Abstract
Everyday consumer choices frequently involve memory, as when we retrieve information about consumer products when making purchasing decisions. In this context, poor memory may affect decision quality, particularly in individuals with memory decline, such as older adults. However, age differences in choice behavior may be reduced if older adults can recruit additional neural resources that support task performance. Although such functional compensation is well documented in other cognitive domains, it is presently unclear whether it can support memory-guided decision making and, if so, which brain regions play a role in compensation. The current study engaged younger and older humans in a memory-dependent choice task in which pairs of consumer products from a popular online-shopping site were evaluated with different delays between the first and second product. Using functional imaging (fMRI), we found that the ventromedial prefrontal cortex (vmPFC) supports compensation as defined by three a priori criteria: (1) increased vmPFC activation was observed in older versus younger adults; (2) age-related increases in vmPFC activity were associated with increased retrieval demands; and (3) increased vmPFC activity was positively associated with performance in older adults—evidence of successful compensation. Extending these results, we observed evidence for compensation in connectivity between vmPFC and the dorsolateral PFC during memory-dependent choice. In contrast, we found no evidence for age differences in value-related processing or age-related compensation for choices without delayed retrieval. Together, these results converge on the conclusion that age-related decline in memory-dependent choice performance can be minimized via functional compensation in vmPFC.
Keywords: aging, compensation, decision making, fMRI, memory, vmPFC
Introduction
Decision research has long focused on how the decision process transforms information into choice outcomes (Dean and Sharfman, 1996); however, the success of that process is determined by the information available for consideration. In everyday life, available information is often constrained by mnemonic limitations (Bettman, 1979; Alba et al., 1991), and thus memory plays a critical, albeit understudied, role in the mechanisms of decision making. Consequences of memory retrieval failures in choice processing include increased bias (for review, see Alba and Hutchinson, 2000) and the inability to effectively execute strategic decision making (Klein and D'Esposito, 2007). Thus, problems with memory function are likely to have an influence on everyday choice processing.
Problems with memory function increase in normal aging (Verhaeghen et al., 1993; Salthouse, 2004), and those effects ramify into age differences in performance on standard economic decision tasks (Henninger et al., 2010; Mata et al., 2011). During decision making, older adults can compensate for declines in fluid intelligence by using decision strategies that focus on the most salient information (Mata et al., 2007), but this strategic approach can fail when decision performance depends on memory for specific contextual details (Skurnik et al., 2005). Thus, previous research indicates age-related change in memory-dependent decision making, but the effect of aging on decision processing cannot be captured fully by behavioral research. Neuroimaging studies can help to address this problem. For example, neuroimaging can be used to determine whether older adults compensate for neural decline through recruitment of additional processes—as evident in distinct patterns of activation or of functional connectivity—to support task performance. This phenomenon, called age-related functional compensation, has been demonstrated in numerous studies in the domains of executive function, perception, emotion, and memory (for review, see Cabeza and Dennis, 2013; St Jacques et al., 2013). Some form of functional compensation, although yet not established, could bolster older adults' performance on everyday decision tasks, providing important insight into age-related individual differences in choice processing and behavior (Venkatraman et al. 2012).
We used fMRI to investigate memory-dependent decision making in younger and older humans. Images of consumer products were taken from a popular online-shopping site and presented with a star rating to represent product value. Two-option choices were made after different delays between the presentation of competing products. We sought evidence for functional compensation in memory-dependent choice processing. Specifically, we determined whether our results met the following criteria for compensation (Cabeza and Dennis, 2013): (1) increased task-related activation is observed in those with greater brain decline; (2) compensatory activity is associated with increased task demands; and (3) compensatory activity is positively associated with performance. Critically, evidence for the third criterion is a requirement for successful compensation. That is, if additional functional activation is enhancing performance, we expect a positive relationship between age-related increases in brain activation and independent measures of performance. Our results indicate that, although a frontoparietal network implicated in executive control supports memory-dependent choice, the ventromedial prefrontal cortex (vmPFC) and its connectivity to that frontoparietal network supports successful functional compensation in older adults.
Materials and Methods
Participants.
The final sample included data from 20 younger (12 males) and 22 older (eight males) adults (for sample characteristics, see Table 1). Participants had normal/corrected vision, were fluent in English, were free of MRI contraindications, and provided informed consent in accordance with rules established by the Institutional Review Board of Duke University Medical Center. All older adults were community dwelling and scored a 27 or higher on the Mini-Mental Status Exam Cognitive (Folstein et al., 1975), which is above standard cut points for cognitive impairment (Mitchell, 2009). At the end of the session, all participants completed a post-experiment questionnaire and received their compensation.
Table 1.
Demographics and behavior
| Younger adults | Older adults | |
|---|---|---|
| Age | 25.4 ± 4.6 | 69.1 ± 5.1*** |
| Education (years) | 16.3 ± 1.7 | 17.8 ± 2.5* |
| Mini-Mental State Exam | — | 29.3 ± 0.9 |
| Decision-making competence: comprehension | 16.5 ± 1.8 | 15.4 ± 2.5 |
| Decision-making competence: cognitive reflection | 4.9 ± 1.1 | 2.6 ± 2.2*** |
| Everyday cognition battery: financial reasoning | 5.9 ± 0.9 | 6.0 ± 0.7 |
| No-delay choice accuracy | 1.00 ± 0.01 | 0.99 ± 0.01 |
| Immediate choice accuracy | 0.93 ± 0.09 | 0.95 ± 0.06 |
| Delayed choice accuracy | 0.86 ± 0.09 | 0.81 ± 0.07 |
| No-delay choice decision speed | 1.14 ± 0.29 | 1.64 ± 0.27 |
| Immediate choice decision speed | 1.37 ± 0.28 | 1.80 ± 0.24 |
| Delayed choice decision speed | 1.52 ± 0.28 | 2.05 ± 0.28 |
Values are mean ± SD. Choice accuracy = proportion correct; decision speed = response time (seconds) for accurate trials.
t test significant at *p < 0.05,
***p < 0.001.
An additional 11 participants were excluded before fMRI data analysis. Three participants were excluded because of technical errors during scanning (two younger), four for poor behavioral performance (all younger, accuracy ≥2 SDs below the mean for immediate choice trials), and four for failing to complete the experiment (two younger; e.g., late to experiment session, discomfort in scanner).
Consumer choice task.
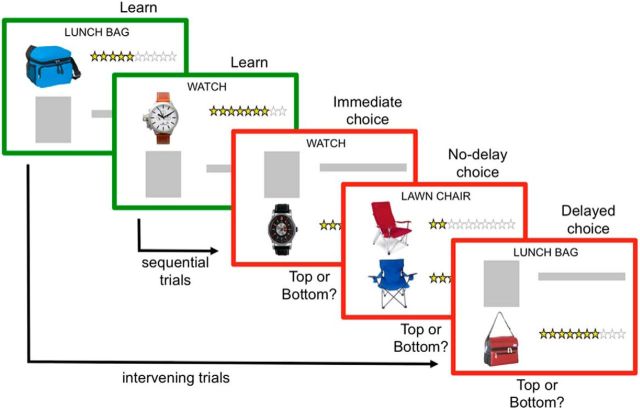
In the consumer choice task (Fig. 1), participants were asked to select the product that had the greater value (i.e., higher star rating). The rating scale ranged from 0 to 10 stars, but actual star values ranged from 1 to 9. Star rating values were pseudorandomly assigned to products from the range of possible values. Stimuli were pictures of consumer products taken from a popular online-shopping site. Product stimuli consisted of 520 pictures of consumer products. Each product type had two associated pictures (260 pairs) across 14 product categories (e.g., clothing, kitchen appliances). There were two task phases: (1) learn and (2) choice. For both task phases, the name of the consumer product was presented at the top of the screen (e.g., sweater, blender). In the learn phase, participants were presented with one product and a star rating for that product. They were instructed to try to remember the value of the product for a later choice. Learn screens were framed in green as a task cue (i.e., “Go. Keep shopping.”). In the choice phase, participants were instructed to make a choice between two competing products and were asked to respond as soon as they knew the correct answer so that decision speeds could be used as a proxy for memory strength.
Figure 1.
Consumer choice task. Participants learned the star ratings for specific products from a popular online-shopping site (green-framed learn screens). In choice trials, participants selected the competing product with the greatest star rating (red-framed choice screens). Products were presented either together or individually. Choices involved different levels of memory-retrieval delay: (1) no-delay (simultaneous presentation); (2) immediate retrieval (consecutive trials); or (3) delayed retrieval (3 intervening trials).
To manipulate memory during choice processing, the task included different delays between the presentation of the first (Product A) and second (Product B) products in a choice pair. There were three choice-delay conditions: (1) no-delay; (2) immediate; and (3) delayed. Choice screens were framed in red as a second task cue (i.e., “Stop. Make a choice.”). For no-delay choices, a pair of competing products appeared on the same task screen and participants selected the product with the highest star rating. For immediate choices, competing products were presented in sequential trials and choices were made during the presentation of Product B. Delayed trials were similar to immediate trials, except that three trials occurred between Products A and B in a given pair. To reduce task demand in immediate and delayed trials, Product A was always presented in the top half of the screen and Product B was presented in the bottom half of the screen. In addition, as mentioned above, all task screens included the name of the consumer product at the top of the screen, allowing participants to use both product pictures and names as memory cues in choice trials. Product pairs (and their names) were not repeated within or across runs, and runs were composed of pairs from each of the 14 product categories to minimize memory interference for similar products (e.g., “sweater” and “jacket” within the “accessories” category). To examine brain regions sensitive to relative-chosen value (Boorman et al., 2009), the task also included two levels of difference in value comparisons: (1) small and (2) large. For small relative-choice values, star ratings for Products A and B differed by two points (A = 4 stars and B = 6 stars). Small relative-choice values were used for no-delay, immediate, and two-thirds of delayed-choice trials. For large relative-choice values, star ratings for Products A and B differed by four points (A = 4 stars and B = 8 stars). Large relative-choice values were used for one-third of delayed trials.
Immediately before scanning, participants completed a practice block. No task stimuli from the practice block appeared in the scanned task. The task was conducted over seven functional runs, each including either 66 or 68 trials. Stimuli were presented for 2500 ms with jittered fixation durations of 2000–4000 ms between trials. The task included a total of 210 learn trials, 50 no-delay choices, 50 immediate choices, and 160 delayed choices. The delayed-choice trials were overrepresented because we expected poorer performance for this condition. The order of trial types was randomized across subjects (within the constraints by delay condition) so that participants could not predict retrieval intervals. Importantly, the consumer choice task was designed to obfuscate delay structure and, thereby, reduce reliance on simple rehearsal of product values to make accurate choices in the delayed condition. Specifically, when Product A was presented alone (learn phase), the associated Product B (choice phase) could be presented on the next task screen (immediate choice) or after three trials of any combination (i.e., three learn, two learn/one choice, one learn/two choice, or three choice). Furthermore, the intervening trials for a given delayed choice could include choices for any of the three retrieval conditions (no-delay, immediate, and delayed).
Decision competence measures.
The current study included measures from two task batteries that have been used to examine age-related changes in decision-related cognitive performance. The current study included two measures from the decision-making competence battery (Finucane and Gullion, 2010): (1) comprehension, which is an index of inductive and arithmetical reasoning abilities (Finucane et al., 2005); and (2) cognitive reflection, which is an index of abilities to engage analytic over intuitive judgments (Frederick, 2005). From the everyday cognition battery (Allaire and Marsiske, 1999), the current study included the financial reasoning subcomponent, which assesses the abilities to obtain information and answer questions about realistic financial scenarios (personal banking).
Behavioral data analysis.
Behavioral data were analyzed using SPSS version 21.0. Repeated-measures ANOVAs assessed group effects for task accuracy and decision speed, with age (younger and older) as a between-subjects factor and choice-delay condition (no-delay, immediate, and delayed) as a within-subject factor. Partial η2 (ηp2) values are included as measures of effect size, and means are presented with their SEs (SEMs). Greenhouse–Geisser values are reported for any analyses in which the homogeneity of variance assumption was not met.
Imaging data acquisition.
Brain imaging was done using a 3 T GE scanner with an eight-channel head coil at the Duke University Brain Imaging and Analysis Center. Participants viewed the experiment screen via a mirror placed in the magnet bore and responded via an MR-compatible button box. Anatomical image acquisition involved a T1-weighted three-dimensional localizer series, with 124 axial slices parallel to the anterior–posterior commissure plane (FOV, 25.6 cm; voxel size, 1 mm2; slice thickness, 1.2 mm). After the structural scan, functional images were acquired using a T2*-weighted inward-spiral pulse sequence (Glover and Law, 2001) sensitive to the blood oxygenation level-dependent (BOLD) signal (TR, 2000 ms; TE, 30 ms; FOV, 24; 34 oblique slices; in-plane voxel size, 3.75 mm2; slice thickness, 3.8 mm). Three volumes acquired at the start of each run were discarded.
Imaging data analyses.
Brain imaging analysis was conducted with the FMRIB (for Functional MRI of the Brain) Software Library [FSL (for FMRIB Software Library); ] using FSL FEAT (for FMRIB fMRI Expert Analysis Tool) version 6.00 (Smith et al., 2004). Preprocessing included the following: (1) motion correction with MCFLIRT (for FMRIB Linear Image Restoration Tool with Motion Correction); (2) spatial smoothing with a 5 mm full-width half-maximum Gaussian kernel; (3) high-pass temporal filtering equivalent to 100 s; and (4) skull stripping of structural images with BET (for FMRIB Brain Extract Tool). Registration was performed with FLIRT such that each functional image was registered to both the participant's high-resolution brain-extracted structural image (6 degrees of freedom) and the FSL Montreal Neurological Institute (MNI) template using an affine transformation (12 degrees of freedom). All reported z-statistic (Gaussianized t) image results survived whole-brain correction (voxelwise threshold of z > 2.3; cluster-corrected threshold of p = 0.05; Worsley, 2001), and cluster coordinates are presented in MNI space. At the first level, preprocessed functional data were analyzed within runs using a general linear model (GLM) with local autocorrelation correction [FILM (for FMRIB Improved Linear Model); Woolrich et al., 2001]. Trial events were convolved with a double-gamma hemodynamic response function, and motion parameters were included as nuisance regressors. Second-level analyses used a fixed-effects model to combine data across runs for each subject. Third-level analyses used a mixed-effects model [FLAME 1 (for FMRIB Local Analysis of Mixed Effects); Beckmann et al., 2003] to combine data across subjects, allowing for examination of group means and differences.
The primary GLM examined BOLD response during correct choices depending on memory-retrieval condition and relative-chosen value (i.e., value difference between the chosen and unchosen options). We modeled the following events: (1) learn-phase immediate choice; (2) learn-phase delayed choice; (3) no-delay choice; (4) immediate choice; (5) delayed choice; and (6) choice response time. Only accurate choice trials were explicitly modeled, and response times were standardized by trial type, within run. The task was designed to allow for high levels of accuracy across conditions and similar levels of accuracy in younger and older adults. Across age groups, mean accuracy rates exceeded 80% for each condition (Table 1), with no significant age differences in accuracy for any of the choice conditions. Each of the regressors was modeled using the trial onset and a duration of 2.5 s (full stimulus presentation). We initially divided delayed choice trials into two events, depending on their relative-choice values (i.e., small or large difference between Products A and B). Our first group-level analysis included bidirectional contrasts for relative-choice value in the delayed condition. Because this analysis yielded no significant age-group differences, subsequent analyses examining brain activation by memory-retrieval condition collapsed across relative-choice value levels (i.e., all delayed choice). Remaining contrasts for the primary GLM examined main effects for each choice-delay condition, bidirectional comparisons of immediate versus delayed choice, and no-delay versus other choice trials.
Region-of-interest (ROI) analyses were conducted using Featquery. Mean z-stat values were extracted at the run level from activation clusters significant in the group-level analysis. To determine whether additional activation in older adults predicted better performance, Pearson's correlations were calculated for clusters indicating greater activation in older versus younger adults and measures of behavior. Behavioral measures were independent of fMRI analyses used to generate ROIs. Specifically, we examined the relationship between parameter estimates during accurate choice trials at different levels of retrieval delay and the following behavioral measures: (1) proportion of correct trials; (2) response time for accurate trials; and (3) decision competence scores from the decision-making competence battery. Correlations with the everyday cognition battery financial reasoning measure could not be conducted because of low variance in scores. ROIs were also used for single-trial analysis to examine relationships between behavioral measures and brain activation within subject. Single-trial analysis was conducted according to the procedure described by Mumford et al. (2012), which obtains an estimate of the activation of each trial using a GLM with one regressor for the current trial and another nuisance regressor that includes all other trials.
Psychophysiological interaction (PPI) analyses examined functional connectivity with seed regions identified as having greater activity in older versus younger adults for the delayed versus immediate contrast, as modulated by memory-retrieval condition. The analysis included all regressors from the primary model with the following additional regressors: (1) the time course for the seed ROI; (2) the interaction between the physiological regressor and immediate choice; and (3) the interaction between the physiological regressor and delayed choice. Contrasts were examined for PPI main effects for the immediate and delayed conditions, as well as bidirectional comparisons of the two conditions.
In addition, to address the possibility that age differences in choice processing arise from age differences in value encoding, a secondary model examined BOLD response to product value during learn trials. Regressors were created for main effects of learn trials and learn trials with value as a parametric modulator. The mean-centered star rating of the current learn trial was used as the parametric modulator. The resulting parametric-value regressor was orthogonalized to the learn regressor to examine activation specifically corresponding to value magnitude. Here, the contrast of interest was the main effect of value magnitude.
Results
Effects of memory retrieval and age on choice behavior
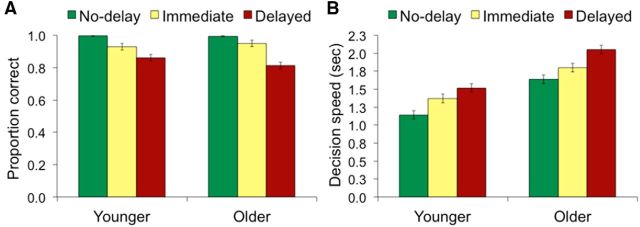
We first examined delay and age effects on accuracy, defined as the proportion of selections for the product with the higher value rating (Fig. 2A; Table 1). There was a main effect of delay such that accuracy was reduced with longer retrieval intervals (F(2,80) = 95.84, p < 0.001, ηp2 = 0.71). Within-subject contrasts revealed that accuracy declined monotonically with delay (F(2,80) = 166.32, p < 0.001, ηp2 = 0.81). Although there was no main effect of age on accuracy (F(1,40) < 1), there was an age × delay interaction (F(2,80) = 5.02, p < 0.01, ηp2 = 0.11). Post hoc t tests indicated that younger and older adults performed similarly on no-delay and immediate choices (t(40) = 0.65, p =0. 52; t(40) = −0.96, p = 0.34), but choice-selection accuracy on delayed choices was marginally lower in older adults (t(40) = 1.92, p = 0.06). Next, we examined decision speed on correct choice trials by age group and delay (Fig. 2B; Table 1). A main effect of delay was observed (F(2,80) = 86.86, p < 0.001, ηp2 = 0.69), as well as a main effect of age (F(1,40) = 39.94, p < 0.001, ηp2 = 0.50), but there was no significant interaction of the two factors (F(2,80) = 1.50, p = 0.23, ηp2 = 0.04). Together, these results suggest a speed–accuracy tradeoff in older adults. That is, older adults took more time to make choices across conditions, which allowed them to reach accuracy levels similar to younger adults, particularly when choices involved no retrieval or immediate retrieval.
Figure 2.
Similar effects of increasing difficulty by choice-delay condition were observed in younger and older adults. Choice accuracy declined (A) and decision speed slowed (B) with increased delay similarly across age groups. Older adults were similar to younger adults in accuracy but had slower decision speeds on correct choices. Error bars reflect SEMs.
Stability in neural response to value-magnitude encoding and relative-choice value with age
In our neuroimaging analysis, we addressed the possibility that age differences in choice result from differential responses to value-related processing in younger and older adults. First, we investigated response to value magnitude (star ratings) for learn trials to determine whether there are age-related changes to neural representations of value magnitude during encoding. Consistent with studies examining brain regions that respond with greater numerical value magnitudes (Arsalidou and Taylor, 2011), we found robust response to parametric value in bilateral parietal regions (e.g., intraparietal sulcus), middle frontal gyrus, insula, precentral gyrus, and cingulate gyrus (Table 2). Age-group comparisons yielded no significant differences in either direction, indicating that aging did not significantly alter signals associated with value magnitudes during encoding. We then examined response to the value difference between chosen and unchosen products (relative-choice value) for delayed-choice trials. Consistent with research on value processing in the absence of episodic retrieval (de Araujo et al., 2005; Boorman et al., 2009; Smith et al., 2010), we found that subgenual vmPFC responded to larger relative-choice values. Larger > smaller relative-choice values were also associated with bilateral activation of the striatum, paracingulate gyrus/anterior cingulate, and middle temporal gyrus (Table 2). The inverse contrasts yielded no significant activation. Critically, we observed no age differences in response to relative-choice value. Combined with results from the analysis of value magnitude at encoding, these findings support the conclusion that value-related processing is maintained in healthy older adults.
Table 2.
Cross age-group cluster peaks (MNI coordinates) for value-related analyses
| x | y | z | Z-max | Voxels | |
|---|---|---|---|---|---|
| Learn trials by star rating magnitude | |||||
| Lingual gyrus/temporal occipital fusiform cortex | 8 | −66 | −8 | 6.9 | 16364 |
| Right superior frontal gyrus/precentral gyrus | 26 | −4 | 48 | 5.0 | 1527 |
| Left middle frontal gyrus/precentral gyrus | −30 | −2 | 54 | 4.8 | 1223 |
| Paracingulate gyrus/anterior cingulate | 2 | 16 | 42 | 4.9 | 806 |
| Delayed choice trials by relative-choice value | |||||
| Paracingulate gyrus/anterior cingulate | −2 | 8 | 46 | 5.5 | 18004 |
| Caudate/subcallosal ventromedial prefrontal cortex | 14 | 16 | −10 | 4.7 | 1625 |
| Middle temporal gyrus | −60 | −46 | −4 | 3.6 | 647 |
Compensatory role of the vmPFC in memory-dependent choice
Our primary neuroimaging analysis examined how reliance on memory affects neural networks supporting choice. Because the previous analysis found no age differences in effects of relative-choice value, we collapsed across value-difference conditions when assessing memory-dependent effects on choice processing. Across age groups, choice processing for the delayed > immediate conditions activated regions in the frontoparietal network, including bilateral dorsolateral PFC (DLPFC; Table 3). Activation for immediate > delayed choices included regions commonly associated with the default-mode network. Of particular interest for the current study were brain regions activated to a greater extent in older adults compared with younger adults (i.e., possible compensatory regions).
Table 3.
Cross age-group cluster peaks (MNI coordinates) for delayed > immediate contrast
| x | y | z | Z-max | Voxels | |
|---|---|---|---|---|---|
| Right dorsolateral prefrontal cortex | 52 | 26 | 30 | 6.1 | 4232 |
| Right angular gyrus/superior parietal lobule | 40 | −54 | 42 | 5.8 | 2211 |
| Left dorsolateral prefrontal cortex | −48 | 12 | 40 | 5.1 | 1766 |
| Paracingulate gyrus/superior frontal gyrus | 2 | 30 | 42 | 7.1 | 1618 |
| Left superior parietal lobule/lateral occipital cortex | −34 | −58 | 40 | 4.8 | 1513 |
| Cerebellum | −8 | −80 | −26 | 4.4 | 909 |
We established three a priori criteria for compensation (Cabeza and Dennis, 2013). The first two criteria are necessary for attempted compensation, and the third is necessary for successful compensation. In attempted compensation, overactivation in the brain is triggered by a mismatch between available processing resources and task demands. In the current study, attempted compensation would be evidenced by regions that (1) increased in activation during execution of the same task in older versus younger adults and (2) increased in activation with increased task demand (in this case, increased memory-retrieval demand). Because age-related increases in brain activation may be associated with no performance benefits (e.g., inefficient processing), we examined evidence for successful compensation, defined as additional brain activation that is beneficial to performance. Specifically, we sought to determine brain regions that (3) showed a positive relationship between additional activation and independent measures of performance.
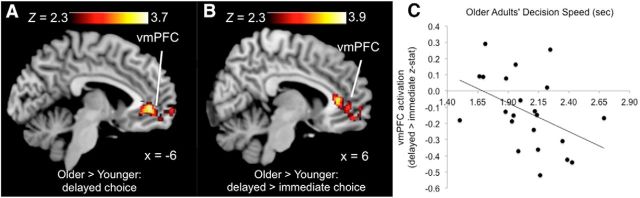
We observed no significant effects for the older > younger contrast in the no-delay or immediate choice conditions but did find one significant cluster for choice processing in the delayed-choice condition (Fig. 3A; peak voxel: Z-max = 3.7, x = −6, y = 42, z = −2). The cluster was located in the vmPFC and extended into a medial portion of the frontal pole. The fact that older adults overactivated the vmPFC during delayed choices is evidence in support of the first criterion (i.e., older > younger for the same task). We acknowledge that, although mean-level age differences in delayed-choice accuracy did not meet statistical significance, fewer delayed-choice trials in older adults could have reduced power to detect an effect in the older > younger contrast. That we still observed significantly greater vmPFC activation in older adults for the delayed condition indicates that mean-level differences in modeled trials did not prevent the observation of, at least some, age differences in this direction.
Figure 3.
Evidence for the role of vmPFC in functional compensation during memory-dependent choice. Relative to younger adults, older adults recruited greater activity in the vmPFC for delayed choices (A). Increased vmPFC activity in older versus younger adults was attributable to the increased memory delay, because the same region was overactivated in older adults for the delayed > immediate contrast (B). Delayed > immediate activity in the vmPFC predicted inter-individual differences in older adults' decision speeds on delayed choices (C).
To test for evidence of the second criterion, we examined age × condition effects corresponding to age-related increases in activation with increased task demand. For this step, we focused on delayed > immediate choices, which allowed examination of memory-retrieval effects in the absence of perceptual and encoding strategy differences between conditions. Results for the older > younger contrast included a region in the vmPFC that primarily overlapped with the region showing an age-related increase in activity in the delayed condition but was more constrained (Fig. 3B; peak voxel: Z-max = 3.8, x = 4, y = 32, z = 8) and also included a region in the postcentral gyrus. These results indicated that, when choices involved increased reliance on memory retrieval, older adults had significantly greater activation than younger adults in the vmPFC. Thus, the vmPFC met both criteria for attempted compensation during memory-dependent choices.
We then investigated evidence for the third criterion, that the vmPFC supports successful compensation. We first conducted an ROI analysis to examine inter-individual differences in vmPFC activity associated with both task performance and external measures of decision competence. ROI analysis revealed that, in older adults, mean parameter estimates for the vmPFC in the delayed > immediate contrast predicted faster mean decision speed on correct delayed-choice trials (Fig. 3C; r(20) = −0.44, p = 0.04) but did not predict decision speed in younger adults (r(18) = 0.00, p = 0.99), or accuracy or decision competence in either group. To confirm that vmPFC activity predicted faster decision speed within older adults, we examined correlations between trial-by-trial activations for delayed choice trials and standardized response times (excluding responses >3 SDs from the subject-level mean and those occurring on the transition from choice trial to fixation). We conducted a t test of correlations between trial-level vmPFC activation and decision times for individual subjects (average of slopes, testing difference from zero). Results were consistent with the previous correlation analysis, indicating that delayed-choice vmPFC activations from the single-trial analysis predicted faster decision speeds in older adults (t(21) = −0.08, p < 0.001). In contrast with younger adults' group-level results, trial-level data analysis in younger adults indicated negative correlations between vmPFC activation and decision times (t(19) = −0.11, p < 0.001). Thus, vmPFC activation during delayed choices predicted faster decision speeds in older adults at the subject and trial levels but only at the trial level in younger adults. We also observed greater activation in older adults in the postcentral gyrus for the delayed > immediate contrast (peak voxel: Z-max = 3.9, x = −42, y = −18, z = 64). However, correlations between activation in this cluster and decision speed indicated that postcentral gyrus activity did not predict faster decision speeds in older adults at the subject level (r(20) = 0.05, p = 0.84) or trial level (t(21) = 0.03, p = 0.17).
Finally, we examined effects of self-reported task strategy that were reported in the post-experiment questionnaire. Older adults were divided into those who reported memorizing the number of stars and those who used a more elaborative strategy (e.g., replacing star ratings with emotional words, associating ratings with personal opinions about products). The analysis revealed that correlations between vmPFC activity and decision speed were stronger in older adults who reported using a more elaborative strategy (r(5) = −0.15) versus those using no elaboration (r(13) = −0.05; F(1,21) = 7.75, p < 0.05, ηp2 = 0.28). These results may reflect greater engagement of self-referential processing during memory-dependent choice in older adults, because the former function relies on the medial PFC and is maintained in healthy aging (Gutchess et al., 2007, 2010).
These results indicate that—unlike other regions exhibiting increased activation in older adults—only the vmPFC supports successful compensation during memory-dependent choice processing. Moreover, the compensatory response in the vmPFC is associated with use of more elaborative and successful memory strategies in older adults.
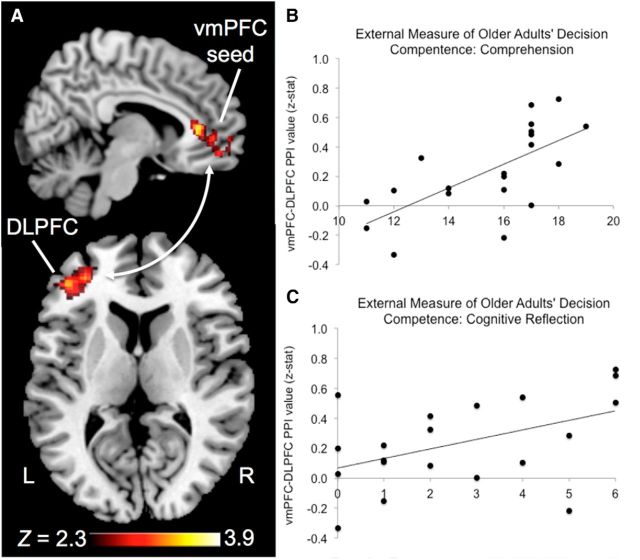
Connectivity between the vmPFC and DLPFC in older adults
Next, we sought to determine whether there was an age-related shift in functional connectivity with the vmPFC. Using the vmPFC ROI from Figure 3B as a seed, we found that, for delayed choices, activity in the vmPFC was correlated with the left DLPFC to a greater extent in older adults than younger adults (Fig. 4A; peak voxel: Z-max = 3.9, x = −30, y = 42, z = 2). Notably, this DLPFC region was located within the frontoparietal network identified in the delayed versus immediate choice contrast across age groups (Table 3). Significant connectivity with the vmPFC was not observed for any other choice-delay condition. In addition, although we did not observe a significant age × condition effect, age differences for the delayed choice condition support the conclusion that older adults have increased vmPFC–DLPFC connectivity during memory-dependent decisions. We then sought evidence that vmPFC–DLPFC connectivity supports decision making in older adults by examining correlations between connectivity values and task behavior and external measures of decision competence. Although older adults' connectivity strength did not predict behavior on the experimental task, it did predict decision competence for measures of comprehension and cognitive reflection (Fig. 4B,C; Table 4). PPI-based brain-behavior correlations were not observed in younger adults for any behavioral measure (Table 4), but this may have been attributable to ceiling effects on the decision competence measures in the younger group.
Figure 4.
vmPFC–DLPFC connectivity enhanced in older adults during memory-dependent decision making. The vmPFC region overactivated in older adults for the delayed > immediate contrast was used as a seed in a PPI analysis, and results revealed greater connectivity with the DLPFC in older versus younger adults during delayed choices (A). This DLPFC region was seated within the network supporting memory-dependent choice across age groups. DLPFC–vmPFC connectivity predicted inter-individual differences in external measures of decision competence in older adults (B, C). L, Left; R, right.
Table 4.
Correlations (r values) for behavior and vmPFC–DLPFC connectivity strength during delayed choice
| Younger adults | Older adults | |
|---|---|---|
| Delayed choice proportion correct | 0.14 | −0.10 |
| Delayed choice decision time | 0.11 | 0.17 |
| Decision-making competence: comprehension | 0.13 | 0.67** |
| Decision-making competence: cognitive reflection | 0.28 | 0.47* |
Connectivity strength = subject-level z-statistics. Correlation significant at *p < 0.05,
**p < 0.01.
Discussion
This study highlights the role of the vmPFC in supporting memory-dependent decision making and in contributing to effective functional compensation during healthy aging. Consistent with previous research (de Araujo et al., 2005; Boorman et al., 2009; Smith et al., 2010), the posterior vmPFC activated with larger choice values; however, such value-related processing was not modulated by age. In contrast, age differences were observed in memory-dependent choice processing, with older adults recruiting additional brain activation with increased memory challenge. Specifically, although increased memory-retrieval demands resulted in activation of a frontoparietal network in both age groups, older adults exhibited additional recruitment of activity in the anterior vmPFC. Our imaging results met the key criteria for concluding that the anterior vmPFC supports compensation (Cabeza and Dennis, 2013) during memory-dependent choice, because vmPFC activity was (1) greater in older than younger adults during delayed choices, (2) increased with greater retrieval demands in older adults, and (3) positively covaried with independent measures of task performance in older adults. Importantly, satisfaction of the third criterion is consistent with the idea that additional activation in the vmPFC enhances memory-dependent choice performance (i.e., supports successful compensation). Furthermore, when making choices involving delayed retrieval, older adults showed increased connectivity between the vmPFC and DLPFC, with the latter region being associated with memory-dependent choice across age groups. Finally, vmPFC–DLPFC connectivity strength values predicted higher scores on external measures of decision competence in older adults. Together, these results provide converging evidence that age-related decline in decision making may be ameliorated via functional compensation in the vmPFC.
Role of the vmPFC in age-related compensation
The vmPFC has been linked with disparate functions in different literatures, including choice valuation in the decision-making literature (Bartra et al., 2013) and episodic retrieval in the memory literature (Buckner and Carroll, 2007; Spreng et al., 2009). An emerging view holds that the vmPFC supports these functions with a common process—specifically, that the vmPFC serves as a hub for connecting systems involved in constructing meaning from conceptual information to guide appropriate affective and behavioral responses (Euston et al., 2012; Roy et al., 2012; Winecoff et al., 2013). This model highlights shared features of value-based decision making and episodic memory and, when applied to our findings, suggests a greater reliance on the integrative functions of the vmPFC with age and dependence on memory, even for tasks that primarily rely on working memory and associated brain regions.
Although the vmPFC is rarely associated with age-related compensation, studies sharing task components with the current experiment have reported overactivation of the vmPFC in older adults. Memory studies have observed greater vmPFC activation during explicit learning in older versus younger adults (Cabez and Dennis, 2011) and during objective recollection in high-performing versus low-performing older adults (Duarte et al., 2008). The latter study found a positive relationship between vmPFC activation and objective recollection exclusively in the high-performing group, suggesting that the vmPFC supported successful compensation. Neuroimaging investigations of age effects on decision making have reported greater activation for older adults in the vmPFC and orbitofrontal cortex during risk taking (Lee et al., 2008; McCarrey et al., 2012), and behavioral research indicates that age differences on laboratory risk-taking tasks are explained by age-related declines in processing speed and memory (Henninger et al., 2010). Notably, the latter study found that immediate and delayed memory loaded onto the same mediating factor, suggesting that increasing working memory, or long-term memory, demand during decision making will trigger the need for compensation in older adults. Whether working or long-term memory is more likely to trigger compensation is an open question.
We also observed activation of a posterior vmPFC region in response to relative-choice values—that is, choices with larger differences in value for chosen versus unchosen products. Activation in a similar vmPFC region has been associated with expected values for chosen and unchosen options (Boorman et al., 2009), willingness-to-pay computations (Plassmann et al., 2007), and decision values (Hare et al., 2008; Plassmann et al. 2010; Smith et al., 2010; Sokol-Hessner et al., 2012). We expand on these findings by demonstrating that the posterior vmPFC—within a larger neural network—tracks relative-choice values for memory-dependent decisions and does so similarly in younger and older adults. These results are consistent with studies showing that age differences in decision making are not simply the result of declines in value representation (Samanez-Larkin et al., 2010, 2014; Chowdhury et al., 2013) and those showing that older adults have intact medial PFC response to reward outcomes that do not involve learning (Samanez-Larkin et al., 2014). In contrast, older adults show diminished vmPFC response to value signals that depend on implicit learning (Chowdhury et al., 2013; Eppinger et al., 2013; Samanez-Larkin et al., 2014). Taken with the findings above, these results suggest that the nature of age effects in the vmPFC during decision making depend on the involvement of specific memory systems.
vmPFC connectivity supports decision making in aging
Recent research has indicated that age-related compensation may also take the form of increased connectivity between brain regions (Reuter-Lorenz, 2002; Tomasi and Volkow, 2012; Cabeza and Dennis, 2013). Increased connectivity between the PFC and the medial temporal lobe in older versus younger adults has been observed in several memory studies (Daselaar et al., 2006; Dennis et al., 2008; St Jacques et al., 2009). Furthermore, increased connectivity between the DLPFC and premotor cortex with increasing working memory load has been shown to predict better performance in both younger and older adults (Nagel et al., 2011). Our findings provide evidence that the vmPFC may be involved in functional compensation via increased connectivity, as we observed increased vmPFC–DLPFC connectivity in older adults that predicted performance on external measures of decision competence.
Our connectivity results are also consistent with the “meaning-centered” view of the vmPFC (Roy et al., 2012). The vmPFC is reciprocally connected to regions involved in higher-level cognition (DLPFC), as well as affective (amygdala) and sensory processing (temporal visual association regions; Wood and Grafman, 2003). Observed vmPFC–DLPFC connectivity during memory-dependent choice may reflect older adults' increased need for coordination of affective signals and goal maintenance to meet task demands. An effect of memory load on vmPFC–DLPFC connectivity has been observed previously in an investigation of relationships between reward processing and memory load during an N-back task (Longe et al., 2009). In that study, vmPFC–DLPFC connectivity was enhanced in response to high memory load when reward was absent. The fact that our study found similar patterns of memory-dependent connectivity when processing abstract representations of value, points to the conclusion that vmPFC–DLPFC connectivity may be particularly important for choices involving high levels of cognitive demand and weak reward or value signals. It has been proposed that age differences in decision processing depend on whether decision making relies primarily on deliberative or automatic/affective processing, with the latter potentially facing less decline in aging (Peters et al., 2007). Thus, an interesting question for future research is whether stronger value signals reduce older adults' need for compensatory vmPFC–DLPFC connectivity during cognitive processing.
Finally, vmPFC–DLPFC connectivity during delayed-choice processing predicted performance on external measures of decision competence, specifically in comprehension and cognitive reflection. The comprehension measure used in the current study indexes inductive and arithmetical reasoning abilities (Allaire and Marsiske, 1999), whereas the cognitive reflection measure indexes abilities to engage analytic over intuitive judgments (Frederick, 2005). Our results suggest that increased vmPFC–DLPFC connectivity supports older adults' decision making for tasks that rely on an integration of abilities in different cognitive domains (Finucane and Gullion, 2010). This fits with the idea that these regions work together in higher-level cognitive processing, with the vmPFC supporting integration of affective, mnemonic, and environmental information and the DLPFC supporting execution of behavioral responses to information integrated by the vmPFC (Wood and Grafman, 2003). Furthermore, previous research has related cognitive reflection with monitoring and inhibition abilities (Del Missier et al., 2012), functions supported by the medial and dorsolateral PFC regions, respectively (for review, see Ridderinkhof et al., 2004). Thus, our results suggest a compensatory role for vmPFC–DLPFC connectivity in decision making, which may be more directly examined in future neuroimaging studies.
Conclusion
Together, the current study suggests that age differences in decision making may be most pronounced when decision performance requires accurate memory retrieval. However, age differences in performance on complex and memory-dependent decision tasks may be minimized with additional recruitment of vmPFC activity and connectivity. Our findings hold implications for a wide range of decision domains that have been found to depend on the neural correlates of working memory and long-term memory, including risk taking (Gupta et al., 2009; Newcombe et al., 2011), delay discounting (Peters and Büchel, 2010), reinforcement learning (Foerde and Shohamy, 2011; Foerde et al., 2013), and use of heuristics (Khader et al., 2011). Future neuroimaging studies can be used to determine whether the vmPFC also supports performance in these domains among older adults or clinical populations with compromised neurocognitive function. Such research will provide important insights into the mechanisms underlying memory–decision interactions and sources of impaired decision making in individuals with neural degeneration.
Footnotes
This work was supported by National Institute on Aging Grant R01 AG034580 (R.C.). Fellowship support was provided by National Institute on Aging Grant T32 AG00029 (N.R.L.). We thank H. Boms, E. Huang, E. Lu, and K. Rand for assistance with task development and data collection.
The authors declare no competing financial interests.
References
- Alba JW, Hutchinson JW. Knowledge calibration: what consumers know and what they think they know. J Consum Res. 2000;27:123–156. doi: 10.1086/314317. [DOI] [Google Scholar]
- Alba JW, Hutchinson JW, Lynch JG. Memory and decision making. In: Robertson TS, Kassarjian HH, editors. Handbook of consumer behavior. Edgewood Cliffs, NJ: Prentice Hall; 1991. pp. 1–49. [Google Scholar]
- Allaire JC, Marsiske M. Everyday cognition: age and intellectual ability correlates. Psychol Aging. 1999;14:627–644. doi: 10.1037/0882-7974.14.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Taylor MJ. Is 2 + 2= 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011;54:2382–2393. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bettman JR. Memory factors in consumer choice: a review. J Marketing. 1979;43:37–53. doi: 10.2307/1250740. [DOI] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. Frontal lobes and aging: deterioration and compensation. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Ed 2. New York: Oxford UP; 2013. pp. 628–652. [Google Scholar]
- Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Düzel E, Dolan RJ. Dopamine restores reward prediction errors in old age. Nat Neurosci. 2013;16:648–653. doi: 10.1038/nn.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JW, Sharfman MP. Does decision process matter? A study of strategic decision-making effectiveness. Acad Manage J. 1996;39:368–392. doi: 10.2307/256784. [DOI] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Del Missier F, Mäntylä T, Bruin WB. Decision-making competence, executive functioning, and general cognitive abilities. J Behav Decis Making. 2012;25:331–351. doi: 10.1002/bdm.731. [DOI] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, Cohen JD. Reduced striatal responses to reward prediction errors in older compared with younger adults. J Neurosci. 2013;33:9905–9912. doi: 10.1523/JNEUROSCI.2942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane ML, Gullion CM. Developing a tool for measuring the decision-making competence of older adults. Psychol Aging. 2010;25:271–288. doi: 10.1037/a0019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane ML, Mertz CK, Slovic P, Schmidt ES. Task complexity and older adults' decision-making competence. Psychol Aging. 2005;20:71–84. doi: 10.1037/0882-7974.20.1.71. [DOI] [PubMed] [Google Scholar]
- Foerde K, Shohamy D. Feedback timing modulates brain systems for learning in humans. J Neurosci. 2011;31:13157–13167. doi: 10.1523/JNEUROSCI.2701-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Braun EK, Shohamy D. A trade-off between feedback-based learning and episodic memory for feedback events: evidence from Parkinson's disease. Neurodegener Dis. 2013;11:93–101. doi: 10.1159/000342000. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frederick S. Cognitive reflection and decision making. J Econ Perspect. 2005;19:25–42. doi: 10.1257/089533005775196732. [DOI] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48:211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger DE, Madden DJ, Huettel SA. Processing speed and memory mediate age-related differences in decision making. Psychol Aging. 2010;25:262–270. doi: 10.1037/a0019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader PH, Pachur T, Meier S, Bien S, Jost K, Rösler F. Memory-based decision-making with heuristics: evidence for a controlled activation of memory representations. J Cogn Neurosci. 2011;23:3540–3554. doi: 10.1162/jocn_a_00059. [DOI] [PubMed] [Google Scholar]
- Klein HE, D'Esposito M. Neurocognitive inefficacy of the strategy process. Ann N Y Acad Sci. 2007;1118:163–185. doi: 10.1196/annals.1412.012. [DOI] [PubMed] [Google Scholar]
- Lee TM, Leung AW, Fox PT, Gao JH, Chan CC. Age-related differences in neural activities during risk taking as revealed by functional MRI. Soc Cogn Affect Neurosci. 2008;3:7–15. doi: 10.1093/scan/nsm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longe O, Senior C, Rippon G. The lateral and ventromedial prefrontal cortex work as a dynamic integrated system: evidence from FMRI connectivity analysis. J Cogn Neurosci. 2009;21:141–154. doi: 10.1162/jocn.2009.21012. [DOI] [PubMed] [Google Scholar]
- Mata R, Schooler LJ, Rieskamp J. The aging decision maker: cognitive aging and the adaptive selection of decision strategies. Psychol Aging. 2007;22:796–810. doi: 10.1037/0882-7974.22.4.796. [DOI] [PubMed] [Google Scholar]
- Mata R, Josef AK, Samanez-Larkin GR, Hertwig R. Age differences in risky choice: a meta-analysis. Ann N Y Acad Sci. 2011;1235:18–29. doi: 10.1111/j.1749-6632.2011.06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, Henry JD, von Hippel W, Weidemann G, Sachdev PS, Wohl MJ, Williams M. Age differences in neural activity during slot machine gambling: an fMRI study. PLoS One. 2012;7:e49787. doi: 10.1371/journal.pone.0049787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Newcombe VF, Outtrim JG, Chatfield DA, Manktelow A, Hutchinson PJ, Coles JP, Williams GB, Sahakian BJ, Menon DK. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain. 2011;134:759–768. doi: 10.1093/brain/awq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Hess TM, Västfjäll D, Auman C. Adult age differences in dual information processes: implications for the role of affective and deliberative processes in older adults' decision making. Perspect Psychol Sci. 2007;2:1–23. doi: 10.1111/j.1745-6916.2007.00025.x. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394–400. doi: 10.1016/S1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Curr Dir Psychol Sci. 2004;13:140–144. doi: 10.1111/j.0963-7214.2004.00293.x. [DOI] [Google Scholar]
- Samanez-Larkin GR, Kuhnen CM, Yoo DJ, Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. J Neurosci. 2010;30:1426–1434. doi: 10.1523/JNEUROSCI.4902-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Worthy DA, Mata R, McClure SM, Knutson B. Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cogn Affect Behav Neurosci. 2014;14:672–682. doi: 10.3758/s13415-014-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik I, Yoon C, Park DC, Schwarz N. How warnings about false claims become recommendations. J Consum Res. 2005;31:713–724. doi: 10.1086/426605. [DOI] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hutcherson C, Hare T, Rangel A. Decision value computation in DLPFC and VMPFC adjusts to the available decision time. Eur J Neurosci. 2012;35:1065–1074. doi: 10.1111/j.1460-9568.2012.08076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Winecoff A, Cabeza R. Emotion and ageing. In: Vuilleumier P, Armony J, editors. Handbook of human affective neuroscience. Cambridge, UK: Cambridge UP; 2013. pp. 635–662. [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:471, 549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Clithero JA, Fitzsimons GJ, Huettel SA. New scanner data for brand marketers: how neuroscience can help better understand differences in brand preferences. J Consum Psychol. 2012;22:143–153. doi: 10.1016/j.jcps.2011.11.008. [DOI] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. J Gerontol B Psychol Sci Soc Sci. 1993;48:P157–P171. doi: 10.1093/geronj/48.4.P157. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. Ventromedial prefrontal cortex encodes emotional value. J Neurosci. 2013;33:11032–11039. doi: 10.1523/JNEUROSCI.4317-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. Oxford, UK: Oxford UP; 2001. pp. 251–270. [Google Scholar]