Abstract
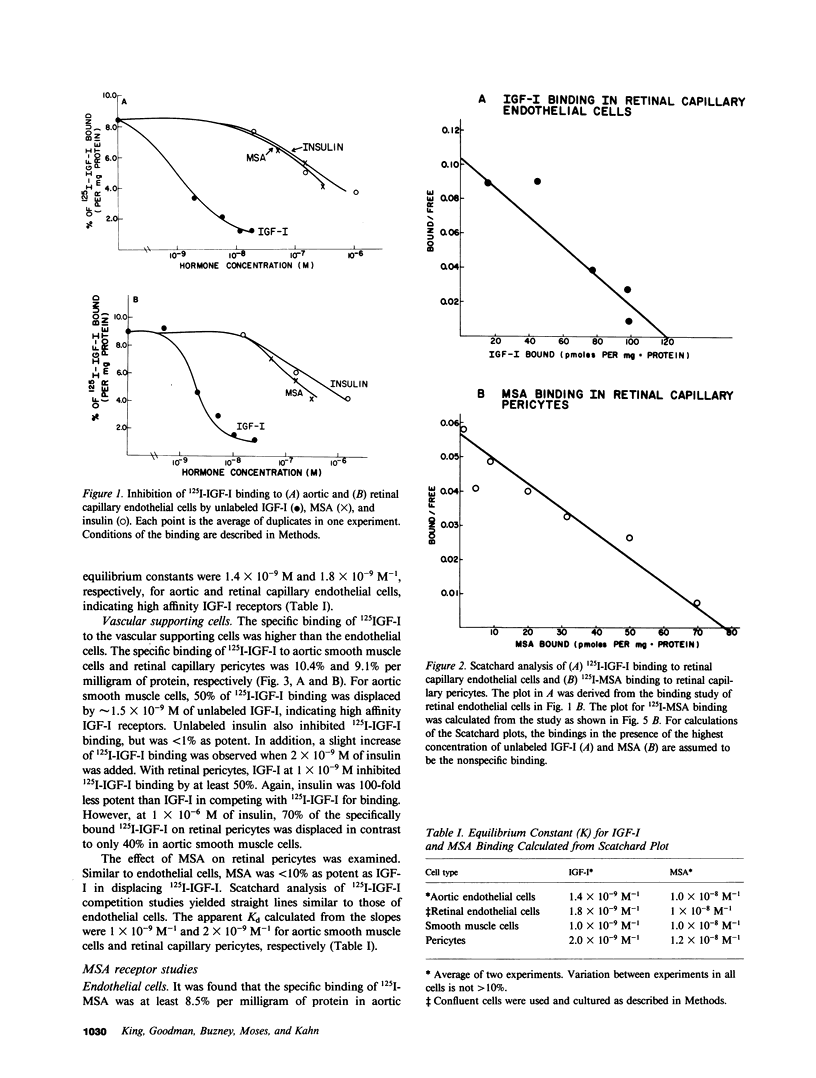
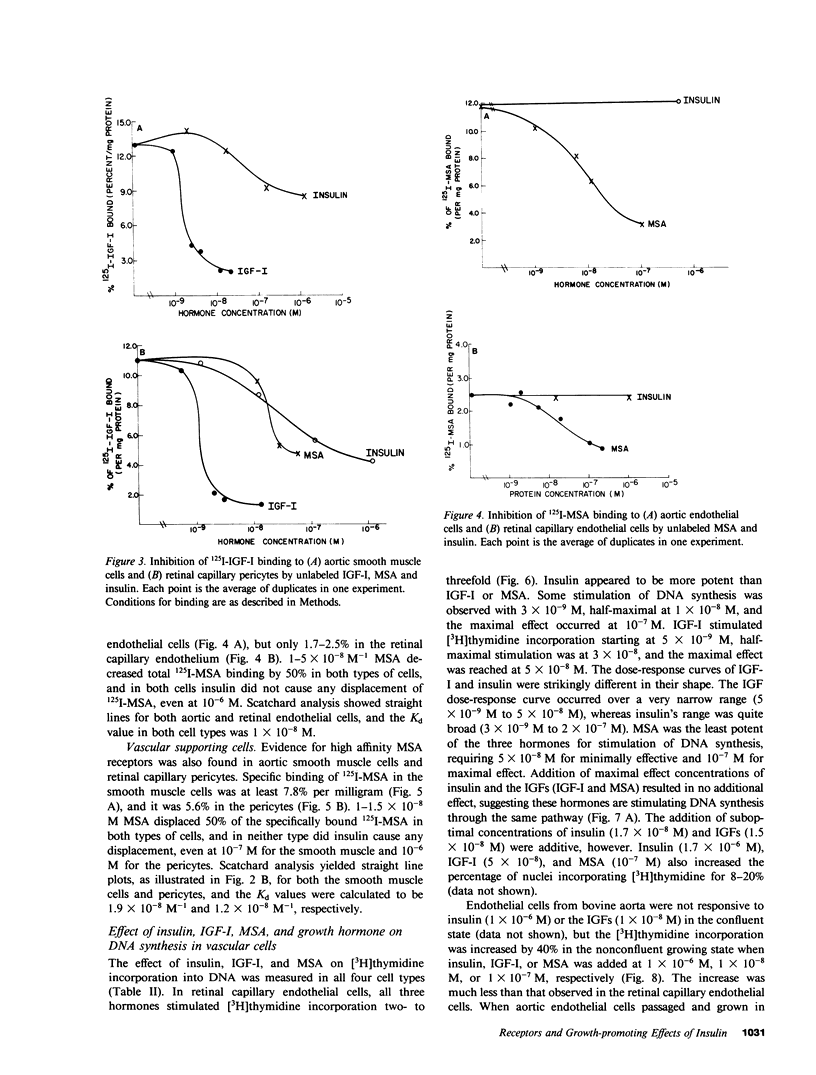
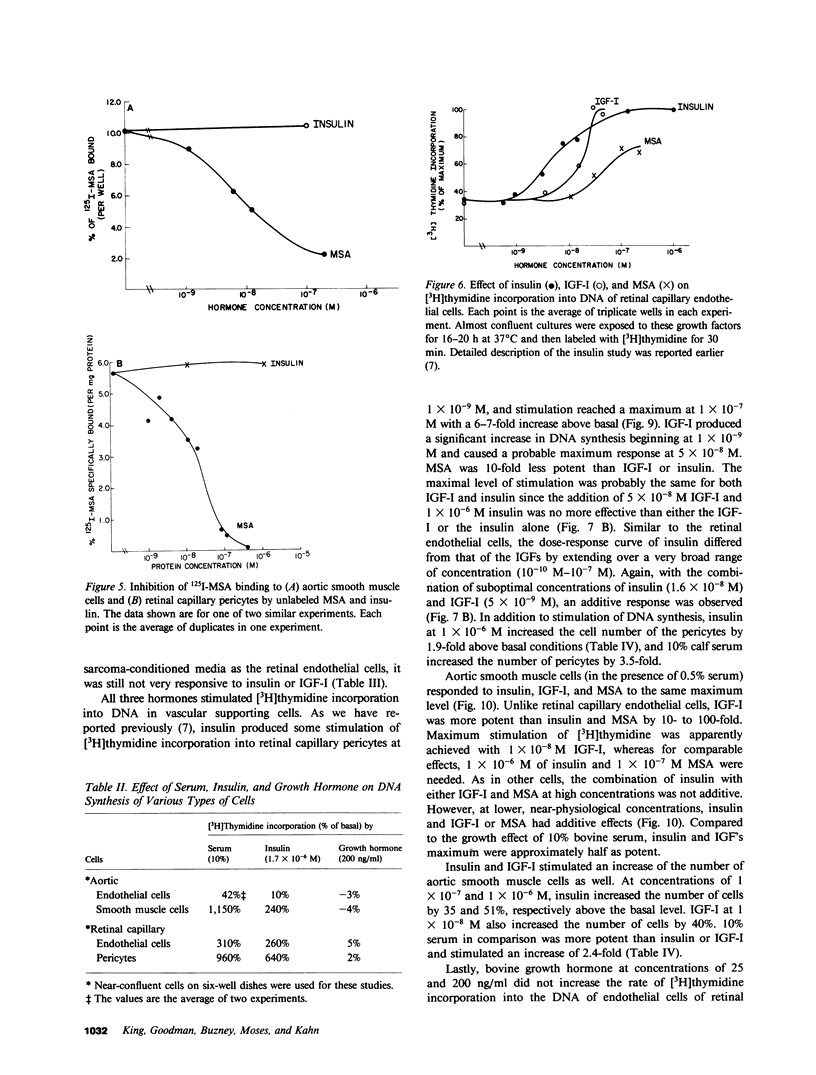
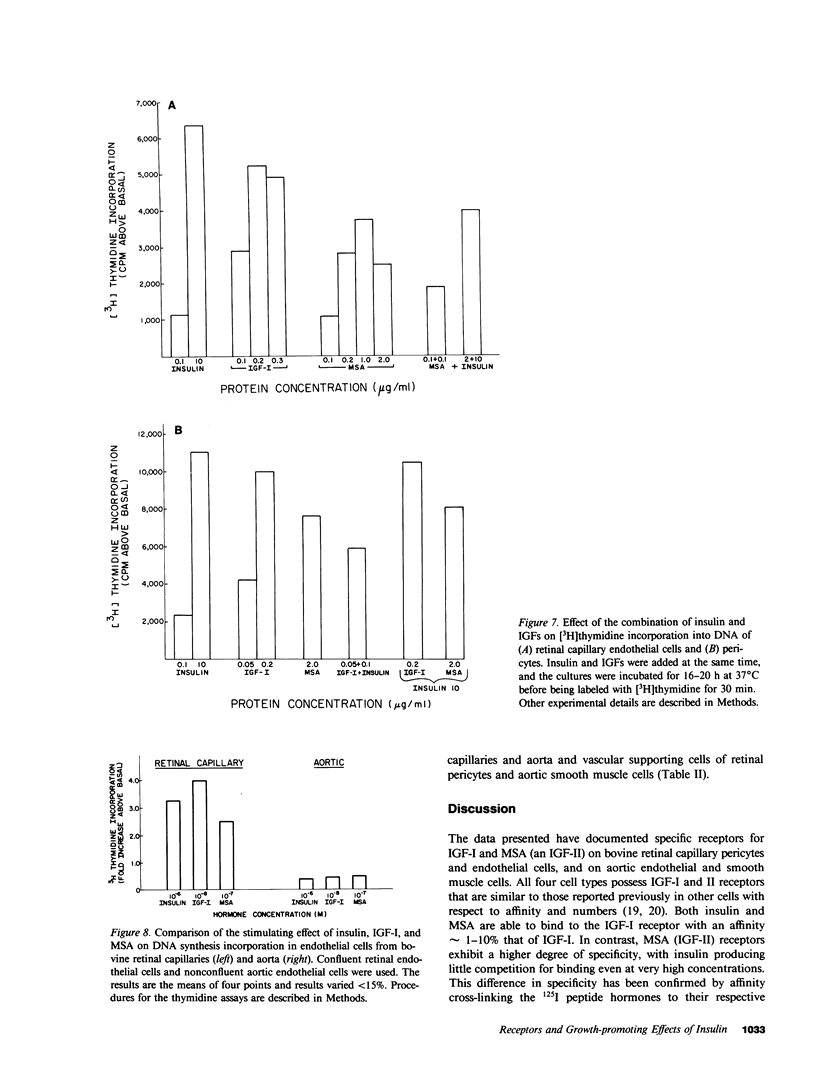
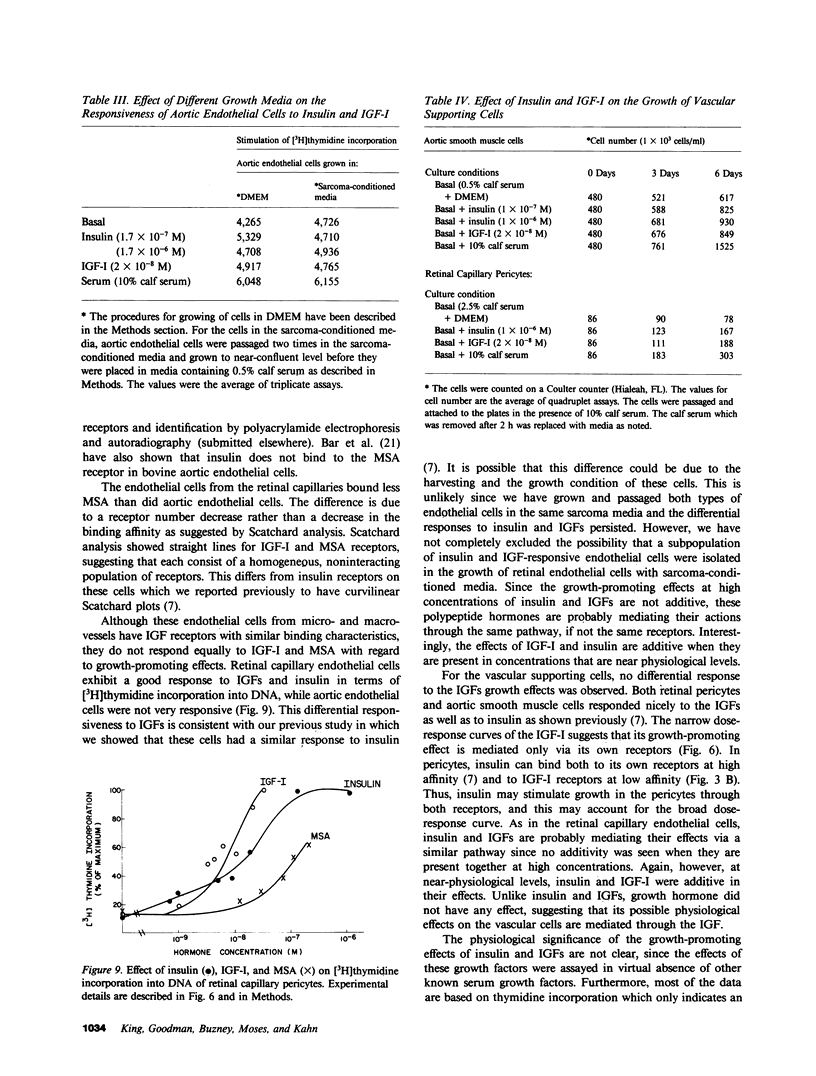
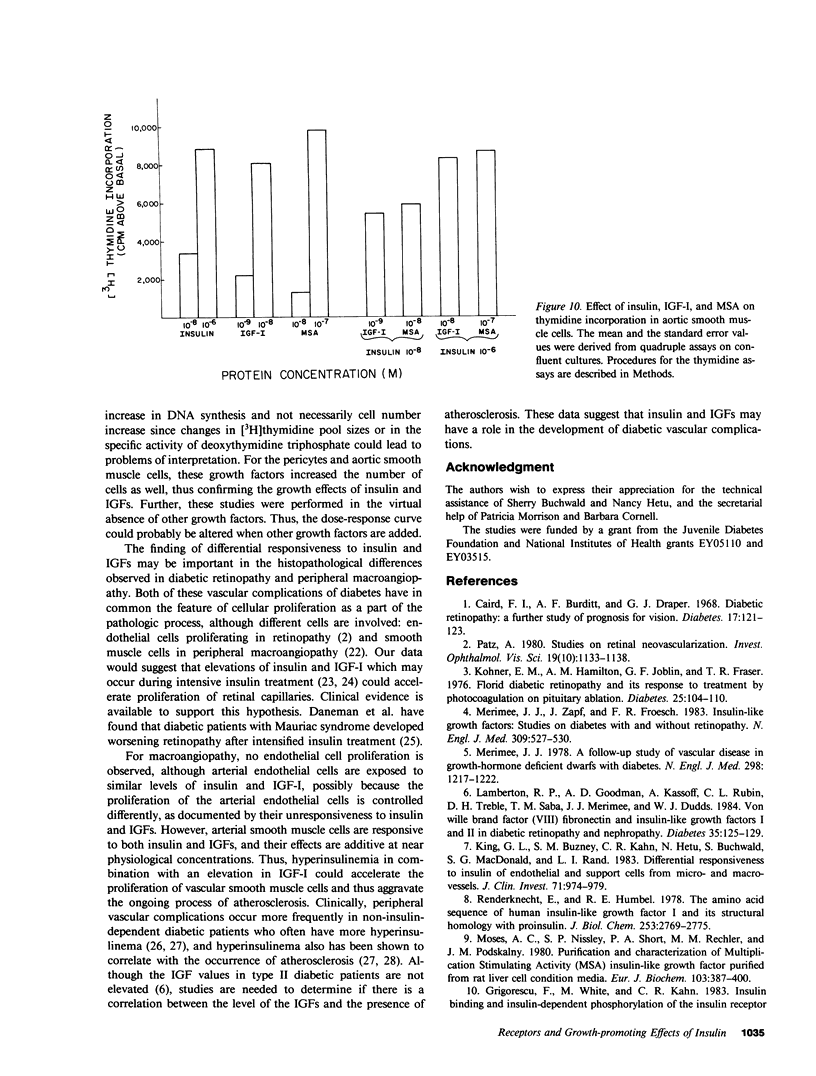
It has been suggested that elevated levels of insulin or insulin-like growth factors (IGFs) play a role in the development of diabetic vascular complications. Previously, we have shown a differential response to insulin between vascular cells from retinal capillaries and large arteries with the former being much more insulin responsive. In the present study, we have characterized the receptors and the growth-promoting effect of insulinlike growth factor I (IGF-I) and multiplication-stimulating activity (MSA, an IGF-II) on endothelial cells and pericytes from calf retinal capillaries and on endothelial and smooth muscle cells from calf aorta. We found single and separate populations of high affinity receptors for IGF-I and MSA with respective affinity constants of 1 X 10(-9) M-1 and 10(-8) M-1 in all four cell types studied. Specific binding of IGF-I was between 7.2 and 7.9% per milligram of protein in endothelial cells and 9.1 and 10.4% in the vascular supporting cells. For 125I-MSA, retinal endothelial cells bound only 1.7-2.5%, whereas the aortic endothelial cells and the vascular supporting cells bound between 5.6 and 8.5% per milligram of protein. The specificity of the receptors for IGF-I and MSA differed, as insulin and MSA was able to compete with 125I-IGF-I for binding to the IGF-I receptors with 0.01-0.1, the potency of unlabeled IGF-I, whereas even 1 X 10(-6) M, insulin did not significantly compete with 125I-MSA for binding to the receptors for MSA. For growth-promoting effects, as measured by the incorporation of [3H]thymidine into DNA, confluent retinal endothelial cells responded to IGF-I and MSA by up to threefold increase in the rate of DNA synthesis, whereas confluent aortic endothelial cells did not respond at all. A similar differential of response to insulin between micro- and macrovascular endothelial cells was reported by us previously. In the retinal endothelium, insulin was more potent than IGF-I and IGF-I was more potent that MSA. In the retinal and aortic supporting cells, no differential response to insulin or the IGFs was observed. In the retinal pericytes, IGF-I, which stimulated significant DNA synthesis beginning at 1 X 10(-9) M, and had a maximal effect at 5 X 10(-8) M, was 10-fold more potent than MSA and equally potent to insulin. In the aortic smooth muscle cells, IGF-I was 10-100 times more potent than insulin or MSA. In the retinal and aortic supporting cells, no differential response to insulin or the IGFs was observed. In the retinal pericytes, IGF-I, which stimulated significant DNA synthesis beginning at 1 X 10(-9) M, and had a maximal effect at 5 X 10(-8) M, was 10-fold more potent than MSA and equally potent to insulin. In the aortic smooth muscle cells, IGF-I was 10-100 times more potent than insulin or MSA. In addition, insulin and IGF-I at 1 X 10(-6) and 1 X 10(-8) M, respectively, stimulated these cells to grow by doubling the number of cells as well. In all responsive tissues, the combination of insulin and IGFs were added together, no further increase in effect was seen. These data showed that vascular cells have insulin and IGF receptors, but have a differential response to these hormones. These differences in biological response between cells from retinal capillaries and large arteries could provide clues to understanding the pathogenesis of diabetic micro- and macroangiopathy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar R. S., Peacock M. L., Rechler M. M., Nissley S. P. Receptors for multiplication-stimulating activity on human arterial and venous endothelial cells. J Clin Endocrinol Metab. 1981 Apr;52(4):814–816. doi: 10.1210/jcem-52-4-814. [DOI] [PubMed] [Google Scholar]
- Buzney S. M., Massicotte S. J. Retinal vessels: proliferation of endothelium in vitro. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1191–1195. [PubMed] [Google Scholar]
- Caird F. I., Burditt A. F., Draper G. J. Diabetic retinopathy. A further study of prognosis for vision. Diabetes. 1968 Mar;17(3):121–123. doi: 10.2337/diab.17.3.121. [DOI] [PubMed] [Google Scholar]
- Daneman D., Drash A. L., Lobes L. A., Becker D. J., Baker L. M., Travis L. B. Progressive retinopathy with improved control in diabetic dwarfism (Mauriac's syndrome). Diabetes Care. 1981 May-Jun;4(3):360–365. doi: 10.2337/diacare.4.3.360. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975 Jul;33(1):16–27. [PubMed] [Google Scholar]
- Gliemann J., Sonne O., Linde S., Hansen B. Biological potency and binding affinity of monoiodoinsulin with iodine in tyrosine A14 or tyrosine A19. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1183–1190. doi: 10.1016/s0006-291x(79)80032-7. [DOI] [PubMed] [Google Scholar]
- Grigorescu F., White M. F., Kahn C. R. Insulin binding and insulin-dependent phosphorylation of the insulin receptor solubilized from human erythrocytes. J Biol Chem. 1983 Nov 25;258(22):13708–13716. [PubMed] [Google Scholar]
- Hanna A. K., Zinman B., Nakhooda A. F., Minuk H. L., Stokes E. F., Albisser A. M., Leibel B. S., Marliss E. B. Insulin, glucagon, and amino acids during glycemic control by the artificial pancreas in diabetic man. Metabolism. 1980 Apr;29(4):321–332. doi: 10.1016/0026-0495(80)90005-0. [DOI] [PubMed] [Google Scholar]
- King G. L., Buzney S. M., Kahn C. R., Hetu N., Buchwald S., Macdonald S. G., Rand L. I. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest. 1983 Apr;71(4):974–979. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohner E. M., Hamilton A. M., Joplin G. F., Fraser T. R. Florid diabetic retinopathy and its response to treatment by photocoagulation or pituitary ablation. Diabetes. 1976 Feb;25(2):104–110. doi: 10.2337/diab.25.2.104. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamberton R. P., Goodman A. D., Kassoff A., Rubin C. L., Treble D. H., Saba T. M., Merimee T. J., Dodds W. J. Von Willebrand factor (VIII R:Ag), fibronectin, and insulin-like growth factors I and II in diabetic retinopathy and nephropathy. Diabetes. 1984 Feb;33(2):125–129. doi: 10.2337/diab.33.2.125. [DOI] [PubMed] [Google Scholar]
- Merimee T. J. A follow-up study of vascular disease in growth-hormone-deficient dwarfs with diabetes. N Engl J Med. 1978 Jun 1;298(22):1217–1222. doi: 10.1056/NEJM197806012982202. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Zapf J., Froesch E. R. Insulin-like growth factors. Studies in diabetics with and without retinopathy. N Engl J Med. 1983 Sep 1;309(9):527–530. doi: 10.1056/NEJM198309013090904. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., Podskalny J. M. Purification and characterization of multiplication-stimulating activity. Insulin-like growth factors purified from rat-liver-cell-conditioned medium. Eur J Biochem. 1980 Jan;103(2):387–400. doi: 10.1111/j.1432-1033.1980.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Miettinen T. A., Vesenne M. R., Pelkonen R. Plasma-insulin in coronary heart-disease: response to oral and intravenous glucose and to tolbutamide. Lancet. 1965 Sep 11;2(7411):508–511. doi: 10.1016/s0140-6736(65)91471-6. [DOI] [PubMed] [Google Scholar]
- Patz A. Studies on retinal neovascularization. Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1980 Oct;19(10):1133–1138. [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Goldfine I. D., Wells C. A. DNA synthesis in human fibroblasts: stimulation by insulin and by nonsuppressible insulin-like activity (NSILA-S). J Clin Endocrinol Metab. 1974 Sep;39(3):512–521. doi: 10.1210/jcem-39-3-512. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Stout R. W., Bierman E. L., Ross R. Effect of insulin on the proliferation of cultured primate arterial smooth muscle cells. Circ Res. 1975 Feb;36(2):319–327. doi: 10.1161/01.res.36.2.319. [DOI] [PubMed] [Google Scholar]
- Stout R. W. The relationship of abnormal circulating insulin levels to atherosclerosis. Atherosclerosis. 1977 May;27(1):1–13. doi: 10.1016/0021-9150(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Tamborlane W. V., Hintz R. L., Bergman M., Genel M., Felig P., Sherwin R. S. Insulin-infusion-pump treatment of diabetes: influence of improved metabolic control on plasma somatomedin levels. N Engl J Med. 1981 Aug 6;305(6):303–307. doi: 10.1056/NEJM198108063050602. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]



