Abstract
We have previously shown that intracerebellar infusion of the neuropeptide secretin enhances the acquisition phase of eyeblink conditioning (EBC). Here, we sought to test whether endogenous secretin also regulates EBC and to test whether the effect of exogenous and endogenous secretin is specific to acquisition. In Experiment 1, rats received intracerebellar infusions of the secretin receptor antagonist 5-27 secretin or vehicle into the lobulus simplex of cerebellar cortex immediately prior to sessions 1–3 of acquisition. Antagonist-infused rats showed a reduction in the percentage of eyeblink CRs compared with vehicle-infused rats. In Experiment 2, rats received intracerebellar infusions of secretin or vehicle immediately prior to sessions 1–2 of extinction. Secretin did not significantly affect extinction performance. In Experiment 3, rats received intracerebellar infusions of 5-27 secretin or vehicle immediately prior to sessions 1–2 of extinction. The secretin antagonist did not significantly affect extinction performance. Together, our current and previous results indicate that both exogenous and endogenous cerebellar secretin modulate acquisition, but not extinction, of EBC. We have previously shown that (1) secretin reduces surface expression of the voltage-gated potassium channel α-subunit Kv1.2 in cerebellar cortex and (2) intracerebellar infusions of a Kv1.2 blocker enhance EBC acquisition, much like secretin. Kv1.2 is almost exclusively expressed in cerebellar cortex at basket cell–Purkinje cell pinceaus and Purkinje cell dendrites; we propose that EBC-induced secretin release from PCs modulates EBC acquisition by reducing surface expression of Kv1.2 at one or both of these sites.
Eyeblink conditioning (EBC) is a form of classical conditioning that is a powerful model for studying the underlying neural mechanisms of learning and memory. In EBC, an initially neutral conditioned stimulus (CS) is paired with an eyeblink-eliciting unconditioned stimulus (US). The CS is typically a tone or a light, while the US is typically a mild periorbital shock or corneal air puff. At the outset of conditioning, the US will elicit a reflexive eyeblink. As training progresses, however, the organism will learn to make an eyeblink conditioned response (CR) to the CS prior to the onset of the US. In delay EBC, the CS and US overlap, with the CS presentation occurring first and the US being presented at the end of the CS period; the two stimuli terminate at the same time. In extinction of EBC, the procedure is similar, but the US is omitted.
Delay EBC engages a discrete brainstem–cerebellar circuit (for review, see Thompson and Steinmetz 2009). Thus far, two key sites of cerebellar synaptic plasticity supporting EBC have been identified: select granule cell-to-PC synapses in cerebellar cortex and pontine nuclei-to-interpositus nucleus neuron synapses in the deep cerebellar nuclei (DCN). Acquisition and expression of an eyeblink CR is dependent upon modulation of PC inhibition of the IPN (Garcia and Mauk 1998; Garcia et al. 1999; Ohyama and Mauk 2001; Bao et al. 2002; Aksenov et al. 2004; Ohyama et al. 2006; Sakamoto and Endo 2008; Parker et al. 2009; Poulos et al. 2009; Vogel et al. 2009; Kalmbach et al. 2010). Since PCs provide the sole output of the cerebellar cortex through inhibitory projections to the DCN, of which the IPN is one (Harvey and Napper 1991), the IPN would be disinhibited through inhibition of PC firing. Disinhibition would allow the expression of eyeblink CRs in response to strengthened pontine nuclei-to-IPN synapses (for review, see Gao et al. 2012).
We have recently shown that infusion of the neuropeptide secretin into cerebellar cortex facilitates delay EBC (Williams et al. 2012). Other research has shown that secretin is expressed in the somatodendritic region of PCs (Yung et al. 2001) and its GS-protein coupled receptor is expressed in PCs and basket cells (BCs) (Yung et al. 2001; Köves et al. 2002; Zhang et al. 2014). Secretin release can be induced by depolarization of cerebellar tissue with KCl and this secretin release is inhibited by an L-type or a P/Q-type calcium channel blocker (Lee et al. 2005). Secretin increases inhibitory postsynaptic currents (IPSCs) recorded from PCs (Yung et al. 2001; Lee et al. 2005). Recently, it has been shown that Pur-Sct−/− mice, in which the secretin coding region is selectively deleted from PCs, are impaired in rota-rod learning (Zhang et al. 2014). All of these data are consistent with our working model in which secretin, released from PCs depolarized by US input, promotes EBC by inhibiting PCs, which reduces inhibition on the IPN. The mechanism for inhibition of PCs may be through a reduction in surface expression of the voltage-gated potassium channel α-subunit Kv1.2 at BC–PC synapses (see Discussion).
In the present set of experiments, we infused a secretin receptor antagonist (5-27 secretin) into lobulus simplex of cerebellar cortex to examine whether endogenous secretin modulates EBC. We also examined whether exogenous and endogenous secretin can modulate extinction of eyeblink CRs. Lobulus simplex (lobule HVI) ipsilateral to the conditioned eye was chosen as the infusion target for these experiments because previous infusion studies in rabbits (Attwell et al. 2001, 2002; Cooke et al. 2004; Kellett et al. 2010) and rats (Cartford et al. 2004) have indicated that this area of cerebellar cortex is important for EBC (Attwell et al. 2001, 2002; Cooke et al. 2004; Kellett et al. 2010). In Experiment 1, infusions of the secretin receptor antagonist, 5-27 secretin, were made into the lobulus simplex of cerebellar cortex prior to each of the first 3 d of EBC. Intracerebellar infusions of exogenous secretin facilitated EBC (Williams et al. 2012), so we predicted that blocking the secretin receptor should impair EBC. Conditioning was continued for an additional 3 d with no infusions in order to examine whether blocking secretin receptors in cerebellar cortex produced more than short-term effects on performance (cf. Attwell et al. 2001).
In Experiments 2 and 3, we examined whether secretin modulates extinction. Despite the importance of extinction as a learning process itself, the cellular mechanisms in the cerebellum that underlie extinction of EBC are mostly unknown. Aspiration lesions of the anterior lobe of the cerebellum impair extinction (Perrett and Mauk 1995). There is a recovery of Purkinje cell simple spiking during extinction (Jirenhed et al. 2007; see also Gould and Steinmetz 1996), which is proposed to be important in suppressing the expression of the CR during extinction. Further support for this idea comes from data showing that infusion of a GABA antagonist into the IPN (pharmacologically disconnecting the IPN from PCs) restores CRs even after extensive extinction training (Medina et al. 2001). Thus, any change in PC modulation of the IPN may in turn affect the rate of extinction. In Experiment 2, intracerebellar infusions of secretin were made prior to extinction sessions 1 or 2. We hypothesized that secretin infused prior to extinction would result in slowing of extinction by maintaining inhibition of PCs and, therefore, downstream disinhibition of the IPN. In Experiment 3, we tested the effect of 5-27 secretin on the extinction of CRs. Since intracerebellar infusions of 5-27 secretin impaired EBC in Experiment 1, these same infusions made prior to session 1 of extinction were predicted to facilitate extinction by reducing inhibitory drive on PCs and, consequently, restoring PC-mediated inhibition of the IPN.
Results
Prior to analyses, we verified all cannula placements for rats in each of the experiments. Any rat whose cannula placement could not be located in the lobulus simplex of the cerebellum ipsilateral to the conditioned eye was removed from data analysis.
Experiment 1: endogenous secretin modulates delay EBC
Experiment 1a: delay EBC with interspersed CS and US probe trials
On the first day, rats were placed in experimental chambers and received 100 no-stimulus trials to measure spontaneous eyeblink activity. The following six conditioning sessions consisted of 80 CS–US paired trials (280-msec delay paradigm), and 10 CS-alone trials and 10 US-alone trials evenly spaced within the CS–US paired trials, so that every five trials alternated between a CS-alone and a US-alone trial. To determine the role of endogenous secretin in cerebellar cortex, the secretin receptor antagonist, 5-27 secretin, (1.0 μL; 1.0 μg/μL; Ant; Anaspec) or vehicle (1.0 μL; phosphate-buffered saline; Veh), was infused into the lobulus simplex of cerebellar cortex ipsilateral to the conditioned eye immediately prior to conditioning sessions 1–3 of six acquisition sessions. A total of 19 rats (9 Ant; 10 Veh) were included in the analyses. A total of 14 rats were removed prior to data analysis due to poor electromyographic (EMG) signals (n = 11), difficulty with infusions (n = 2), or not being able to locate the cannula placement (n = 1).
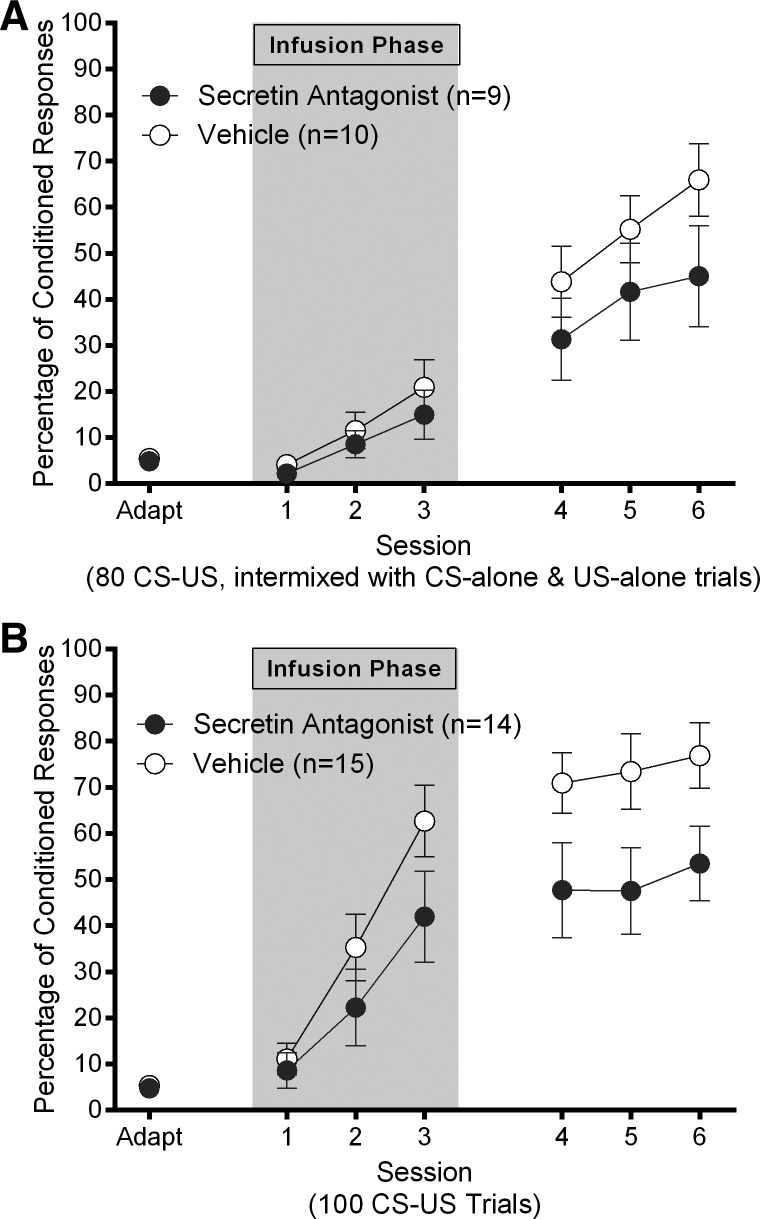
The results suggest that while both groups learned, and there was a trend for Group Veh rats to outperform Group Ant rats by the last session, there were no statistically significant differences between groups. A 2 (group: Ant; Veh) by 3 (session: infusion days 1–3) repeated-measures ANOVA on the percentage of CRs in conditioning sessions that took place immediately after infusions yielded a significant main effect of session (F(2,34) = 15.72, P < 0.001), but no significant main effect of group (P = 0.451) and no significant interaction effect (P = 0.735; Fig. 1A; gray area). A second 2 (group: Ant; Veh) by 3 (session: noninfusion days 4–6) repeated-measures ANOVA on percentage of CRs for the remaining three conditioning days yielded a significant main effect of session (F(2,34) = 16.13, P < 0.001), but no significant main effect of group (P = 0.211) and no significant interaction effect (P = 0.363; Fig. 1A). This finding was corroborated by performance during CS-alone trials (data not shown) where CRs were scored up to 150-msec after CS offset.
Figure 1.
(A) Experiment 1A: mean ± SEM percentage of eyeblink CRs as a function of session for rats who received 1.0 μL intracerebellar infusions of 5-27 secretin (1 μg/μL) or vehicle prior to the first three sessions of acquisition (gray area) in the first 5-27 secretin and acquisition experiment (80 CS–US trials per session, with interspersed CS and US probe trials). (B) Experiment 1B: mean ± SEM percentage of eyeblink CRs as a function of session for rats who received 1.0 μL intracerebellar infusions of 5-27 secretin (1 μg/μL) or vehicle prior to the first three sessions of acquisition (gray area) in the second 5-27 secretin and acquisition experiment (100 CS–US trials per session with no interspersed probe trials).
Next, we examined whether infusions had an effect on reflexive responding to the CS or US. A series of 2 (group: Ant; Veh) by 3 (session: infusion days 1–3 or noninfusion days 4–6) repeated-measures ANOVAs on the percentage of startle responses on CS–US paired trials or UR amplitude on US-alone trials yielded no significant session (P’s > 0.05) or group (P’s > 0.05) main effects and no significant interaction effects (P’s > 0.05), confirming that there were no differences between the groups on reflexive responses to the stimuli. Finally, we did not observe any systematic differences between the groups on other secondary measures, including CR amplitude during CS–US trials (P’s > 0.05), CR onset latency during CS–US trials (P’s > 0.05), and CR peak latency during CS-alone trials (P’s > 0.05).
The results from this experiment did not support our hypothesis that infusions of 5-27 secretin would impair EBC. We did, however, observe slower learning than expected from Group Veh for a 280-msec delay procedure. This may be a function of 20% of the trials in each session being unreinforced probe trials. In addition, it appeared that there were fewer CRs in Group Ant in the final session of acquisition, although this did not reach the conventional level of statistical significance (P = 0.14). Given our hypothesis that 5-27 secretin would impair EBC, the slow learning in Group Veh may have made it more difficult to observe slower learning in Group Ant, at least within the six sessions of EBC that we conducted. In order to examine this possibility, a second experiment was conducted in which we used a procedure to increase the rate of EBC.
Experiment 1b: delay EBC without interspersed probe trials
In Experiment 1b, we used the same procedure as in Experiment 1a but replaced the probe trials with CS–US trials so that all 100 trials in each session were CS–US paired trials. Six rats were removed prior to data analysis due to poor EMG signals (n = 5) or poor bipolar electrode placement (n = 1). A total of 29 rats (14 Ant; 15 Veh) were included in the analyses.
Overall, the findings from Experiment 1b showed that intracerebellar infusion of 5-27 secretin did impair EBC, supporting the hypothesis that secretin release is important for normal acquisition of EBC. A 2 (group: Ant; Veh) by 3 (session: infusion days 1–3) repeated-measures ANOVA on percentage of CRs revealed a significant main effect of session (F(2,54) = 42.90, P < 0.001), but no significant main effect of group (P = 0.167) and no significant interaction effect (P = 0.148; Fig. 1B; gray area). A second 2 (group: Ant; Veh) by 3 (session: noninfusion days 4–6) repeated-measures ANOVA on percentage of CRs revealed a significant main effect of group (F(1,27) = 4.60, P = 0.041) but no significant main effect of session (P = 0.114) and no significant interaction effect (P = 0.876; Fig. 1B; white area). The fact that we did not observe a significant main effect of session during the last 3 d of conditioning suggested that groups had reached asymptote with regard to percentage of CRs. The main effect of group suggested that infusions of 5-27 secretin impaired conditioning in Group Ant (Fig. 1B).
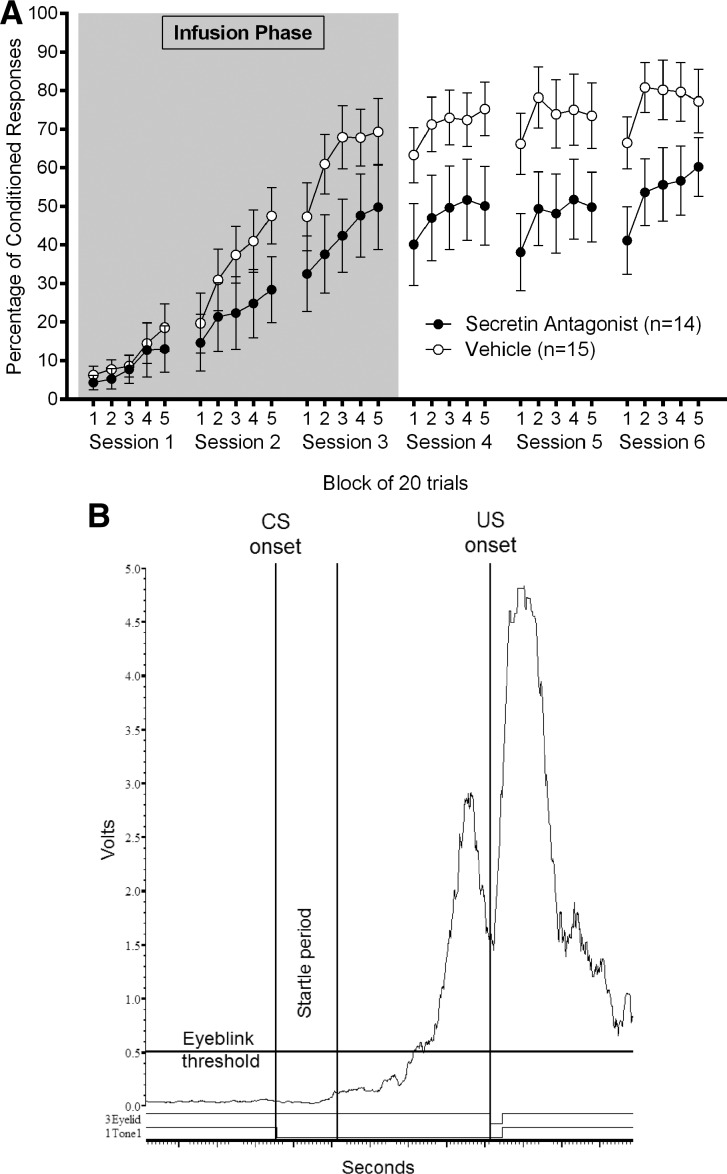
To further investigate the difference between groups in post-infusion sessions 4–6 of Experiment 1b, we conducted additional analyses. It appeared that vehicle-infused rats had begun to outperform antagonist-infused rats in the final infusion session (acquisition session 3), although a one-way ANOVA comparing groups in session 3 did not attain statistical significance (P = 0.108). Figure 2 illustrates this even more clearly by showing the percentage of CRs in each session as a function of 20-trial block within the session; it is clear from these data that antagonist-infused rats are already showing fewer CRs than vehicle-infused rats. Thus, differences in percentage of CRs between groups in sessions 4–6 were likely due to carryover from session 3. Consistent with this interpretation, a 2 (group: Ant; Veh) by 3 (session: noninfusion days 4–6) repeated-measures ANOVA on percentage of CRs, with percentage of CRs in session 3 as a covariate, abolished the significant group effect (F(1,26) = 1.66, P = 0.209). This strongly suggests that differences in performance in sessions 4–6 were a function of differences in performance in the final infusion session, session 3.
Figure 2.
Experiment 1B: (A) Mean ± SEM percentage of eyeblink CRs as a function of 20 trial block of trials within sessions for rats who received 1.0 μL intracerebellar infusions of 5-27 secretin (1 μg/μL) or vehicle prior to the first three sessions of acquisition (gray area) in the second 5-27 secretin and acquisition experiment (100 CS–US trials per session with no interspersed probe trials). (B) Individual trial of a vehicle-infused rat in session 2 of acquisition. Amplified, full-wave rectified, smoothed (10-msec time constant), and timed-shifted (10-msec to compensate for smoothing) eyelid EMG of an eyeblink CR to the tone CS.
To confirm that intracerebellar infusions of 5-27 secretin prior to acquisition did not change the reflexive startle response to the CS, we analyzed the percentage of startle responses to the CS. Two 2 (group: Ant; Veh) by 3 (session: infusion days 1–3 or noninfusion days 4–6) repeated-measures ANOVAs yielded no significant main effects of group (P’s > 0.05) or session (P’s > 0.05). Furthermore, there were no systematic differences between the groups on CR onset latency (P’s > 0.05). The only other difference observed between the groups was a trend for CR amplitude to be larger in Group Veh compared with Group Ant in noninfusion sessions 4–6 (F(1,27) = 3.84 P = 0.06).
Experiment 2: exogenous secretin does not modulate extinction
Infusing secretin or 5-27 secretin into cerebellar cortex prior to EBC facilitates (Williams et al. 2012) or impairs (Experiment 1b) acquisition, respectively. We hypothesized that infusions of secretin into cerebellar cortex would slow extinction by prolonging expression of CRs. Rats underwent one adaptation session and six sessions of 280-msec delay EBC without infusions. Subsequently, rats were assigned to one of three conditions: Secretin–Vehicle (Sec–Veh), Vehicle–Secretin (Veh–Sec), or Vehicle–Vehicle (Veh–Veh). These conditions correspond to what intracerebellar infusions the groups received on the first 2 d of extinction. For example, rats in the Sec–Veh group received an infusion of secretin on the first day of extinction and an infusion of vehicle on the second day. No infusions were made on the third day of extinction. These groups were formed in order to compare between extinction learning and expression of extinction. Forty-three rats were included in the analyses for this experiment (15 Sec–Veh; 14 Veh–Sec; 14 Veh–Veh). Rats were excluded from the analyses if they did not meet the learning criterion of 60% CRs in session 6 of acquisition (n = 9), if they exhibited poor EMG signals (n = 4), or if cannula placement could not be verified (n = 5).
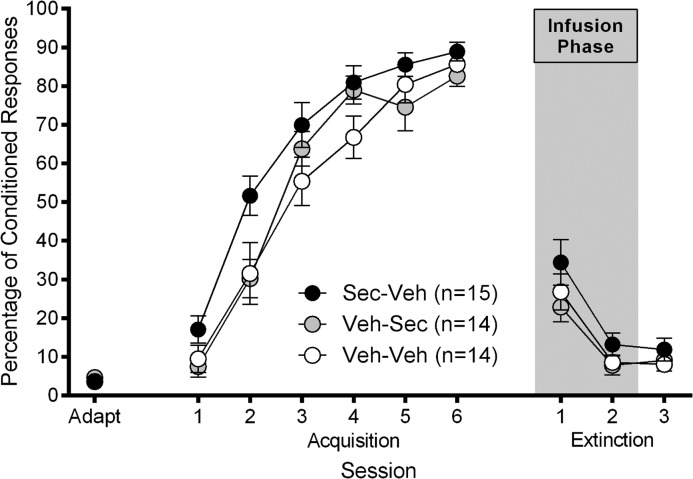
During the acquisition phase of the experiment (when no infusions were made), all groups reached an asymptote of ∼80% CRs (Fig. 3) and a one-way ANOVA comparing groups on day 6 of acquisition showed that there was no difference between the groups in percentage of CRs on the final day of acquisition (F(2,40) = 1.90, P = 0.163). Intracerebellar infusions of either secretin (1.0 μL; 1.0 μg/μL) or vehicle (1.0 μL; phosphate-buffered saline), based on group assignment, were made immediately prior to the first 2 d of extinction. The two infusion sessions were analyzed separately from the final day of extinction. A 3 (group: Sec–Veh; Veh–Sec; Veh–Veh) by 2 (session: extinction sessions 1–2) repeated-measures ANOVA revealed a significant main effect of session (F(1,40) = 51.38, P < 0.001). However, there was no significant main effect of group (F(2,40) = 1.83, P = 0.173) or interaction effect (F(2,40) = 0.494, P = 0.614) (Fig. 3). Since the Veh–Sec and Veh–Veh groups were treated identically in extinction session 1, we also compared the percentage of CRs in extinction session 1 between Group Sec–Veh and a vehicle-treated group comprised of Groups Veh–Sec and Veh–Veh rats. A one-way ANOVA revealed no significant difference between these reconfigured groups in extinction session 1 (F(1,41) = 2.58, P = 0.116). A one-way ANOVA was also conducted to compare Group Veh–Sec and Group Veh–Veh in percentage of CRs in extinction session 2; this ANOVA revealed no significant effect of intracerebellar secretin on expression of CRs in extinction (F(1,26) = 0.062, P = 0.806). A one-way ANOVA revealed no significant difference between the groups on the last day of extinction (F(2,39) = 0.668, P = 0.519). Analyses of other dependent measures during extinction did not yield any significant group differences (P’s > 0.05). The results from this experiment show that, contrary to our hypothesis, secretin did not impair extinction of eyeblink CRs, although a (nonsignificant) slowing trend was evident in Group Sec–Veh, which underwent intracerebellar secretin infusion prior to the first session of extinction.
Figure 3.
Experiment 2: mean ± SEM percentage of eyeblink CRs as a function of session for adaptation, acquisition and extinction. Sec–Veh rats received 1.0 μL intracerebellar infusion of secretin (1 μg/μL) prior to extinction session 1 and 1.0 μL intracerebellar infusion of vehicle prior to extinction session 2 (gray area). Veh–Sec rats received 1.0 μL intracerebellar infusion of vehicle prior to extinction session 1 and 1.0 μL intracerebellar infusion of secretin (1 μg/μL) prior to extinction session 2 (gray area). Veh–Veh rats received 1.0 μL intracerebellar infusion of vehicle prior to extinction sessions 1 and 2.
Experiment 3: endogenous secretin does not modulate extinction
The criterion for inclusion in this experiment was the same as in Experiment 2. Infusions were only made on the first 2 d of extinction and there were two groups in this experiment: 5-27 Secretin–Vehicle (Ant–Veh) and Vehicle–Vehicle (Veh–Veh), indicating what was infused on each of the two infusion days. A total of 23 rats were included in the analyses (12 Ant–Veh; 11 Veh–Veh). Three rats were removed from the analysis because they either did not meet the criterion for acquisition (n = 1) or because we were unable to determine the cannula placement in the lobulus simplex of the cerebellar cortex (n = 2).
During each of the conditioning sessions, rats were presented with 80 CS–US paired trials and 20 CS-alone trials. The CS-alone trials were intermixed with the CS–US trials so that in each block of 10 trials, eight were CS–US paired trials and two were CS-alone trials. The CS-alone trials were pseudorandomly spaced in each block of 10 trials so that the animal was not able to “anticipate” the next CS-alone trial. This was done in an attempt to slow subsequent extinction so that any facilitation of extinction by intracerebellar infusion of 5-27 secretin could be revealed. Both groups reached an asymptote of ∼80% CRs (Fig. 4) and a one-way ANOVA comparing groups on day 6 of acquisition showed that there was no difference between the groups in percentage of CRs on the final day of acquisition (F(1,21) = 0.084, P = 0.775).
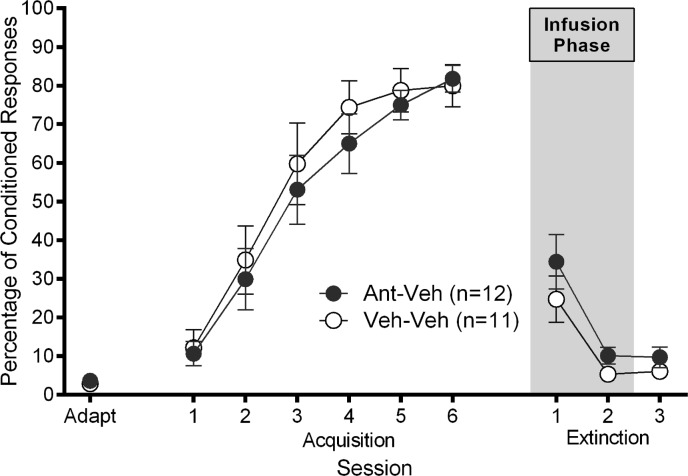
Figure 4.
Experiment 3: mean ± SEM percentage of eyeblink CRs as a function of session for adaptation, acquisition and extinction. Ant–Veh rats received 1.0 μL intracerebellar infusion of 5-27 secretin (1 μg/μL) prior to extinction session 1 and 1.0 μL intracerebellar infusion of vehicle prior to extinction session 2 (gray area). Veh–Veh rats received 1.0 μL intracerebellar infusion of vehicle prior to extinction sessions 1 and 2.
Intracerebellar infusions of either 5-27 secretin (1.0 μL; 1.0 μg/μL) or vehicle (1.0 μL; phosphate-buffered saline), based on group assignment, were made immediately prior to the first 2 d of extinction. As can be seen in Figure 4, there was no difference between the groups in the rate of extinction. This finding was confirmed by a 2 (group: Ant–Veh; Veh–Veh) by 2 (session: extinction session 1–2) repeated-measures ANOVA on the percentage of CRs, which yielded a significant main effect of session (F(1,21) = 34.61, P < 0.001), but no main effect of group (F(1,21) = 1.55, P = 0.227) or interaction effect (F(1,21) = 0.436, P = 0.516). A one-way ANOVA revealed no significant difference between the groups on the last day of extinction (F(1,20) = 1.264, P = 0.274). Analyses of secondary measures during extinction did not yield any significant group differences (P’s > 0.05). The results show that infusions of 5-27 secretin into the cerebellar cortex of rats did not alter extinction of eyeblink CRs.
Histological analysis
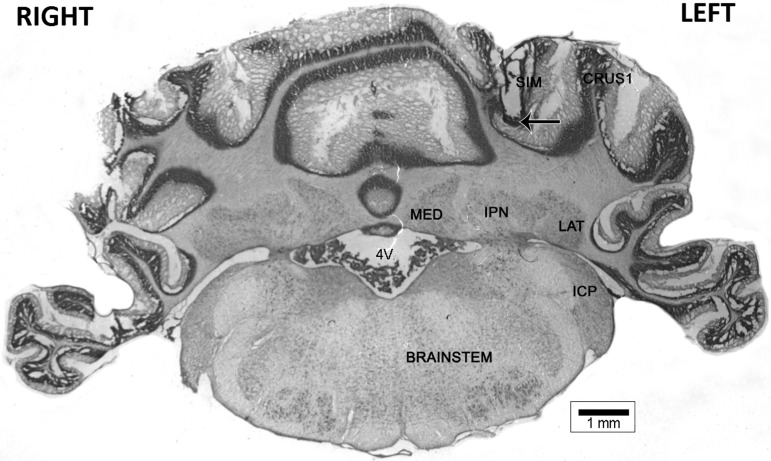
Figure 5 shows an example of a confirmed cannula placement in the lobulus simplex of cerebellar cortex. Cannula placements of rats included in the analyses were confirmed to be within the lobulus simplex of the cerebellar cortex; otherwise, the rat was removed from data analysis.
Figure 5.
Representative internal cannula tip placement (arrow). (SIM) lobulus simplex of cerebellar cortex; (CRUS1) Crus I of cerebellar cortex; (LAT) lateral deep cerebellar nucleus; (IPN) interpositus deep cerebellar nucleus; (MED) medial deep cerebellar nucleus; (ICP) inferior cerebellar peduncle; (4V) fourth ventricle.
Discussion
In Experiment 1, we showed that 5-27 secretin, a secretin receptor antagonist, impaired EBC when infused into the lobulus simplex of cerebellar cortex prior to acquisition sessions 1–3. This finding compliments our previous finding that secretin facilitated EBC when infused into lobulus simplex of cerebellar cortex prior to acquisition sessions 1–3 (Williams et al. 2012). Since secretin is endogenously expressed in PCs in cerebellar cortex (Yung et al. 2001; Köves et al. 2002; Zhang et al. 2014), is released when PCs are depolarized (Lee et al. 2005), and increases IPSCs in PCs (Yung et al. 2001; Lee et al. 2005), secretin may modulate EBC via inhibition of PCs and a concomitant disinhibition of the IPN.
Secretin's effects on EBC may be through the regulation of Kv1 channels containing the α-subunit Kv1.2 at BC–PC synapses in cerebellar cortex. Kv1.2 is densely expressed in the cerebellum at BC–PC synapses and in PC dendrites (Laube et al. 1996; Koch et al. 1997; Chung et al. 2001). Blocking these channels increases the rate and amplitude of miniature IPSCs (mIPSCs) recorded in PCs (Southan and Robertson 1998a,b; 2000). We have shown that secretin reduces surface Kv1.2 and increases internalized Kv1.2 in cerebellum, without affecting total Kv1.2 (Williams et al. 2012). Secretin-induced reduction in surface Kv1.2 is mediated by an adenyl cyclase (AC)-protein kinase A (PKA) signaling cascade that induces Kv1.2 endocytosis; secretin does not reduce surface Kv1.2 in the presence of an AC inhibitor (SQ-22536) or a PKA inhibitor (KT-5720) and the reduction in surface Kv1.2 is dynamin-dependent (Williams et al. 2012). Stimulation of PCs by blocking inhibitory input also reduced surface Kv1.2, an effect that was blocked by the secretin receptor antagonist 5-27 secretin (Williams et al. 2012). The reduction in surface Kv1.2 occurs at both the BC–PC pinceaus and in PC dendrites (Williams et al. 2012).
All of the above results are consistent with a model in which PCs, strongly depolarized by US input during EBC, release secretin. Secretin, acting as a retrograde messenger, decreases surface expression of Kv1.2 on BC axon terminals. Subsequently, when the CS activates BCs via parallel fibers (PFs), those BCs with reduced surface Kv1.2 will provide greater inhibitory input to PCs than other BCs with more surface Kv1.2. Thus, BCs with reduced surface expression of Kv1.2 will be in a position to more strongly inhibit the PCs with which they form synapses, PCs that also receive the US via climbing fiber input. Increased inhibition of PCs would be expected to disinhibit the IPN. When combined with an increase in the number of mossy fiber axons from the auditory region of the pontine nuclei to the IPN and the number of excitatory synapses in the IPN (Kleim et al. 2002; Boele et al. 2013), this would allow acquisition and/or expression of eyeblink CRs. Mittmann et al. (2005) have shown that feed-forward inhibition (FFI) is a mechanism by which spike output from PCs may be regulated. Thus, FFI could complement long-term depression (LTD) at PF–PC synapses as a learning mechanism for EBC, by providing inhibition of PC spontaneous activity. Without FFI, PC spontaneous activity would still be present after PF–PC LTD, making an inhibitory mechanism seem necessary (Hesslow et al. 2013).
It is increasingly unclear exactly what role PF–PC LTD plays in EBC. On the one hand, EBC is impaired in mice with altered function or expression of mGluR1 (Aiba et al. 1994; Kishimoto et al. 2002; Ohtani et al. 2014) or GluRδ2 (GRID2) (Kishimoto et al. 2001a,b; Kakegawa et al. 2008), both of which are important for PF–PC LTD (Gao et al. 2012). On the other hand, mice lacking PF–PC AMPA receptor suppression, a mechanism of LTD expression, show normal EBC (Schonewille et al. 2011). EBC is associated with an increase in PC dendritic excitability, not the decrease in excitability that would be expected with PF–PC LTD (Schreurs et al. 1997). Interestingly, both secretin, which suppresses surface Kv1.2 in PC dendrites (Williams et al. 2012) and tityustoxin-Kα, a selective Kv1.2 blocker, also increase PC dendritic excitability (Khavandgar et al. 2005; Williams et al. 2012). Combined with the current data showing that intracerebellar infusion of a secretin antagonist impairs EBC and our previous data showing that intracerebellar infusion of TsTX or secretin facilitates EBC (Williams et al. 2012), it may be the case that secretin and Kv1.2 are important for EBC through signaling pathways that are independent of AMPAR suppression at PF–PC synapses. EBC may suppress Kv1.2 in PC dendrites, enhancing the influence of inputs to those PCs, which might be related to the finding that EBC is impaired in mice with dysfunctional PF–PC long-term potentiation (i.e., Schonewille et al. 2010). EBC might also suppress Kv1.2 in BC–PC pinceaus, reducing excitability of outputs from PCs to the IPN, which may be related to the finding that adaptation of the vestibular-ocular reflex (another form of cerebellar-dependent motor learning) is impaired with dysfunctional inhibitory interneuron input to PCs (Wulff et al. 2009). Finally, mGluR1 and GluRδ2 might be important for EBC not because their activation suppresses PF–PC AMPARs but because their activation reduces surface Kv1.2 in PC dendrites and BC–PC pinceaus.
Unlike EBC acquisition, secretin does not appear to play a strong role in extinction of EBC. We reasoned that infusing secretin prior to extinction training would artificially maintain inhibition of PCs and downstream disinhibition of the IPN, thereby slowing extinction. However, we observed neither an impairment of extinction with intracerebellar infusions of secretin nor a facilitation of extinction with intracerebellar infusions of the secretin receptor antagonist 5-27 secretin. Within the cerebellar cortex, the cellular mechanisms underlying extinction of eyeblink CRs remain elusive; a recent study showed that, unlike acquisition, extinction of eyeblink CRs can occur even without functional PF–PC synapses (Emi et al. 2013). What is known about a role for the cerebellar cortex in extinction of eyeblink CRs is that large lesions of the anterior lobe impair extinction (Perrett and Mauk 1995), there is recovery of simple spiking by PCs during extinction training (Jirenhed et al. 2007; see also Gould and Steinmetz 1996), and infusion of a GABA antagonist into the IPN, which putatively disconnects the IPN from PCs, restores CRs even after extensive extinction training (Medina et al. 2001). However, the cerebellar cortical mechanism underlying the recovery of PC simple spiking and restoration of PC inhibition of the IPN accompanying extinction may not be the opposite of what occurs during acquisition; indeed, the rapid rate of extinction of eyeblink CRs compared with the relatively slow rate of acquisition of eyeblink CRs may be a behavioral indicator that acquisition and extinction are not mediated by opposite mechanisms at the cerebellar cortical level.
Secretin release from depolarized PCs, presynaptic reduction of surface Kv1.2 and subsequent increased inhibition of PCs appears similar to depolarization-induced potentiation of inhibition (DPI) in which the release of glutamate from depolarized PCs activates presynaptic NMDA receptors and increases inhibition of PCs (Duguid and Smart 2004; Duguid et al. 2007). For example, depolarization of PCs increases mIPSC amplitude in PCs (“rebound potentiation” of GABA receptors) for at least 10-min after the depolarization ends, and also produces an initial and brief decrease in mIPSC frequency (depolarization-induced suppression of inhibition; DSI) followed by an increase in mIPSC frequency (DPI) that lasts for several minutes (Duguid and Smart 2004). DPI, but not DSI, is blocked by an NMDA antagonist, while the reverse was true with a CB1 antagonist. Glutamate release by depolarized PCs also involves autocrine activation of metabotropic glutamate receptors on PC dendrites (Duguid et al. 2007); given the presence of secretin receptors on PCs (Yung et al. 2001), secretin release by depolarized PCs would also be able to act as an autocrine signal. These autocrine effects may provide a link with PF–PC postsynaptic LTD.
In conclusion, we have shown that intracerebellar infusion of a secretin receptor antagonist (5-27 secretin) impairs EBC, complementing our previous results showing that intracerebellar infusion of secretin facilitates EBC. In contrast, neither intracerebellar infusion of secretin nor intracerebellar infusion of 5-27 secretin had any effect on extinction of EBC. Collectively, this and our previous work (Williams et al. 2012), suggest that the facilitatory effect of cerebellar secretin in EBC, whether endogenous or exogenous, is specific to acquisition. We propose that secretin's effects on EBC acquisition are through a reduction of surface Kv1.2 in BC–PC pinceaus and PC dendrites.
Materials and Methods
Subjects
Male Wistar rats were purchased from Harlan (Indianapolis, IN) or Charles River (Quebec, Canada) and housed in pairs upon arrival with access to food and water ad libitum. Rats were single housed after surgery. The colony room was maintained on a 12-h light–dark cycle (lights on at 7:00 a.m. and off at 7:00 p.m.). Rats weighed 300–400 g prior to surgery. All testing took place during the light phase of the schedule and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Vermont.
Surgery
Surgeries took place 4–6 d after arrival. Surgeries were performed under aseptic conditions. Rats were anesthetized with 3% isoflurane in oxygen. During the surgery, a 7-mm stainless steel 22-gauge guide cannula (Plastics One) was implanted into the lobulus simplex of the cerebellar cortex (AP: −11.3 from bregma; ML: −2.5; DV: −3.1; infusion at DV: −4.1). After securing the cannula to the skull with dental cement, an 8-mm dummy cannula was placed in the guide cannula and secured with screw threads. The dummy cannula served to seal the guide cannula to prevent infection and to prevent the guide cannula from becoming obstructed prior to infusions. A bipolar stimulation electrode (Plastics One) was positioned subdermally immediately dorsocaudal to the ipsilateral eye. Two EMG wires for recording activity of the external muscles of the eyelid, the orbicularis oculi, were constructed from two strands of 75-μm Teflon coated stainless steel wire soldered at one end to a ministrip connector. The other end of the wire was passed subdermally to penetrate the skin of the upper eyelid of the eye ipsilateral to the guide cannula. A ground wire was wrapped around two skull screws at one end and the other end was soldered to the ministrip connector. The cannula, ministrip connector, and stimulation bipolar electrode were cemented to the skull with dental cement. The wound was numbed with a local injection of 0.1 mL bupivacaine, spread out over three points around the wound. The wound was salved with antibiotic ointment (Povidone), and an analgesic (buprenorphine) and fluids (lactated Ringers) were administered (s.c.) immediately after surgery. Analgesic was administered twice the following day. Rats were given 5–6 d to recover prior to eyeblink conditioning.
Apparatus
Eyeblink conditioning took place in one of four identical testing chambers (30.5 × 24.1 × 29.2 cm; Med-Associates), each with a grid floor. The top of the chamber was altered so that a 25-channel tether/commutator could be mounted to it. Each testing chamber was kept within a separate electrically shielded, sound-attenuating chamber (45.7 × 91.4 × 50.8 cm; BRS-LVE, Laurel, MD). A fan in each sound-attenuating chamber provided background noise of ∼60 dB sound pressure level. A speaker was mounted in each corner of the rear wall and a light (off during testing) was mounted in the center of the rear wall of each chamber. The sound-attenuating chambers were housed within a walk-in sound-proof chamber.
Stimulus delivery was controlled by a computer running Spike2 software (CED). A 2.8 kHz, 80 dB tone, delivered through the left speaker of the sound-attenuating chamber, served as the CS. The CS was 295-msec in duration. A 15-msec, 4.0-mA uniphasic periorbital stimulation, delivered from a constant current stimulator (model A365D; World Precision Instruments), served as the US during conditioning. Recording of the eyelid EMG activity was controlled by a computer interfaced with a Power 1401 high-speed data acquisition unit and running Spike2 software (CED). Eyelid EMG signals were amplified (10K) and bandpass filtered (100–1000 Hz) prior to being passed to the Power 1401 and from there to the computer running Spike2. Sampling rate was 2 kHz for EMG activity. The Spike2 software was used to full-wave rectify, smooth (10 msec time constant), and time shift (10 msec, to compensate for smoothing) the amplified EMG signal to facilitate behavioral data analysis.
Eyeblink conditioning procedure
At the beginning of each session, each rat was plugged in, via the connectors cemented to its head, to the 25-channel tether/commutator, which carried leads to and from peripheral equipment and allowed the rat to move freely within the testing box. On day 1 (adaptation), rats were plugged in but no stimuli were delivered. They remained in the chamber for 60 min (the approximate length of a training session). Spontaneous eyelid EMG activity was sampled for the same duration and at the same time points as during the subsequent conditioning sessions (i.e., 2 sec samples with an average intertrial interval (ITI) of 30 sec and a range of 20–40 sec). On days 2–7 (conditioning), rats received 100 trials per day, with an average ITI of 30 sec (range = 20–40 sec). In Experiment 1a, each block of 10 trials consisted of the following trial sequence: 4 CS–US trials (CS preceding and coterminating with the US), 1 CS-alone trial, 4 CS–US trials, and 1 US-alone trial. In Experiments 1b and 2, all 100 trials were CS–US trials. In Experiment 3, each block consisted of 2 CS-alone trials and 8 CS–US trials. The position of the CS-alone trials within each block changed across blocks. Finally, in Experiments 2 and 3, rats underwent extinction training on days 8–10. Extinction sessions each consisted of 100 CS-alone trials.
Prior to the first 3 d of conditioning (Experiments 1a and 1b) or the first 2 d of extinction (Experiments 2 and 3), rats received an intracerebellar infusion of 1 µL of either 1 µg/1 µL 5-27 secretin (Ant; Experiments 1a, 1b, and 3), 1 µg/1 µL secretin (Sec; Experiment 2) or phosphate-buffered saline vehicle (Veh; all experiments). The volume and concentration of secretin was the same as was used in Williams et al. (2012), where we observed a facilitation of EBC acquisition; we elected to use the same volume and concentration of 5-27 secretin in the current experiments. For infusions, the dummy cannula was removed and a 28-gauge internal cannula was inserted into the guide cannula. The internal cannula protruded 1 mm below the guide cannula tip, making the final infusion depth 4.1 mm below bregma. Infusions were made with a 10-µL Hamilton syringe loaded onto an infusion pump (KD Scientific) set to deliver 1 µL of solution over 2 min. At the end of the infusion period, the internal cannula remained in place an additional 1 min to allow diffusion of the infused solution away from the cannula tip. Subsequently, the internal cannula was removed, the dummy cannula was replaced, the rats were plugged in, and the EBC session began. Rats were infused and tested in groups of four (two from each group). Infusions took place ∼15 min prior to the start of the EBC session.
Histology
Within ∼24 h after the final session, rats were overdosed with sodium pentobarbital (150 mg/kg) and transcardially perfused with 0.9% saline followed by 10% buffered formalin. A small DC electrolytic lesion (100 μA, ∼10 sec) was made by passing current through an electrode made from a 000 gauge insect pin insulated (except for 0.5 mm on the tip) with nail polish, that was placed into the guide cannula, with the tip extending out of the guide cannula by ∼1.0 mm. The brain was removed and stored in 10% buffered formalin. Four or 5 d prior to sectioning, the brain was transferred to a 30% sucrose/10% buffered formalin solution. Before sectioning, cerebella were embedded in albumin–gelatin. Frozen sections of the cerebellum were taken at 60 μm. Tissue was mounted on gelatin-coated glass slides, stained with Prussian blue (for iron deposits left by the marking lesions) and cresyl violet (for cell bodies) and cover slipped with Permount.
Behavior analysis
For the conditioning sessions, CS–US trials were subdivided into four time periods: (1) a “baseline” period, 280 msec prior to CS onset; (2) a nonassociative “startle” period, 0–80 msec after CS onset; (3) a “CR” period, 81–280 msec after CS onset; and (4) a “UR period,” 65–165 msec after US onset (the first 65 msec is obscured by the stimulation artifact). On CS-alone trials, the “CR” period extended for 150 msec after CS offset to capture CRs that may normally be masked by the US. In order for a response to be scored as a CR, eyeblinks had to exceed the mean baseline activity for that trial by 0.5 arbitrary units (where these units had a range of 0.0–5.0) during the CR period. Eyeblinks that met this threshold during the startle period were scored as startle responses and were analyzed separately. Trials in which eyeblinks exceeded 1.0 arbitrary unit during the baseline period were discarded. Comparable scoring intervals and criteria were used to evaluate spontaneous blink rate during the initial adaptation day when no stimuli were administered. The primary dependent measure for all experiments was the percentage of CRs across all CS–US (acquisition) or CS-alone (extinction) trials of each session.
For the percentage of CRs for all experiments, data were analyzed using repeated-measures ANOVAs. Separate ANOVAs were conducted on data from infusion sessions and noninfusion sessions. We computed all statistical analyses using SPSS 21.0. An α level of 0.05 was set as the rejection criterion for all statistical tests.
Acknowledgments
This research was supported by funding from NIH/NINDS R21 NS085471, the University of Vermont Neuroscience Behavior and Health Initiative, and the University of Vermont College of Arts and Sciences. We thank Willard Gove for his help with data collection and histology, and Nicole Bishop and Nicole Bouffard for help with the histology image.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.035766.114.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S 1994. Deficient long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377–388. [PubMed] [Google Scholar]
- Aksenov D, Serdyukova N, Irwin K, Bracha V 2004. GABA neurotransmission in the cerebellar interposed nuclei: involvement in classically conditioned eyeblinks and neuronal activity. J Neurophysiol 91: 719–727. [DOI] [PubMed] [Google Scholar]
- Attwell PJE, Rahman S, Yeo CH 2001. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J Neurosci 21: 5715–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell PJE, Cooke SF, Yeo CH 2002. Cerebellar function in consolidation of a motor memory. Neuron 34: 1011–1020. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF 2002. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci 99: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele H-J, Koekkoek SKE, De Zeeuw CI, Ruigrok TJH 2013. Axonal sprouting and formation of terminals in the adult cerebellum during associative motor learning. J Neurosci 33: 17897–17907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartford MC, Samec A, Fister M, Bickford PC 2004. Cerebellar norepinephrine modulates learning of delay classical eyeblink conditioning: evidence for post-synaptic signaling via PKA. Learn Mem 11: 732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Shin C, Kim MJ, Lee BK, Cha CI 2001. Immunohistochemical study on the distribution of six members of the Kv1 channel subunits in the rat cerebellum. Brain Res 895: 173–177. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Attwell PJE, Yeo CH 2004. Temporal properties of cerebellar-dependent memory consolidation. J Neurosci 24: 2934–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC, Smart TG 2004. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron–Purkinje cell synapses. Nat Neurosci 7: 525–533. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Pankratov Y, Moss GWJ, Smart TG 2007. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci 27: 12464–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi K, Kakegawa W, Miura E, Ito-Ishida A, Kohda K, Yuzaki M 2013. Reevaluation of the role of parallel fiber synapses in delay eyeblink conditioning in mice using Cbln1 as a tool. Front Neural Circuits 7: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, van Beugen BJ, De Zeeuw CI 2012. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13: 619–635. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD 1998. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 37: 471–480. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD 1999. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci 19: 10940–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Steinmetz JE 1996. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiol Learn Mem 65: 17–34. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Napper RM 1991. Quantitative studies on the mammalian cerebellum. Prog Neurobiol 36: 437–463. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Jirenhed D-A, Rasmussen A, Johansson F 2013. Classical conditioning of motor responses: what is the learning mechanism? Neural Netw 47: 81–87. [DOI] [PubMed] [Google Scholar]
- Jirenhed D-A, Bengtsson F, Hesslow G 2007. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 27: 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Emi K, Matsuda K, Kohda K, Motohashi J, Mishina M, Kawahara S, Watanabe M, Yuzaki M 2008. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRδ2. J Neurosci 28: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Davis T, Ohyama T, Riusech F, Nores WL, Mauk MD 2010. Cerebellar cortex contributions to the expression and timing of conditioned eyelid responses. J Neurophysiol 103: 2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett DO, Fukunaga I, Chen-Kubota E, Dean P, Yeo CH 2010. Memory consolidation in the cerebellar cortex. PLoS One 5: e11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavandgar S, Walter JT, Sageser K, Khodakhah K 2005. Kv1 channels selectively prevent dendritic hyperexcitability in rat Purkinje cells. J Physiol 569: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y 2001a. Impairment of eyeblink conditioning in GluRd2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. Eur J Neurosci 14: 1515–1521. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y 2001b. Classical eyeblink conditioning in glutamate receptor subunit d2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci 13: 1249–1253. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y 2002. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur J Neurosci 16: 2416–2424. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D 2002. Synapse formation is associated with memory storage in the cerebellum. Proc Natl Acad Sci 99: 13228–13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch RO, Wanner SG, Koschak A, Hanner M, Schwarzer C, Kaczorowski GJ, Slaughter RS, Garcia ML, Knaus HG 1997. Complex subunit assembly of neuronal voltage-gated K+ channels. Basis for high-affinity toxin interactions and pharmacology. J Biol Chem 272: 27577–27581. [DOI] [PubMed] [Google Scholar]
- Köves K, Kausz M, Reser D, Horváth K 2002. What may be the anatomical basis that secretin can improve the mental functions in autism? Regul Pept 109: 167–172. [DOI] [PubMed] [Google Scholar]
- Laube G, Röper J, Christian Pitt J, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW 1996. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Mol Brain Res 42: 51–61. [DOI] [PubMed] [Google Scholar]
- Lee SMY, Chen L, Chow BKC, Yung WH 2005. Endogenous release and multiple actions of secretin in the rat cerebellum. Neuroscience 134: 377–386. [DOI] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD 2001. A mechanism for savings in the cerebellum. J Neurosci 21: 4081–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann W, Koch U, Häusser M 2005. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol 563: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani Y, Miyata M, Hashimoto K, Tabata T, Kishimoto Y, Fukaya M, Kase D, Kassai H, Nakao K, Hirata T, et al. 2014. The synaptic targeting of mGluR1 by its carboxyl-terminal domain is crucial for cerebellar function. J Neurosci 34: 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mauk M 2001. Latent acquisition of timed responses in cerebellar cortex. J Neurosci 21: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD 2006. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci 26: 12656–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Zbarska S, Carrel AJ, Bracha V 2009. Blocking GABAA neurotransmission in the interposed nuclei: effects on conditioned and unconditioned eyeblinks. Brain Res 1292: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Mauk MD 1995. Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. J Neurosci 15: 2074–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Nobuta H, Thompson RF 2009. Disruption of cerebellar cortical inhibition in the absence of learning promotes sensory-evoked eyeblink responses. Behav Neurosci 123: 694–700. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Endo S 2008. GABAA receptors in deep cerebellar nuclei play important roles in mouse eyeblink conditioning. Brain Res 1230: 125–137. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, et al. 2010. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 67: 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele H-J, Veloz MFV, Amerika WE, Simek AAM, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, et al. 2011. Reevaluating the role of LTD in cerebellar motor learning. Neuron 70: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Tomsic D, Gusev PA, Alkon DL 1997. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit's nictitating membrane response. J Neurophysiol 77: 86–92. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B 1998a. Modulation of inhibitory post-synaptic currents (IPSCs) in mouse cerebellar Purkinje and basket cells by snake and scorpion toxin K+ channel blockers. Br J Pharmacol 125: 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B 1998b. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci 18: 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B 2000. Electrophysiological characterization of voltage-gated K(+) currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci 20: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE 2009. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 162: 732–755. [DOI] [PubMed] [Google Scholar]
- Vogel RW, Amundson JC, Lindquist DH, Steinmetz JE 2009. Eyeblink conditioning during an interstimulus interval switch in rabbits (Oryctolagus cuniculus) using picrotoxin to disrupt cerebellar cortical input to the interpositus nucleus. Behav Neurosci 123: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Fuchs JR, Green JT, Morielli AD 2012. Cellular mechanisms and behavioral consequences of Kv1.2 regulation in the rat cerebellum. J Neurosci 32: 9228–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Schonewille M, Renzi M, Viltono L, Sassoe-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, et al. 2009. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci 12: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WH, Leung PS, Ng SS, Zhang J, Chan SC, Chow BK 2001. Secretin facilitates GABA transmission in the cerebellum. J Neurosci 21: 7063–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chung SK, Chow BKC 2014. The knockout of secretin in cerebellar Purkinje cells impairs mouse motor coordination and motor learning. Neuropsychopharmacology 39: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]