Abstract
The liver has a strong regenerative capacity. After injury, quiescent hepatocytes can reenter the mitotic cell cycle to restore tissue homeostasis. This G0/G1-S cell-cycle transition of primed hepatocytes is regulated by complexes of cyclin-dependent kinase 2 (Cdk2) with E-type cyclins (CcnE1 or CcnE2). However, single genetic ablation of either E-cyclin or Cdk2 does not affect overall liver regeneration. Here, we systematically investigated the contribution of CcnE1, CcnE2, and Cdk2 for liver regeneration after partial hepatectomy (PH) by generating corresponding double- and triple-knockout (KO) mouse mutants. We demonstrate that conditional deletion of Cdk2 alone in hepatocytes resulted in accelerated induction of CcnE1, but otherwise normal initiation of S phase in vivo and in vitro. Excessive CcnE1 did not contribute to a noncanonical kinase activity, but was located at chromatin together with components of the pre-replication complex (pre-RC), such as the minichromosome maintenance (MCM) helicase. Concomitant ablation of Cdk2 and CcnE1 in hepatocytes caused a defect in pre-RC formation and further led to dramatically impaired S-phase progression by down-regulation of cyclin A2 and cell death in vitro and substantially reduced hepatocyte proliferation and liver regeneration after PH in vivo. Similarly, combined loss of CcnE1 and CcnE2, but also the Cdk2/CcnE1/CcnE2 triple KO in liver, significantly inhibited S-phase initiation and liver mass reconstitution after PH, whereas concomitant ablation of CcnE2 and Cdk2 had no effect.
Conclusion
In the absence of Cdk2, CcnE1 performs crucial kinase-independent functions in hepatocytes, which are capable of driving MCM loading on chromatin, cyclin A2 expression, and S-phase progression. Thus, combined inactivation of Cdk2 and CcnE1 is the minimal requirement for blocking S-phase machinery in vivo.
The mammalian cell-cycle machinery is controlled by cyclin-dependent kinases (Cdks) and cyclins (Ccn), which act as Cdk-regulatory subunits.1 In the presence of extracellular mitogenic signals, D-type cyclins (CcnD1, CcnD2, and CcnD3) drive G1-phase progression through activation of Cdk4 and Cdk6, leading to phosphorylation and thus inactivation of the retinoblastoma protein (Rb). Rb phosphorylation is completed by CcnE/Cdk2 kinase complexes shortly before entry into S phase,2 eventually leading to activation of E2F transcription factors and induction of cell-cycle–related genes. After initiation of DNA synthesis, Cdk2 interacts with CcnA and triggers S-phase progression. Accurate DNA replication depends on assembly of pre-replicative complexes (pre-RCs), which are formed immediately after exit from mitosis in continuously dividing cells,3 or in the G1 phase of previously quiescent cells.4 Pre-RC formation involves recruitment of the origin recognition complex (ORC), chromatin licensing and DNA replication factor 1 (Cdt1), and Cdc6 to replication origins and subsequent loading of the minichromosome maintenance complex (MCM2-7), which acts as the replicative helicase.5-8
Surprisingly, genetic knockout (KO) experiments in mice revealed that none of the interphase Cdks (Cdk2, Cdk4, and Cdk6) are essential for cell proliferation or development in vivo.9,10 Moreover, Cdk2 and Cdk4 are largely dispensable for liver regeneration.11-13 Similarly, single genetic inactivation of D-type cyclins (CcnD1-3) or E-type cyclins (CcnE1-2) does not affect viability or development in mice.14,15 However, E-cyclins were shown to be essential for the transition from the quiescent state into the active cell cycle, because CcnE1/E2 double-deficient fibroblasts are unable to reenter the cell cycle from starvation-induced quiescence.15
Liver regeneration after partial (70%) hepatectomy (PH) in mice is one of the best experimental models for analyzing cell-cycle regulation in vivo. Because of the high regenerative capacity of the liver, resting remnant hepatocytes leave their quiescent state and undergo one to two synchronized rounds of cell cycle with a peak of DNA synthesis after approximately 40-48 hours, resulting in restoration of original liver mass within 7-10 days.16 We have recently demonstrated that CcnE1 and CcnE2 have unique functions after PH and even play antagonistic roles.17 CcnE2−/− livers showed accelerated and sustained DNA synthesis and hepatomegaly, whereas ablation of CcnE1 provoked only a minor delay of hepatocyte proliferation.
Here, we aimed to define the relevance of all Cdk2/CcnE complex components (Cdk2, CcnE1, and CcnE2) for DNA synthesis and cell-cycle reentry in vivo. We generated viable mouse mutants harboring all possible combinations of CcnE1/CcnE2 and Cdk2 KO alleles and studied the consequences for hepatocyte proliferation in vitro and in vivo. We demonstrate that CcnE1—but not its homolog, CcnE2—is essential for driving G0/S-phase transition in hepatocytes lacking Cdk2 and identify minimal genetic requirements concerning Cdk2 and E-cyclins to drive the hepatic cell cycle.
Materials and Methods
Generation of Mice and Animal Experiments
Constitutive CcnE1−/− and CcnE2−/− KO mice were recently generated and characterized.15,17,18 Mice with floxed alleles of Cdk2 (Cdk2f/f) and CcnE1 (CcnE1f/f) have been described elsewhere.10,19 To generate hepatocyte-specific Cdk2 KO mice (Cdk2Δhepa) and CcnE1 KO mice (CcnE1Δhepa), respectively, we crossed Cdk2f/f and CcnE1f/f animals with transgenic alfp-cre mice expressing cre-recombinase under control of albumin promoter and alpha-fetoprotein (AFP) enhancer elements in a C57BL6 background.20 Matching Cdk2f/f and CcnE1f/f littermates were used as wildtype (WT) controls. Double- and triple-KO strains (Cdk2ΔhepaCcnE1−/−, CcnE1ΔhepaCcnE2−/−, and Cdk2ΔhepaCcnE1ΔhepaCcnE2−/−) were generated by crossing of appropriate single-KO mice. Besides male sterility in mice lacking Cdk2 and/or CcnE2, all animals developed normally and displayed regular liver morphology and size under homeostatic conditions. Animal experiments were carried out according to German legal requirements and animal protection law and were approved by the authority for environment conservation and consumer protection of the state of North Rhine-Westfalia (LANUV, Germany, AZ 8.87-50.10.35.08.284). PH was performed as recently described.17
Isolation of Primary Hepatocytes and Cell Culture
Primary mouse hepatocytes were isolated from 7- to 8-week-old mice by collagenase perfusion.21 Live cells were plated on collagen type 1–coated Petri dishes at a density of 1.2 × 104/cm2 in Dulbecco’s modified Eagle’s medium (PAA Laboratories GmbH, Pasching, Austria) supplemented with L-glutamine, high glucose (4.5 g/L), and 100 U/mL of penicillin/streptomycin. After 4-hour incubation (37 °C, 5% CO2) and every 24 hours, cells were stimulated with 10 ng/mL of epidermal growth factor (EGF; Sigma-Aldrich, St. Louis, MO) and 20 mIU/mL of insulin (Novo Nordisk, Mainz, Germany).
Determination of Bromodeoxyuridine Incorporation and Immunofluorescence Staining
Two hours before killing, all mice were injected with bromodeoxyuridine (BrdU; AppliChem, Cheshire, CT). For determination of S phase in the regenerating liver, incorporation of BrdU was measured as described recently.17 Fluorescence-labeled liver cryosections were analyzed and documented using an Imager Z1 fluorescence microscope together with Axiovision software (Carl Zeiss, Jena, Germany).
Statistical Analysis
Data are presented as mean- ± standard deviation of the mean. Statistical significance was determined by the Student t test.
Results
Hepatocyte-Specific Ablation of Cdk2 Does Not Affect S Phase and Liver Regeneration After PH
Cdk2Δhepa mice revealed efficient Cdk2 gene inactivation in ex vivo isolated primary hepatocytes, whereas slight residual Cdk2 gene expression was detectable in whole liver tissue reflecting Cdk2 expression of cre-negative nonparenchymal liver cells (Supporting Fig. 1A). However, ablation of Cdk2 in hepatocytes did not impair DNA replication or liver regeneration after PH, in comparison to WT controls, as evidenced by BrdU analysis and liver mass restoration (Supporting Fig. 1B-D). Consistently, we did not detect major differences in regulation of most interphase cyclins, associated Cdks, and target proteins in Cdk2Δhepa mice after PH (Supporting Fig. 1E-H). Of note, Rb phosphorylation (Ser807 and Ser811), which is usually mediated by CcnD-Cdk4/6 and CcnE/Cdk2 kinases, was also normal in Cdk2Δhepa mice (Supporting Fig. 1G), hinting at a compensatory kinase activity in these animals. These findings are in agreement with recent studies using constitutive Cdk2 KO mice,12,13 suggesting that Cdk2-deficient hepatocytes retained the full capacity to reenter the cell cycle after liver resection.
CcnE1 Mediates Kinase-Independent Functions in Hepatocytes and Is Essential for MCM2 Loading on Chromatin in the Absence of Cdk2
The main focus of our study was to identify mechanisms allowing hepatocyte proliferation in the absence of Cdk2. To this end, we thoroughly analyzed the regulation and activity of the canonical Cdk2 regulators, CcnE1 and CcnE2. After PH, CcnE1 gene and protein expression was prematurely induced in Cdk2Δhepa mice (Fig. 1A,B), which was not the case for its homolog, CcnE2 (Supporting Fig. 1E). It was recently demonstrated that in the absence of Cdk2, CcnE1 can mediate noncanonical kinase activities (e.g., by association with Cdk1) at least in embryo, spleen, and thymus.22,23 However, extensive analysis of Cdk activities in regenerating Cdk2Δhepa livers revealed that both CcnE1 and CcnE2 did not contribute to any kinase activity during the S phase (Fig. 1C). Instead, we detected increased kinase activity of CcnD-related complexes (CcnD/Cdk4 and CcnD/Cdk6) 36 hours after PH and enhanced Cdk1 kinase activity 48 hours post-PH, suggesting that these kinases are sufficient to phosphorylate S-phase-specific substrates in the absence of Cdk2.
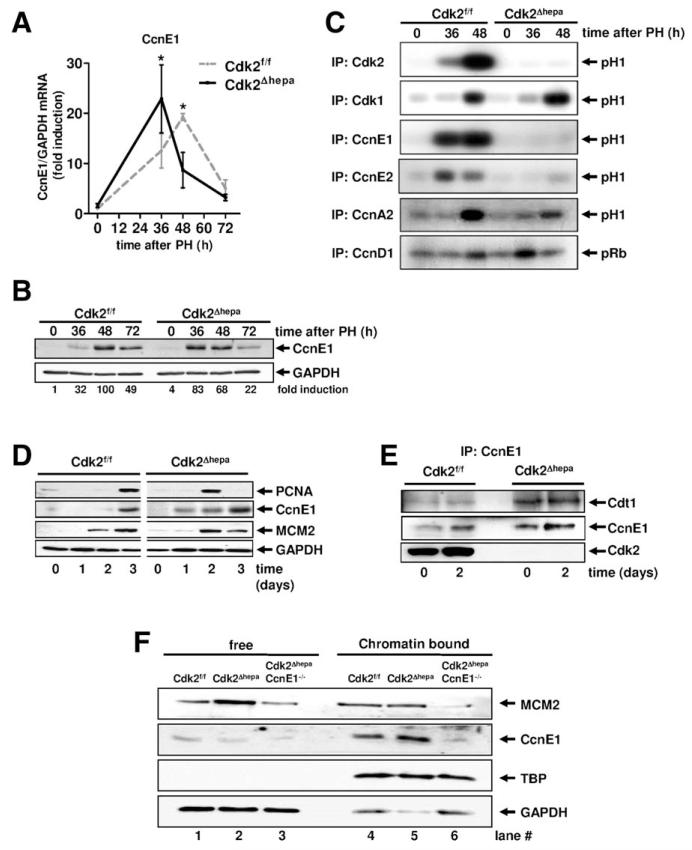
Fig. 1.
CcnE1 mediates kinase-independent functions in hepatocytes and is essential for MCM2 loading on chromatin in the absence of Cdk2. (A and C) Cdk2f/f and Cdk2Δhepa mice were subjected to PH and sacrificed at indicated time points. (A and B) Gene and protein expression profile of CcnE1 after PH. *P < 0.05. Protein levels (fold induction) were quantified using electronic densitometry and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. (C) Liver extracts were subjected to immunoprecipitation (IP) with antibodies directed against indicated cyclins and Cdks. Precipitated cyclin/kinase complexes were used for in vitro kinase assays using recombinant histone H1 (H1) or Rb as substrate. Phosphorylated proteins are highlighted by arrows. (D and F) Primary mouse hepatocytes were isolated from indicated strains (Cdk2f/f, Cdk2Δhepa, or Cdk2ΔhepaCcnE1−/−) and cultured for up to 3 days in the presence of EGF and insulin. (D) Total protein expression of PCNA, CcnE1, and MCM2. (E) CcnE1 complexes were isolated from total proteins by IP (CcnE1) and probed for Cdt1, CcnE1, and Cdk2. (F) Expression and cellular localization of MCM2 and CcnE1 was analyzed by western blotting using fractionated cell extracts representing whole cytoplasmic protein (free) or the chromatin-bound proteins, respectively. TATA binding protein (TBP) and GAPDH represent loading controls for chromatin and cytoplasmic fractions, respectively.
These data excluded the possibility that excessive CcnE1 in regenerating Cdk2Δhepa mice contributes to S-phase initiation by formation of a noncanonical kinase complex, pointing to a kinase-independent function of CcnE1. In fibroblasts, CcnE1 facilitates the formation of the pre-RC and MCM loading onto chromatin in a Cdk2-independent manner.18 Thus, we hypothesized that CcnE1 could be especially relevant for MCM loading if Cdk2 is not available. Therefore, we isolated and cultivated quiescent primary hepatocytes from Cdk2Δhepa mice and Cdk2f/f controls and forced these cells to reenter the cell cycle in vitro by mitogen stimulation using EGF and insulin. Interestingly, Cdk2-deficient hepatocytes revealed accelerated onset of CcnE1, which was associated with premature induction of the replication-related factors, MCM2 and proliferating cell nuclear antigen (PCNA; Fig. 1D). Using coprecipitation analysis, we demonstrated that CcnE1 constitutively interacted with Cdt1 in the absence of Cdk2, which was not the case in control cells (Fig. 1E).
In purified chromatin fractions of Cdk2Δhepa cells, we detected normal loading of MCM2 on chromatin, but increased chromatin-bound CcnE1 (Fig. 1F, compare lanes 4 and 5), suggesting that loss of Cdk2 results in augmented association of CcnE1 with the MCM-Cdt1 complex at chromatin. To confirm this hypothesis, we also depleted CcnE1 and analyzed MCM loading in hepatocytes lacking both Cdk2 and CcnE1 (derived from Cdk2ΔhepaCcnE1−/− mice). Interestingly, combined loss of Cdk2 and CcnE1 substantially down-regulated MCM expression (Fig. 1F, lane 3) and prevented MCM loading on chromatin (Fig. 1F, lane 6). Collectively, these key data indicated that a kinase-independent function of CcnE1 is crucial for loading of MCM2 at chromatin in the absence of Cdk2.
Combined Depletion of Cdk2 and CcnE1 in Hepatocytes Results in Strongly Reduced DNA Synthesis and Severely Impaired Viability
We determined further potential cell-cycle defects in primary hepatocytes lacking Cdk2 and CcnE1. Gene and protein expression of CcnA2 (indicating S-phase progression) was largely abolished in Cdk2−/−CcnE1−/− cells (Fig. 2A,B). Therefore, we tested whether CcnE1 could be involved in CcnA2 promoter regulation. In fact, we detected increased association of CcnE1 with the CcnA2 promoter in mitogen-stimulated WT hepatocytes and further found that Cdk2 and CcnE1 are relevant for loading a yet unknown protein complex at a previously characterized E2F binding site (Supporting Fig. 2A-C).
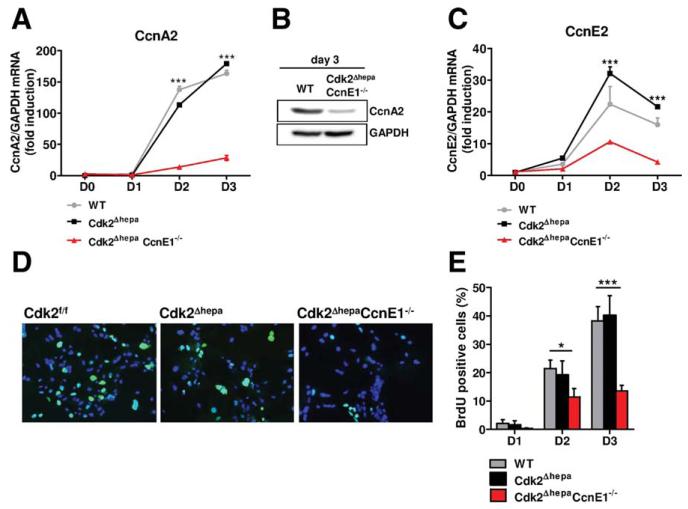
Fig. 2.
Combined depletion of Cdk2 and CcnE1 results in strongly reduced CcnA2 expression and impaired DNA synthesis. Primary mouse hepatocytes from Cdk2f/f (WT), Cdk2Δhepa, and Cdk2ΔhepaCcnE1−/− mice were isolated and cultured for up to 3 days in the presence of EGF and insulin. (A) qPCR analysis of CcnA2 gene expression. (B) Down-regulation of CcnA2 in Cdk2ΔhepaCcnE1−/− mice at day 3 was confirmed by western blotting. (C) qPCR analysis of CcnE2 gene expression. (D) Determination of BrdU incorporation (green) in primary hepatocytes after 3 days in culture on coverslips. Total nuclei are counterstained with 4′, 6-diamidino-2-phenylindole (blue). (E) Quantification of BrdU-positive hepatocytes. *P < 0.05; ***P < 0.001.
Of note, CcnE2 was also strongly down-regulated in Cdk2ΔhepaCcnE1−/− cells (Fig. 2C), suggesting that CcnE1 is involved in CcnE2 and CcnA2 gene induction. Impaired CcnA2 gene expression in Cdk2Δhepa CcnE1−/− hepatocytes affected S-phase entry and elongation. The S phase in Cdk2ΔhepaCcnE−/− hepatocytes was reduced to 35%, compared to WT or Cdk2Δhepa cells, after 3 days in culture, corresponding to the peak of DNA synthesis, as evidenced by measurement of BrdU incorporation (Fig. 2D,E).
Primary Cdk2ΔhepaCcnE1−/− hepatocytes showed normal morphology directly after isolation (Fig. 3A). However, after mitogen stimulation, these cells grew poorly and revealed continuously decreasing cell numbers over time (Fig. 3B). Flow cytometry (FCM) analysis demonstrated that the majority of Cdk2ΔhepaCcnE1−/− hepatocytes had a DNA content of less than 2n (sub-G1), indicating massive apoptotic cell death (Fig. 3C). In addition, surviving cells also had substantial reduced DNA content, compared to controls (Fig. 3D). Thus, in the absence of Cdk2, CcnE1 is crucial for CcnA expression, proper S-phase progression, and survival of primary hepatocytes in vitro.
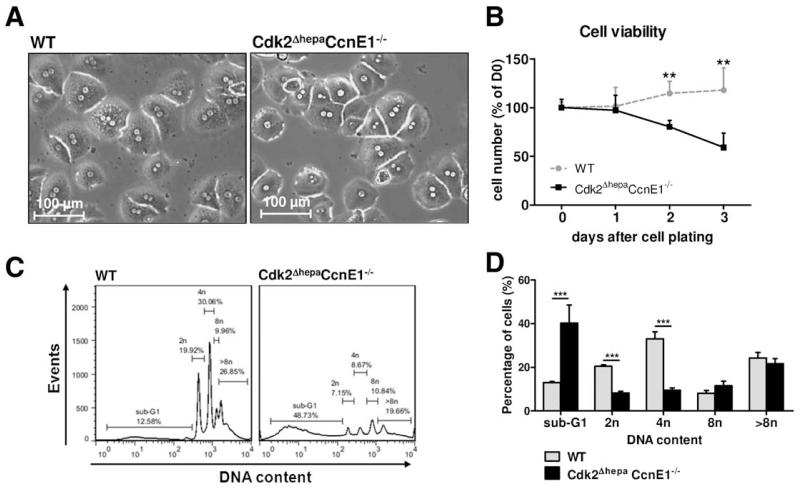
Fig. 3.
Hepatocytes lacking Cdk2 and CcnE1 display severely impaired viability. Primary hepatocytes from Cdk2f/f (WT) and Cdk2ΔhepaCcnE1−/− mice were isolated and cultured for up to 3 days (D0-D3) in the presence of EGF and insulin. (A) Microscopic images of cultured hepatocytes at D0 showing comparable density and morphology. (B) Quantification of living hepatocytes per magnification field. Cell numbers were normalized to the average cell numbers determined at D0. (C) DNA content of propidium-iodide-stained hepatocytes was determined by FCM analysis at D3. Representative histograms show the distribution of apoptotic cell fractions (sub-G1), diploid (2n), tetraploid (4n), octoploid (8n), and high polyploid (>8n) cells. (D) Quantification of different cell fractions. ***P < 0.001.
Concomitant Ablation of Cdk2 and CcnE1 In Vivo Impairs Liver Regeneration After PH
We next performed PH in Cdk2ΔhepaCcnE−/− mice and corresponding WT controls. As anticipated from our in vitro experiments, Cdk2ΔhepaCcnE1−/− mice showed dramatically reduced hepatic DNA synthesis 40-48 hours after PH (Fig. 4A,B). As a consequence, Cdk2ΔhepaCcnE1−/− mice showed significantly reduced liver mass restoration, which was most evident 7 days after PH (Fig. 4C). However, impaired liver regeneration was not associated with signs of elevated liver injury, such as increased transaminase values (aspartate aminotransferase and alanine aminotransferase) or apoptosis (Supporting Fig. 3A-C). We analyzed hepatic protein expression of cell-cycle–related factors at distinct time points after PH. Deletion of Cdk2/CcnE1 was associated with pronounced down-regulation of CcnA2 and CcnB1 and marginal Rb phosphorylation, whereas PCNA was normally regulated in these animals (Fig. 4D). In agreement with poor expression of CcnA2 and pRb, CcnA/Cdk1 kinase activity was substantially impaired in Cdk2ΔhepaCcnE1−/− livers, whereas CcnD-related kinase activity was up-regulated at onset of S phase (36 hours post-PH; Fig. 4E).
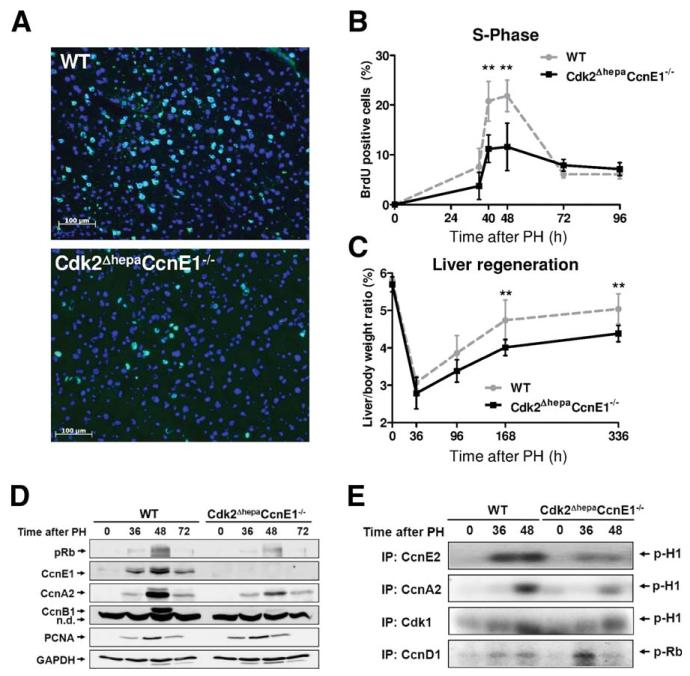
Fig. 4.
Simultaneous ablation of Cdk2 and CcnE1 in hepatocytes restrains liver regeneration after PH. Cdk2f/f (WT) and Cdk2ΔhepaCcnE1−/− mice were subjected to PH and analyzed at 0-336 hours after surgery. (A) Immunofluorescence staining for nuclear BrdU incorporation (green) on liver cryosections 48 hours after PH representing the peak of S-phase. Total nuclei are counter-stained with 4′, 6-diamidino-2-phenylindole (blue). (B) Quantification of BrdU-positive hepatocytes 0-96 hours after PH. (C) Liver-weight/body-weight index (%) was determined for up to 2 weeks (336 hours) after PH. **P < 0.01. (D) Protein expression profiles of phospho-Rb, CcnE1, CcnA2, CcnB1, and PCNA in WT and Cdk2ΔhepaCcnE1−/− mice after PH. (E) Whole-liver cell extracts were collected from WT and Cdk2ΔhepaCcnE1−/− mice at indicated time points after PH. Native cyclin/Cdk kinase complexes were immunoprecipitated (IP) with antibodies, as indicated, and subjected to in vitro kinase assays using either recombinant Rb or histone H1 as substrate.
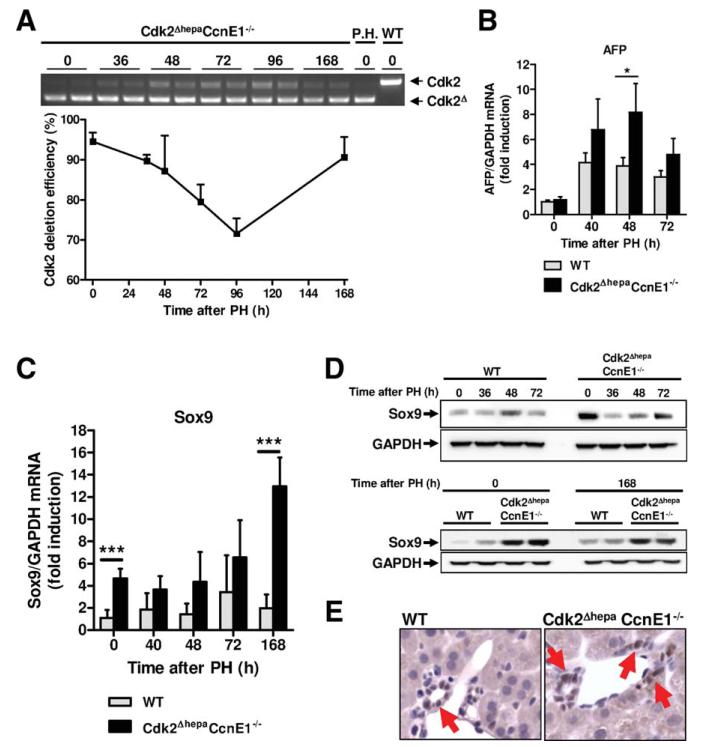
Residual Cell-Cycle Progression in Cdk2Δhepa CcnE1−/− Liver Is Associated With Transient Reex-pression of Cdk2 and Up-regulation of Progenitor Cell Markers
Although Cdk2ΔhepaCcnE1−/− livers revealed strongly impaired regeneration post-PH, the observed phenotype in vivo was less severe, compared to ex vivo isolated hepatocytes, which were apparently predisposed to cell death after mitogen stimulation. Therefore, we hypothesized that in vivo, a Cdk2-proficient hepatic cell population, such as liver progenitor cells, may provide protective effects, resulting in residual liver regeneration, which is not possible in purified Cdk2-deficient hepatocytes. In fact, we observed transient Cdk2 reexpression in regenerating livers of Cdk2ΔhepaCcnE1−/− mice (Fig. 5A), suggesting that a nonparenchymal liver cell compartment may compensate for loss of Cdk2 in hepatocytes. Thus, we measured expression of four established markers for liver progenitor cells (cytokeratin 19 [CK19], Prominin/CD133, AFP, and sex-determining region Y box 9 [Sox9]) by quantitative real-time polymerase chain reaction (qPCR). CK19 and CD133 did not show elevated expression in Cdk2ΔhepaCcnE1−/− liver (Supporting Fig. 3D) and were not analyzed in more detail. However, AFP was significantly more highly expressed in Cdk2ΔhepaCcnE1−/− liver 48 hours after PH (Fig. 5B), correlating with Cdk2 reexpression. Additionally, Sox9 was significantly more highly expressed in Cdk2ΔhepaCcnE1−/− mice, especially before and 7 days after PH (Fig. 5C,D). Immunohistological analysis confirmed these findings and localized a Sox9-positive nonparenchymal cell population at the ductal epithelia of Cdk2ΔhepaCcnE1−/− livers (Fig. 5E).
Fig. 5.
Transient Cdk2 reexpression in Cdk2ΔhepaCcnE1−/− liver after PH is associated with up-regulation of progenitor cell markers. (A) Cdk2ΔhepaCcnE1−/− mice were subjected to PH. At indicated time points, Cdk2 deletion efficiency was determined from complementary DNA using primers specific for exon 1 and 4, respectively. Upper band: WT Cdk2 gene expression; lower band: truncated Cdk2 lacking exon 2. Deletion efficiency was calculated by digital quantification of WT and KO bands. P.H., primary hepatocytes derived from Cdk2Δhepa mice were used as positive control for complete Cdk2 deletion. (B and C) Gene expression profile of AFP and Sox9 in WT and Cdk2ΔhepaCcnE1−/− mice post-PH was determined by qPCR. For each group, 3-5 animals were analyzed. *P < 0.05; ***P < 0.001. (D) Protein expression profiles of Sox9 in WT and Cdk2ΔhepaCcnE1−/− mice before and after PH. (E) Sox9 staining of liver sections from untreated WT and Cdk2ΔhepaCcnE1−/− mice. Sox9-positive cells show brown nuclear staining and are highlighted by arrows.
In addition, morphometric analysis of Cdk2ΔhepaCcnE1−/− liver revealed that cells lacking CcnE1 and Cdk2 showed an overall enlargement in cell size (Supporting Fig. 4A,B), combined with overall lower density of nuclei after liver regeneration (Supporting Fig. 4C). Altogether, our results implicate that combined deletion of Cdk2 and CcnE1 blocks cell-cycle progression and proliferation of hepatocytes, whereas liver function is preserved as a result of liver progenitor cell activation and—in part—compensatory liver hypertrophy.
Genetic Dissection of the Complete Cdk2/CcnE Complex
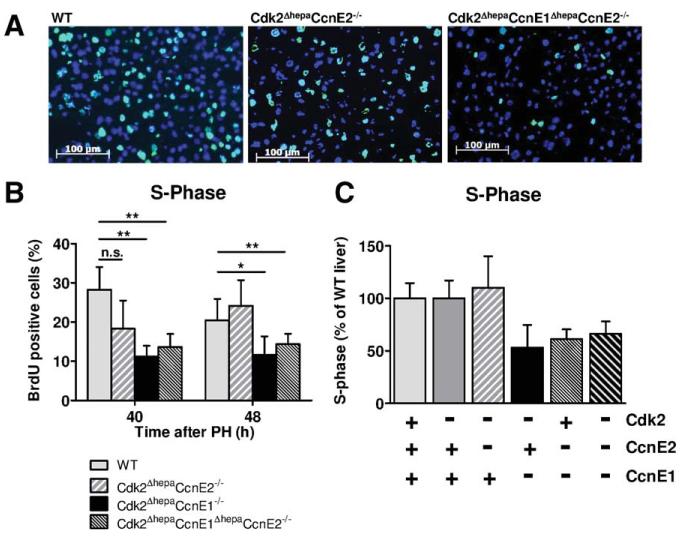
To fully understand the role of the Cdk2/CcnE complex for S-phase transition in vivo, we generated Cdk2ΔhepaCcnE2−/− and Cdk2ΔhepaCcnE1Δhepa CcnE2−/− mice, respectively, which both developed normally. After PH, Cdk2ΔhepaCcnE1ΔhepaCcnE2−/− triple-KO mice showed a similar defect in DNA synthesis, as observed in Cdk2ΔhepaCcnE1−/− mice, whereas Cdk2ΔhepaCcnE2−/− mice showed normal DNA synthesis after PH, which was not significantly different, compared to matching WT animals (Fig. 6A,B). These results demonstrate that CcnE2 does not contribute to S-phase control in the absence of Cdk2.
Fig. 6.
CcnE2 is dispensable for DNA-synthesis after PH in Cdk2-deficient liver. WT, Cdk2ΔhepaCcnE2−/− and Cdk2ΔhepaCcnE1ΔhepaCcnE2−/− mice were subjected to PH and sacrificed 40 and 48 hours after resection. A. Immunofluorescence staining for BrdU incorporation (green) on liver cryosections 48 h after PH. B. Quantification of BrdU-positive hepatocytes. *: p<0.05; **: p<0.01; n.s.: not significant. C. The relative BrdU incorporation for each investigated strain 48 h after PH was calculated as percentage in comparison to matching WT controls from the same strain.
We finally analyzed CcnE1ΔhepaCcnE2−/− mice in the PH model, which revealed very similar results, compared to Cdk2ΔhepaCcnE1−/− animals. Combined loss of CcnE1 and CcnE2 resulted in an approximately 50% reduction of S phase in hepatic cells 40-48 hours after PH (Fig. 6C and Supporting Fig. 5A,B) and severely impaired liver mass reconstitution (Supporting Fig. 5C). Interestingly, CcnE1ΔhepaCcnE2−/− mice and Cdk2Δhepa CcnE1ΔhepaCcnE2−/− mice revealed strong basal up-regulation of hepatic Sox9 (Supporting Fig. 5D), suggesting that general inhibition of S-phase machinery in the liver triggers the enrichment of a yet not characterized progenitor cell niche.
Discussion
The classical paradigm of mammalian cell-cycle control suggested that Cdk2 and its associated cyclins should be absolutely essential for G0/G1/S transition. However, extensive work on gene KO mouse models in vivo revealed that Cdk2, CcnE1, and CcnE2 are largely dispensable for mitotic cell-cycle regulation, presumably because of high redundancy and overlapping functions of residual interphase Cdks and cyclins.24
Here, we used the model of PH in mice to study S-phase reentry and cell-cycle progression in vivo. For our study, we used combinations of conditional and constitutive mouse mutants that allowed the stepwise deletion of the complete CcnE/Cdk2 complex, including CcnE1, CcnE2, and Cdk2, in hepatocytes in vitro and in vivo. Our ultimate aim was to identify the minimal requirements essential for blocking S-phase initiation in liver.
As a starting point, we generated mice with conditional Cdk2 deletion specifically in hepatocytes. In agreement with earlier studies using constitutive Cdk2 KO mice,13 we confirmed that hepatocytes do not require Cdk2 for S-phase transition and liver regeneration after PH. However, our experiments revealed several novel findings. In WT cells, Cdk2 forms kinase complexes with CcnE (CcnE1 or CcnE2) at the late G1 phase and completes Rb phosphorylation.2 Surprisingly, Rb phosphorylation (Ser807/811) was performed in Cdk2Δhepa mice to the same extent as observed in WT mice after PH. Our systematic analysis of residual Cdk activities suggests that early Cdk2 function (36 hours post-PH) is substituted by CcnD1/Cdk4 and CcnD1/Cdk6 kinases, whereas at the peak of G0/S-phase transition (48 hours post-PH), only Cdk1 kinase activity was detectable in Cdk2Δhepa liver. Therefore, we conclude that Cdk1 substitutes loss of Cdk2 in this setting, presumably by interacting with residual CcnA2. It has been suggested that in the absence of Cdk2, more CcnA2 molecules are available for binding to Cdk1 and thus enhance Cdk1 activity. Consistent with this idea, a recent study using hepatocyte-specific Cdk1 KO mice demonstrated that livers lacking Cdk1 have elevated Cdk2/CcnA2 activity resulting from enhanced binding of CcnA2 to Cdk2.25
One of our key findings was the accelerated induction and enhanced expression of CcnE1 in regenerating livers and primary hepatocytes of Cdk2Δhepa mice. Previous work demonstrated that in the absence of Cdk2, G1/S-phase transition is regulated by a noncanonical interaction of CcnE1 with Cdk1 at least in spleen and thymus.22 However, we did not detect any CcnE1-related noncanonical kinase activity in Cdk2-deficient liver after PH. Instead, this excessive CcnE1 physically interacts with Cdt1 and is predominantly localized at pre-RCs on chromatin. This kinase-independent, enhanced association of CcnE1 with chromatin is absolutely essential for S-phase reentry in Cdk2-deficient hepatocytes, because combined ablation of Cdk2 and CcnE1 abolished MCM loading and induction of the S phase. In addition, isolated hepatocytes lacking Cdk2 and CcnE1 revealed poor survival after onset of the S phase, which was not observed in vivo, as discussed below. Therefore, we conclude that CcnE1 contributes to liver regeneration in a kinase-independent manner in Cdk2Δhepa mice through enhanced MCM loading.
Our data further indicate that CcnE1 is required for inducing CcnA2 and CcnE2 gene expression in a Cdk2-independent manner, although the underlying mechanisms remain, in part, unclear. Individual loss of Cdk2 allowed normal CcnA2/CcnE2 gene induction in liver after PH and in mitogen-stimulated hepatocytes, whereas combined ablation of both factors resulted in a substantial inhibition of CcnA2 and CcnE2. Our data suggest that CcnE1 physically interacts with the CcnA2 promoter, whereas concomitant lack of CcnE1 and Cdk2 completely abrogated the formation of a yet unknown protein complex at a well-characterized E2F-binding element described earlier.26 Therefore, we speculate that in the absence of Cdk2, CcnE1 might be important for the activation of the CcnA2 promoter and may also regulate CcnE2 expression through a similar mechanism, as indicated by our earlier studies.17 However, detailed analysis of the CcnA2/CcnE2 promoters was beyond the scope of this study and will be addressed in future work. We conclude that S-phase inhibition in cells lacking Cdk2 and CcnE1 is the consequence of at least two independent mechanisms involving poor MCM loading and blocking of transcriptional CcnA2 and CcnE2 activation.
In agreement with our results from isolated hepatocytes, Cdk2ΔhepaCcnE1−/− livers reveal strongly impaired CcnA2 expression, resulting in substantially reduced S phase and poor liver mass reconstitution. However, we did not observe excessive cell death or enhanced liver injury after PH, as anticipated from our results in primary hepatocytes. Because Cdk2 is only deleted in albumin-expressing parenchymal cells, we hypothesize that, in vivo, Cdk2-expressing progenitor cells could contribute to residual hepatic cell proliferation, improved survival, and liver function until these cells are fully differentiated into mature (albumin-expressing), but Cdk2-deficient hepatocytes. This hypothesis is supported by transient Cdk2 reexpression and significantly higher expression of AFP and Sox9 in Cdk2ΔhepaCcnE1−/− mice. Involvement of Sox9-positive precursors in liver regeneration after injury was recently demonstrated.27 Thus, we speculate that Sox9- or AFP-positive, Cdk2-expressing progenitor cells could contribute to residual cell proliferation Cdk2ΔhepaCcnE1−/− liver. Besides that, our data suggest that partial liver mass reconstitution in Cdk2ΔhepaCcnE1−/− mice is mediated through hypertrophic cell growth of hepatocytes.
The contribution of CcnE2 for liver regeneration appears complex. Cdk2ΔhepaCcnE2−/− mice displayed normal DNA synthesis and liver regeneration, pointing to a negligible role of CcnE2 for this process. CcnE2 induction was already largely abolished in Cdk2ΔhepaCcnE1−/− hepatocytes after mitogen stimulation. This could explain why additional genetic ablation of CcnE2 in Cdk2ΔhepaCcnE1ΔhepaCcnE2−/− mice did not result in further impairment of S phase and liver regeneration after PH, but rather reflected the phenotype observed in Cdk2ΔhepaCcnE1−/− mice. However, the severely impaired DNA replication and liver regeneration observed in CcnE1ΔhepaCcnE2−/− mice—which was not observed in CcnE1−/− or CcnE2−/− mice17—clearly demonstrated that CcnE2 shares at least some overlapping functions with CcnE1. This result represents the first in vivo confirmation of recent in vitro studies, which demonstrated that combined genetic ablation of both CcnE1 and CcnE2 blocks cell-cycle reentry of mouse embryonic fibroblasts from quiescence.15
Our main conclusions are illustrated in Supporting Fig 6. In WT hepatocytes, Cdk2 interacts with CcnE1, CcnE2, and CcnA2 and contributes to G1/S transition and S-phase progression, respectively. In addition, CcnE1 performs a second, Cdk2-independent function in MCM loading. In the absence of CcnE1, CcnA2/Cdk2—and, to a much lesser extent, CcnE2/Cdk2—substitute kinase activity.17 Proper S-phase transition without Cdk2 requires at least the kinase-independent function of CcnE1 for pre-RC assembly and CcnA2/Cdk1 kinase, as postulated earlier.15 Simultaneous genetic inactivation of CcnE1 and Cdk2 in hepatocytes results in secondary inhibition of CcnA2 and CcnE2. As a consequence, MCM proteins cannot be sufficiently loaded on replication origins and DNA replication is further inhibited as a result of the lack of CcnA and related kinase activity, eventually leading to reduced DNA synthesis of hepatocytes and inefficient liver regeneration.
In summary, combined inactivation of Cdk2 and CcnE1 is the minimal requirement for blocking S-phase machinery. It remains to be elucidated whether this mechanism is restricted to hepatocytes and the liver or is also effective in other cell types and organs.
Supplementary Material
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (LI1045/2-4; to C.T. and C.L.). M.B. was supported by a grant from the European Research Council (ERC-AG/250297-RAS AHEAD).
FUNDING STATEMENT:
The NIH grant is Peter Sicinski’s R01CA108950.
Abbreviations
- AFP
alpha-fetoprotein
- BrdU
bromdesoxyuridine
- Ccn
cyclin
- Cdk
cyclin-dependent kinase
- Cdt1
chromatin licensing and DNA replication factor 1
- CK19
cytokeratin 19
- EGF
epidermal growth factor
- FCM
flow cytometry
- KO
knockout
- MCM
minichromosome maintenance complex
- ORC
origin recognition complex
- PCNA
proliferating cell nuclear antigen
- PH
partial hepatectomy
- pre-RC
pre-replicative complex
- qPCR
quantitative real-time polymerase chain reaction
- Rb
retinoblastoma protein
- Sox9
sex-determining region Y box 9
- WT
wild type
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madine MA, Swietlik M, Pelizon C, Romanowski P, Mills AD, Laskey RA. The roles of the MCM, ORC, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J Struct Biol. 2000;129:198–210. doi: 10.1006/jsbi.2000.4218. [DOI] [PubMed] [Google Scholar]
- 5.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 6.Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene. 2005;24:2827–2843. doi: 10.1038/sj.onc.1208616. [DOI] [PubMed] [Google Scholar]
- 8.Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14:R778–R786. doi: 10.1016/j.cub.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 11.Barriere C, Santamaria D, Cerqueira A, Galan J, Martin A, Ortega S, et al. Mice thrive without Cdk4 and Cdk2. Mol Oncol. 2007;1:72–83. doi: 10.1016/j.molonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanse EA, Nelsen CJ, Goggin MM, Anttila CK, Mullany LK, Berthet C, et al. Cdk2 plays a critical role in hepatocyte cell cycle progression and survival in the setting of cyclin D1 expression in vivo. Cell Cycle. 2009;8:2802–2809. doi: 10.4161/cc.8.17.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satyanarayana A, Hilton MB, Kaldis P. p21 Inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase DNA damage checkpoint. Mol Biol Cell. 2008;19:65–77. doi: 10.1091/mbc.E07-06-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, et al. Development of mice expressing a single D-type cyclin. Genes Dev. 2002;16:3277–3289. doi: 10.1101/gad.1023602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 16.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 17.Nevzorova YA, Tschaharganeh D, Gassler N, Geng Y, Weiskirchen R, Sicinski P, et al. Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin. Gastroenterology. 2009;137:691–703. e1-6. doi: 10.1053/j.gastro.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng Y, Lee YM, Welcker M, Swanger J, Zagozdzon A, Winer JD, et al. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–139. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Odajima J, Wills ZP, Ndassa YM, Terunuma M, Kretschmannova K, Deeb TZ, et al. Cyclin E constrains Cdk5 activity to regulate synaptic plasticity and memory formation. Dev Cell. 2011;21:655–668. doi: 10.1016/j.devcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellendonk C, Opherk C, Anlag K, Schutz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- 23.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 24.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins, and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 25.Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, et al. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A. 2012;109:3826–3831. doi: 10.1073/pnas.1115201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulze A, Zerfass K, Spitkovsky D, Middendorp S, Berges J, Helin K, et al. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci U S A. 1995;92:11264–11268. doi: 10.1073/pnas.92.24.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.