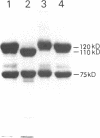
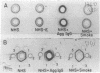
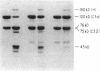
Abstract
Cigarette smoking is associated with significant increases in the number of pulmonary mononuclear phagocytes and neutrophils. A potent chemoattractant for these cells is C5a, a peptide generated during complement (C) activation. We, therefore, investigated the possibility that cigarette smoke could activate the complement system in vitro. Our results show that factor(s) (mol wt less than 1,000) present in an aqueous solution of whole, unfiltered cigarette smoke can deplete the hemolytic capacity of whole human serum in a dose-dependent manner. The particle-free, filtered gas phase of cigarette smoke is inactive. The smoke factor(s) do not activate serum C1, but do deplete serum C4 activity. Treatment of purified human C3 with whole smoke solution modifies the molecule such that its subsequent addition to serum (containing Mg/EGTA to block the classical pathway) results in consumption of hemolytic complement by activation of the alternative pathway. Smoke-modified C3 shows increased anodal migration in agarose electrophoresis, but this is not due to proteolytic cleavage of the molecule as evidenced by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In contrast to methylamine-treated C3, C3 treated with smoke is only partially susceptible to the action of the complement regulatory proteins Factors H and I. In addition, smoke-modified C3 has diminished binding to Factor H as compared with methylamine-treated C3. Finally, smoke-modified C3 incorporates [14C]methylamine which suggests that the thiolester bond may be intact. These data indicate that aqueous whole cigarette smoke solution can modify C3 and activate the alternative pathway of complement, perhaps by a previously unrecognized mechanism. Should this occur in vivo, complement activation might partly account for the extensive pulmonary leukocyte recruitment observed in smokers.
Full text
PDF

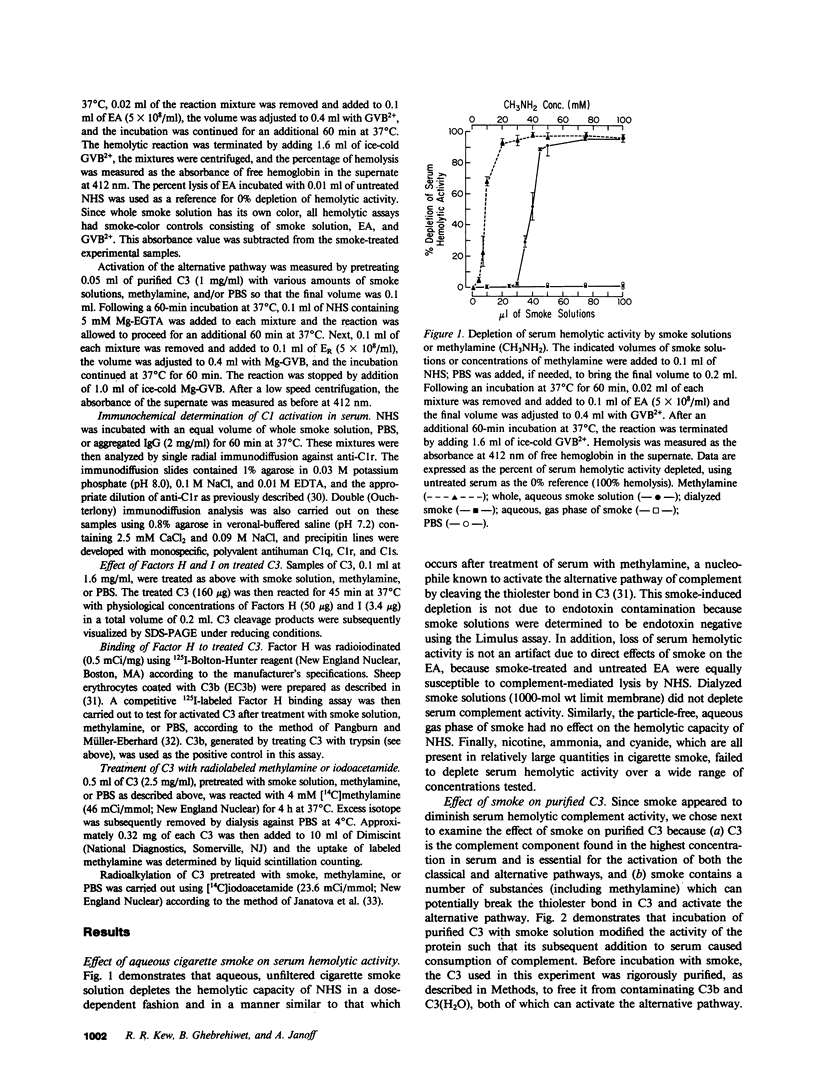
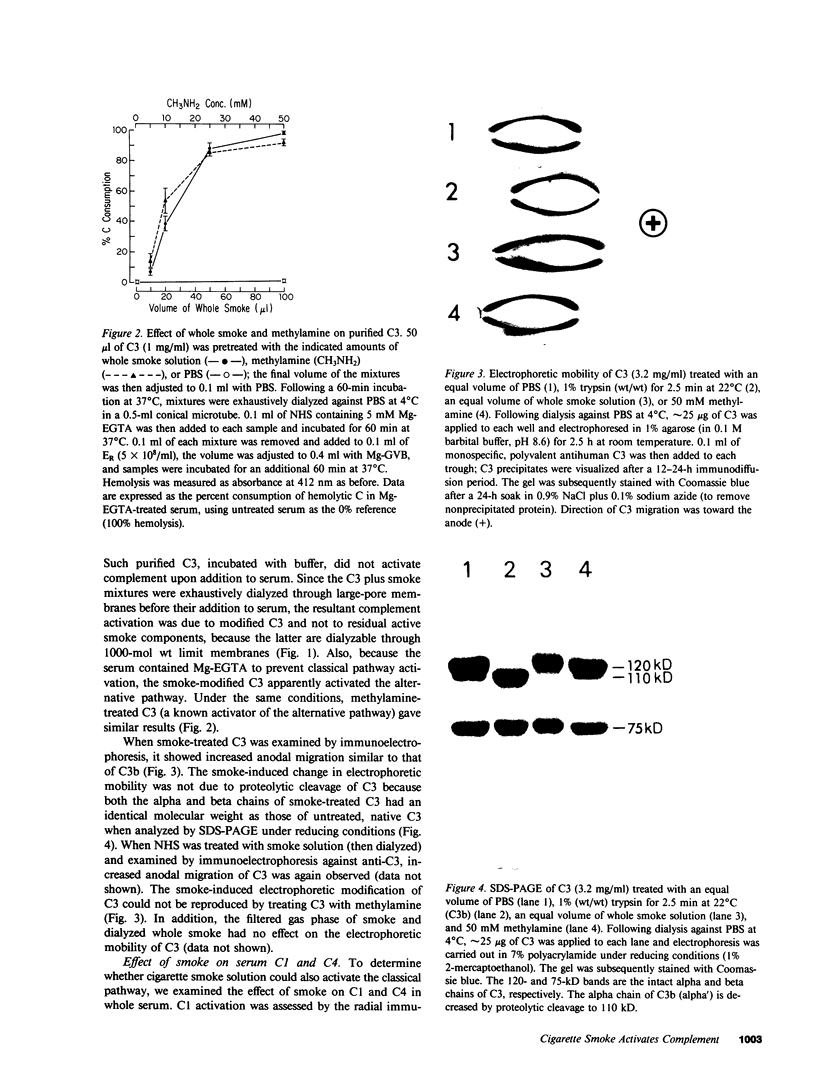
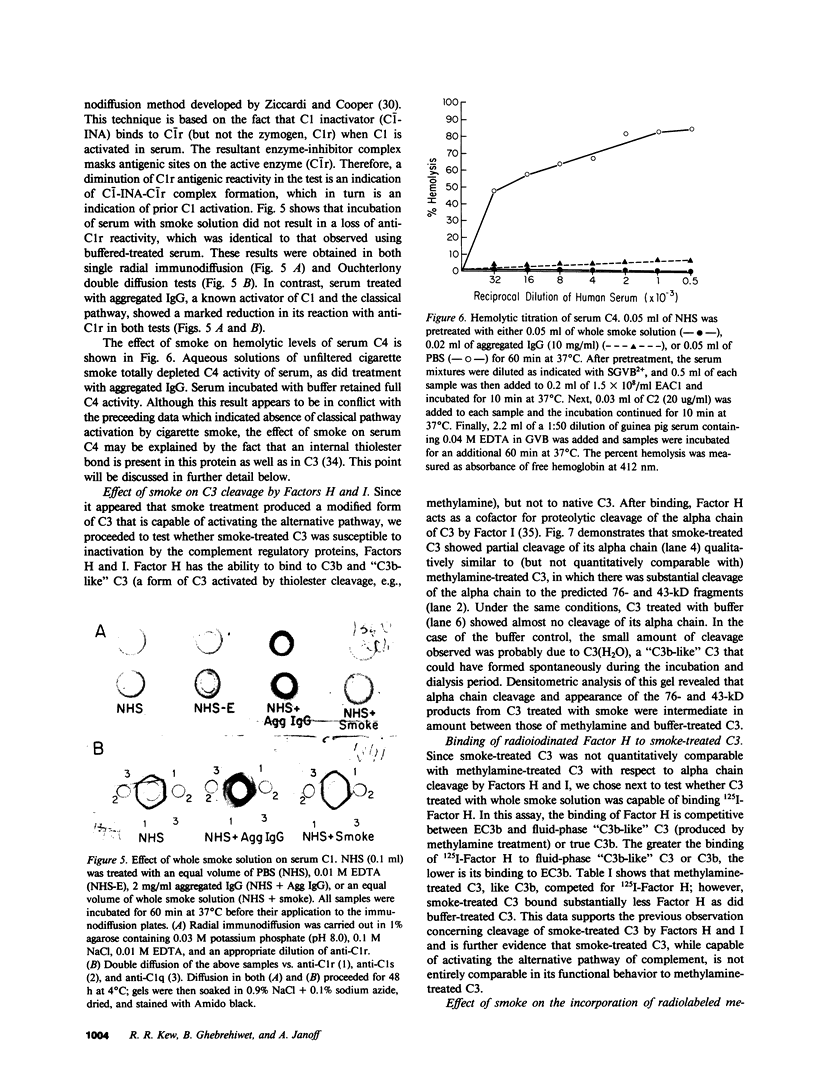



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the proposed thiolester bond of human complement component C4 and comparison with the corresponding sequences from C3 and alpha 2-macroglobulin. Biosci Rep. 1981 May;1(5):423–429. doi: 10.1007/BF01116192. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Chenoweth D. E., Hugli T. E. The release of elastase, myeloperoxidase, and lysozyme from human alveolar macrophages. Am Rev Respir Dis. 1982 Aug;126(2):241–247. doi: 10.1164/arrd.1982.126.2.241. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. A comparison of methods for the molecular quantitation of the fourth component of human complement. Immunochemistry. 1968 Mar;5(2):155–169. doi: 10.1016/0019-2791(68)90100-6. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Harrison R. A. Structural characterization of factor I mediated cleavage of the third component of complement. Biochemistry. 1982 Nov 9;21(23):5745–5749. doi: 10.1021/bi00266a003. [DOI] [PubMed] [Google Scholar]
- Hinman L. M., Stevens C. A., Matthay R. A., Gee J. B. Elastase and lysozyme activities in human alveolar macrophages. Effects of cigarette smoking. Am Rev Respir Dis. 1980 Feb;121(2):263–271. doi: 10.1164/arrd.1980.121.2.263. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Niewoehner D. E. Pathogenesis of emphysema. Chest. 1983 Apr;83(4):679–685. doi: 10.1378/chest.83.4.679. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F. Fourth component of human complement: studies of an amine-sensitive site comprised of a thiol component. Biochemistry. 1981 Apr 28;20(9):2394–2402. doi: 10.1021/bi00512a005. [DOI] [PubMed] [Google Scholar]
- Janatova J., Tack B. F., Prahl J. W. Third component of human complement: structural requirements for its function. Biochemistry. 1980 Sep 16;19(19):4479–4485. doi: 10.1021/bi00560a015. [DOI] [PubMed] [Google Scholar]
- Janatova J. The third (C3) and the fourth (C4) components of complement: labile binding site and covalent bond formation. Ann N Y Acad Sci. 1983;421:218–234. doi: 10.1111/j.1749-6632.1983.tb18111.x. [DOI] [PubMed] [Google Scholar]
- Janoff A. Biochemical links between cigarette smoking and pulmonary emphysema. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):285–293. doi: 10.1152/jappl.1983.55.2.285. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Isolation of a thermolabile serum protein which precipitates gamma-globulin aggregates and participates in immune hemolysis. Proc Soc Exp Biol Med. 1961 Feb;106:291–295. doi: 10.3181/00379727-106-26313. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone R., de Carolis C., de Sanctis G., Fontana L. Complement activation by cigarette smoke condensate and tobacco infusion. Arch Environ Health. 1983 May-Jun;38(3):176–179. doi: 10.1080/00039896.1983.10544001. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman R. L. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968 Apr;68(2):153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Totti N., 3rd, McCusker K. T., Campbell E. J., Griffin G. L., Senior R. M. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science. 1984 Jan 13;223(4632):169–171. doi: 10.1126/science.6318317. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Development of an immunochemical test to assess C1 inactivator function in human serum and its use for the diagnosis of hereditary angioedema. Clin Immunol Immunopathol. 1980 Mar;15(3):465–471. doi: 10.1016/0090-1229(80)90058-6. [DOI] [PubMed] [Google Scholar]