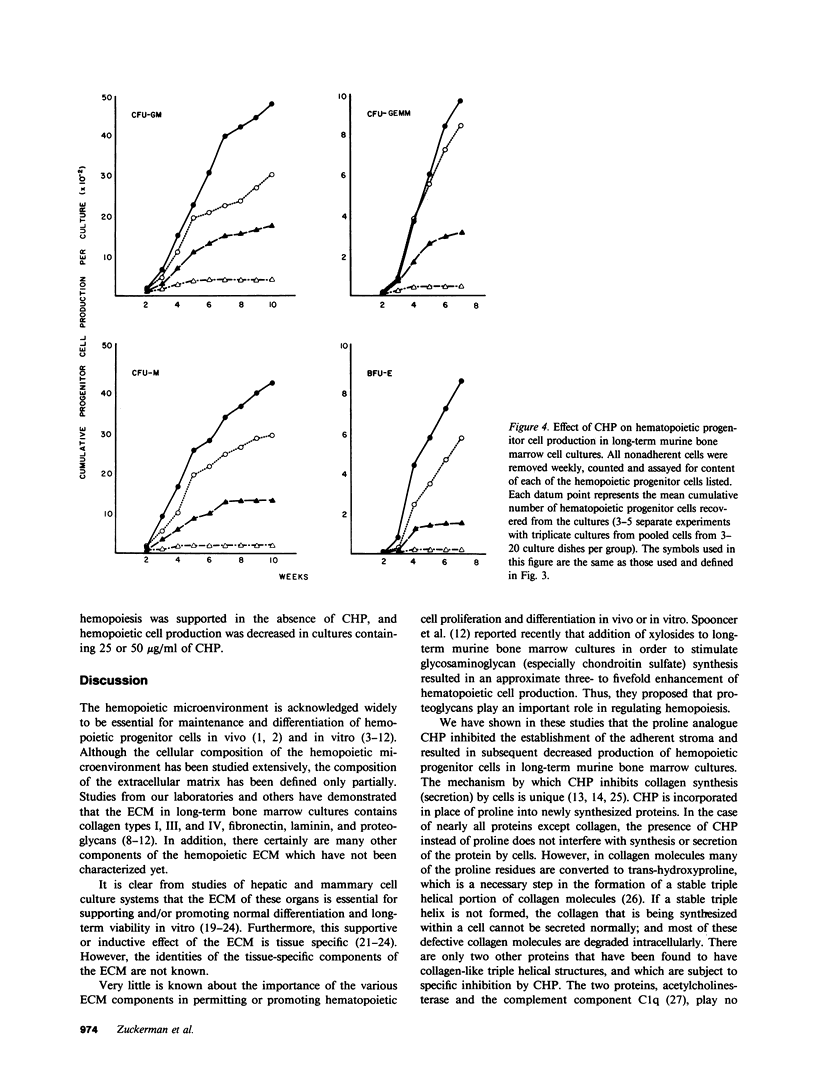

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley S. A. Collagen synthesis by bone marrow stromal cells: a quantitative study. Br J Haematol. 1982 Mar;50(3):491–497. doi: 10.1111/j.1365-2141.1982.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Bentley S. A., Foidart J. M. Some properties of marrow derived adherent cells in tissue culture. Blood. 1980 Dec;56(6):1006–1012. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G., Allen T. D., Rutherford T., Scolnick E. Molecular and cell biologic aspects of erythropoiesis in long-term bone marrow cultures. Blood. 1981 Oct;58(4):699–707. [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G. Differentiation and proliferation of hemopoietic cells in culture. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Ponce P., Cordero J., Rojkind M. A noncollagenous matrix for attachment of rat hepatocytes in culture. Hepatology. 1981 May-Jun;1(3):204–210. doi: 10.1002/hep.1840010303. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Gaitmaitan Z., Arias I., Ponce P., Rojkind M. Long-term cultures of normal rat hepatocytes on liver biomatrix. Ann N Y Acad Sci. 1980;349:70–76. doi: 10.1111/j.1749-6632.1980.tb29516.x. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Rojkind M. New techniques for culturing differentiated cells: reconstituted basement membrane rafts. Methods Enzymol. 1979;58:263–278. doi: 10.1016/s0076-6879(79)58142-7. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Gallagher J. T., Krizsa F., Dexter T. M. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983 Feb;96(2):510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Studies on hemopoietic microenvironments. Report of a workshop held in La Jolla,, California, August 8-9, 1974. Exp Hematol. 1975 Aug;3(4):213–226. [PubMed] [Google Scholar]
- Tavassoli M., Takahashi K. Morphological studies on long-term culture of marrow cells: characterization of the adherent stromal cells and their interactions in maintaining the proliferation of hemopoietic stem cells. Am J Anat. 1982 Jun;164(2):91–111. doi: 10.1002/aja.1001640202. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Incorporation of proline analogs into procollagen. Assay for replacement of imino acids by cis-4-hydroxy-L-proline and cis-4-fluoro-L-proline. Arch Biochem Biophys. 1977 May;181(1):293–299. doi: 10.1016/0003-9861(77)90507-0. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. S. The haemopoietic microenvironment. Clin Haematol. 1979 Jun;8(2):469–500. [PubMed] [Google Scholar]
- Zuckerman K. S., Wicha M. S. Extracellular matrix production by the adherent cells of long-term murine bone marrow cultures. Blood. 1983 Mar;61(3):540–547. [PubMed] [Google Scholar]











