Abstract
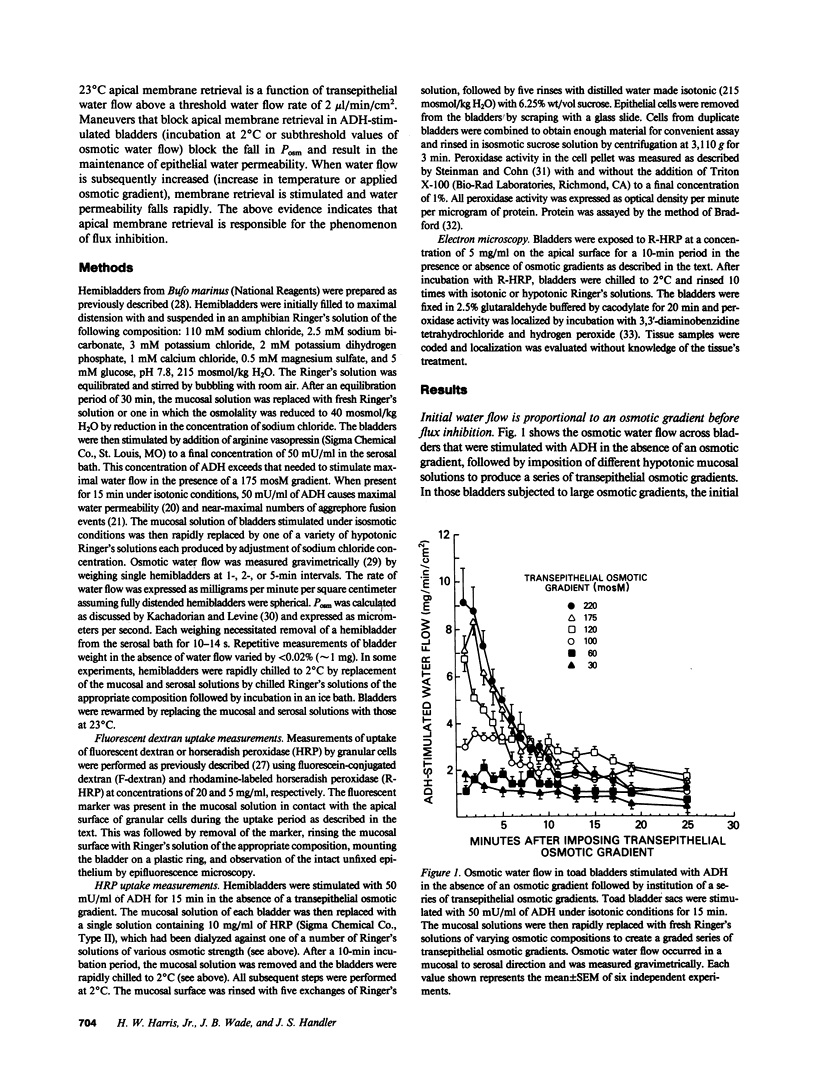
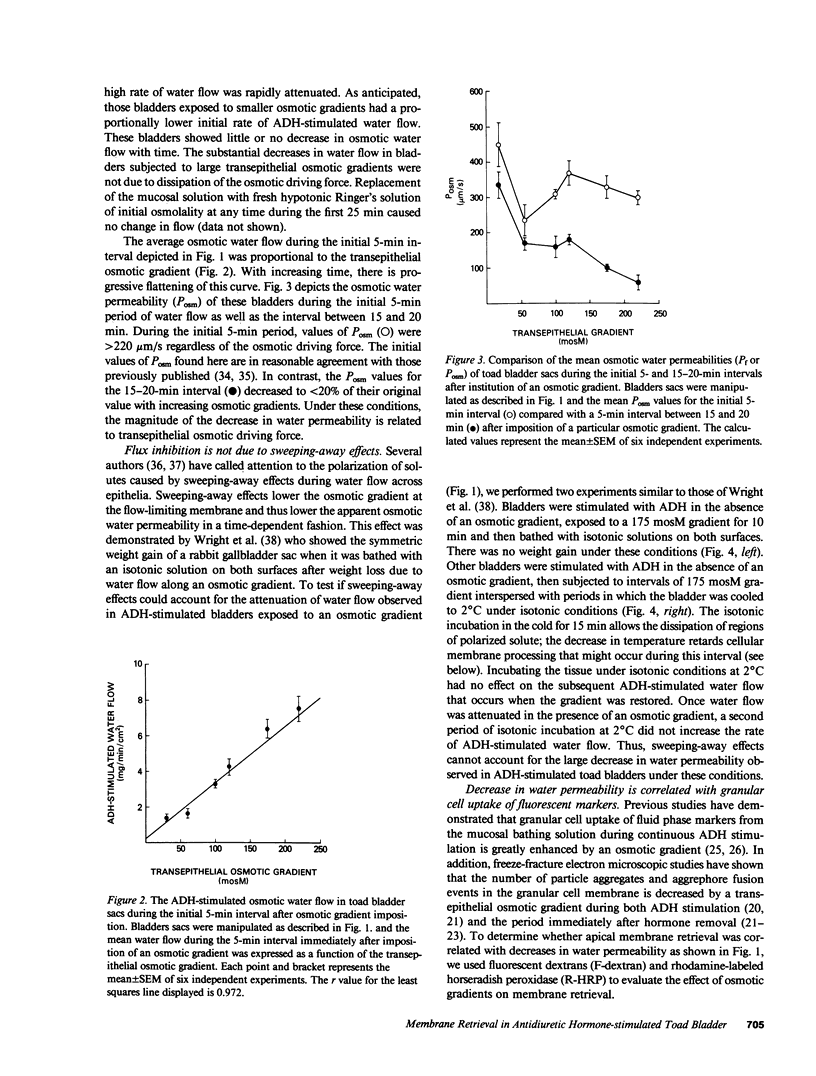
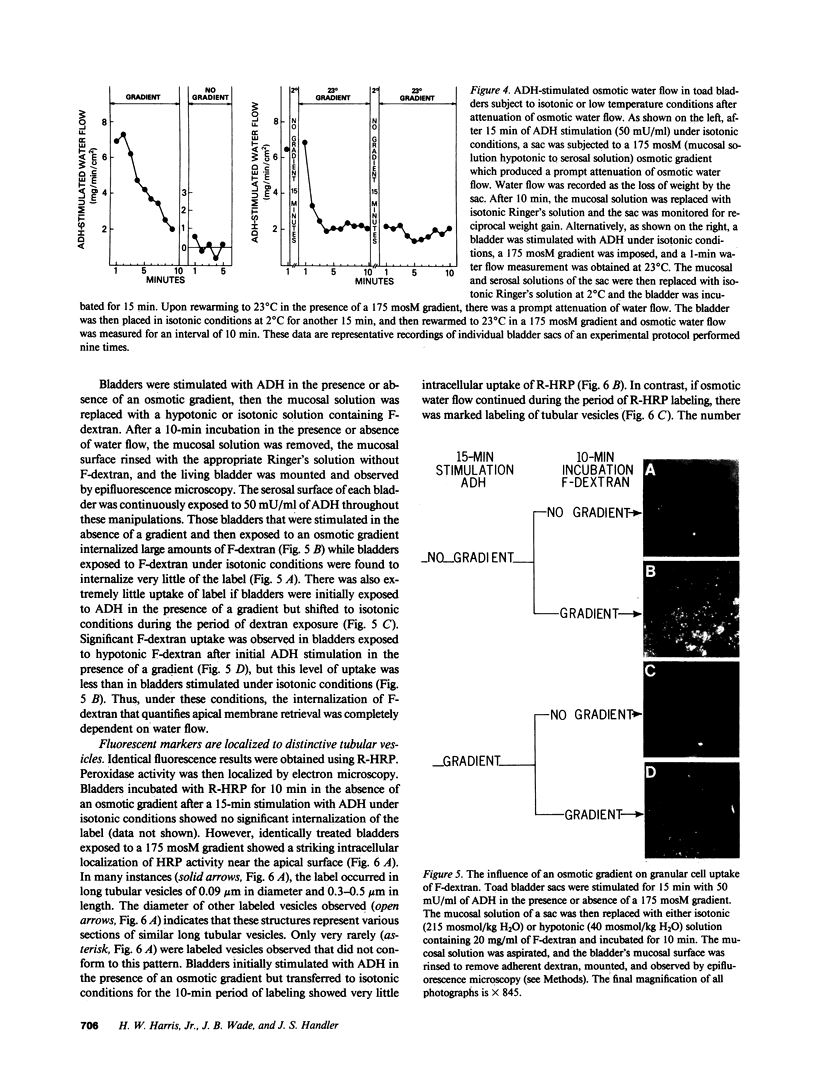
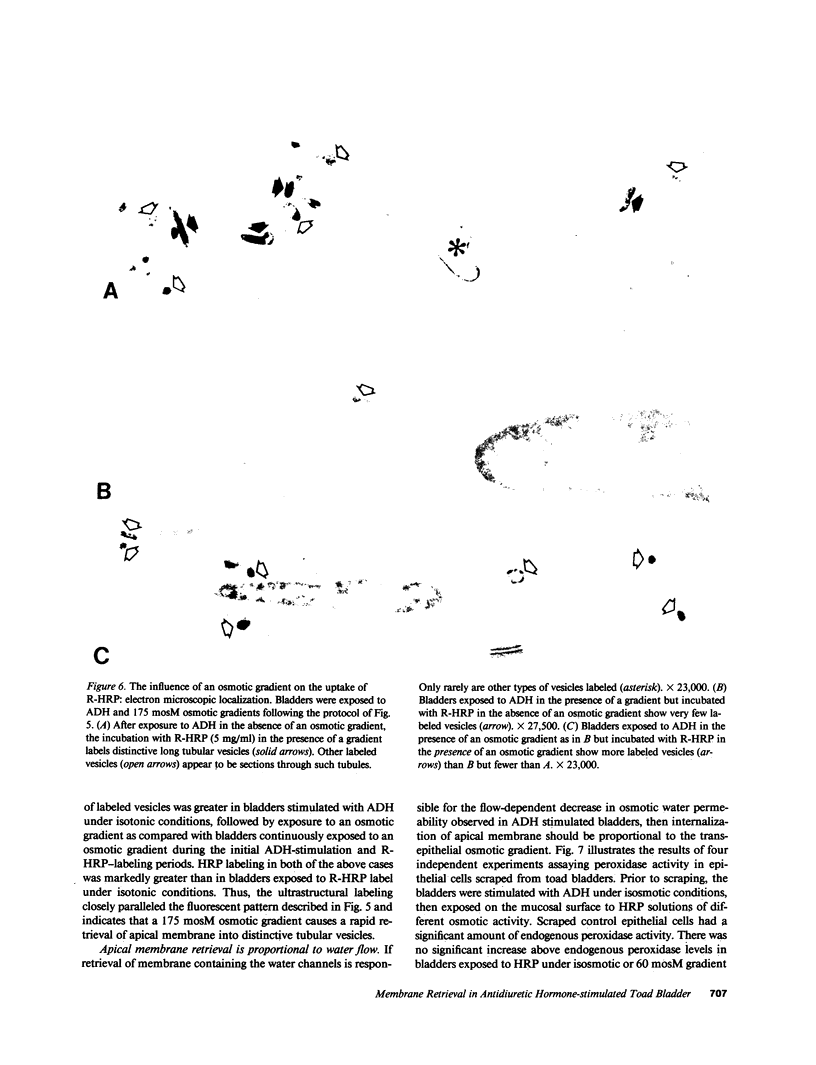
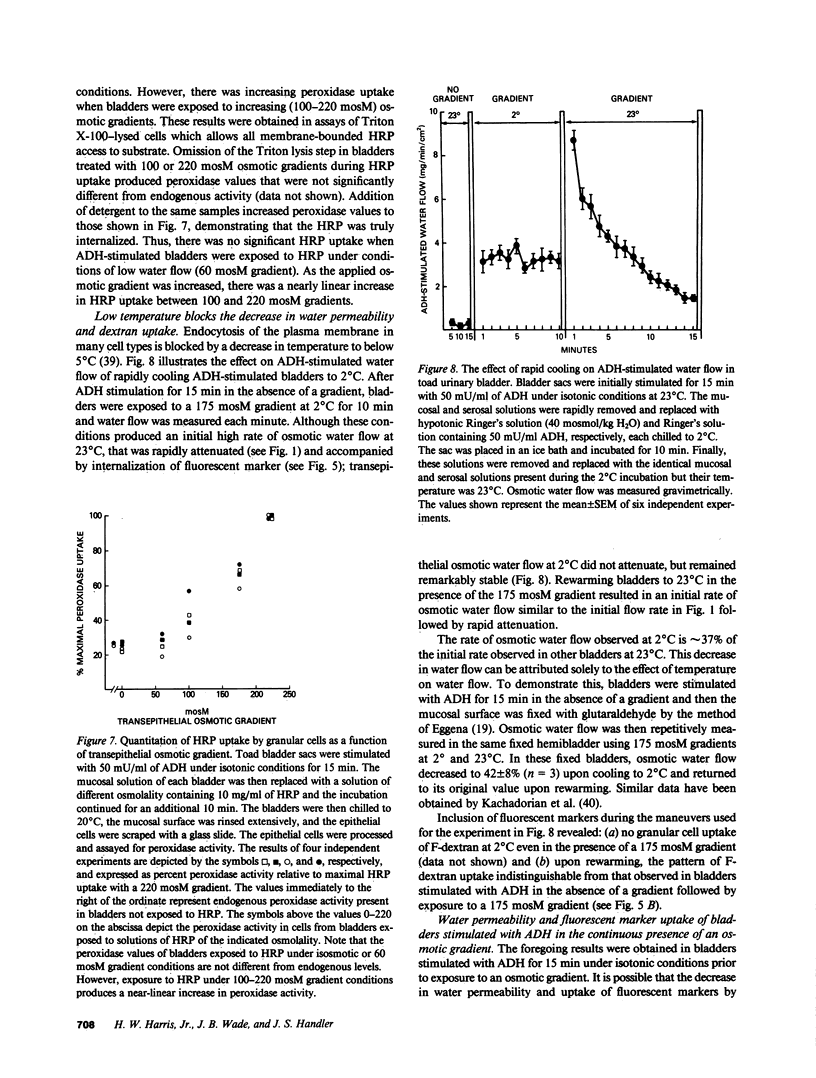
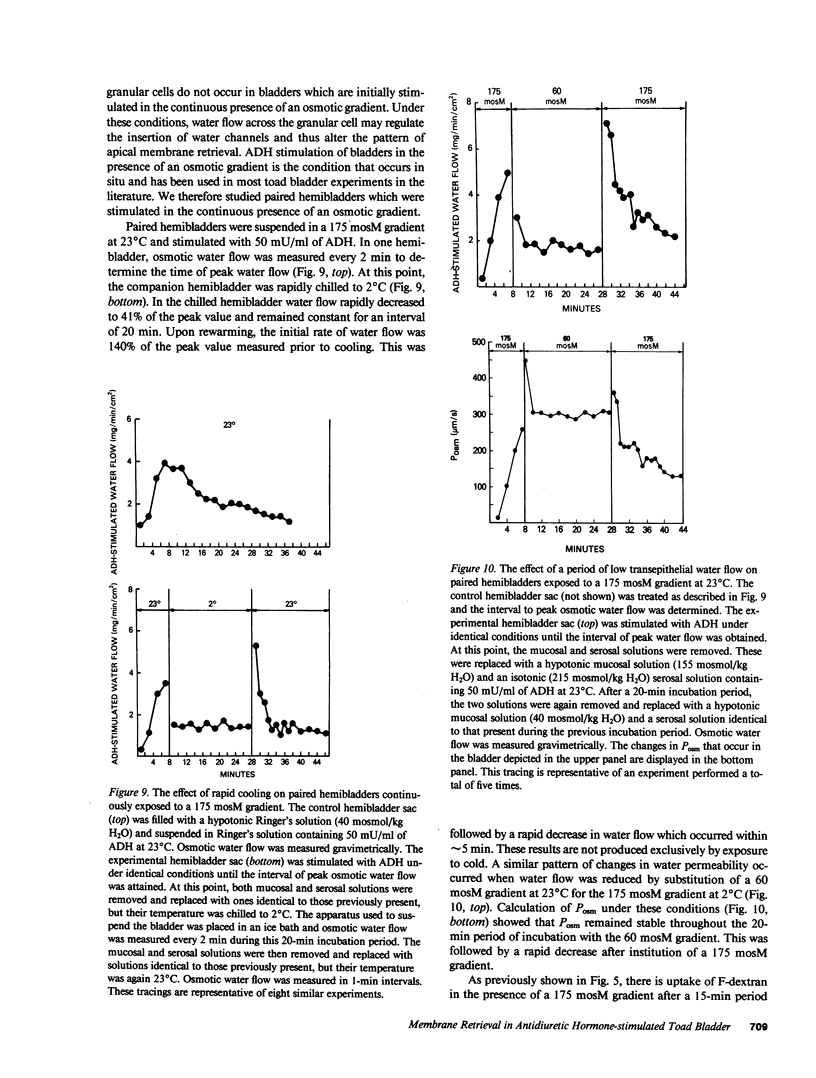
Antidiuretic hormone (ADH) increases the osmotic water permeability (Posm) of toad urinary bladder. This increase is believed to be produced by fusion of intracellular vesicles called aggrephores with the granular cell apical plasma membrane. Aggrephores contain intramembrane particle aggregates postulated to be water channels. ADH-stimulated Posm is decreased by osmotic gradient exposure, which is termed flux inhibition. We studied flux inhibition by exposing ADH-stimulated bladders to various osmotic gradients. Osmotic water flow was initially proportional to the applied osmotic gradient, but Posm decreased with time. Ultrastructural and quantitative studies of endocytosis demonstrate that apical membrane retrieval was a direct function of the transepithelial osmotic gradient. Posm remained unchanged when apical membrane retrieval was blocked by incubation of bladders at 2 degrees C, or under low water-flow conditions. These effects were reversed by increases in temperature or the applied osmotic gradient. We conclude that apical membrane retrieval causes the phenomenon of flux inhibition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Bourguet J., Chevalier J., Hugon J. S. Alterations in membrane-associated particle distribution during antidiuretic challenge in frog urinary bladder epithelium. Biophys J. 1976 Jun;16(6):627–639. doi: 10.1016/S0006-3495(76)85717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown D., Shields G. I., Valtin H., Morris J. F., Orci L. Lack of intramembranous particle clusters in collecting ducts of mice with nephrogenic diabetes insipidus. Am J Physiol. 1985 Oct;249(4 Pt 2):F582–F589. doi: 10.1152/ajprenal.1985.249.4.F582. [DOI] [PubMed] [Google Scholar]
- DiBona D. R. Cytoplasmic involvement in ADH-mediated osmosis across toad urinary bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):C297–C307. doi: 10.1152/ajpcell.1983.245.5.C297. [DOI] [PubMed] [Google Scholar]
- Diamond J. M. Osmotic water flow in leaky epithelia. J Membr Biol. 1979 Dec 31;51(3-4):195–216. doi: 10.1007/BF01869084. [DOI] [PubMed] [Google Scholar]
- EDELMAN I. S., PETERSEN M. J., GULYASSY P. F. KINETIC ANALYSIS OF THE ANTIDIURETIC ACTION OF VASOPRESSIN AND ADENOSINE-3',5'-MONOPHOSPHATE. J Clin Invest. 1964 Nov;43:2185–2194. doi: 10.1172/JCI105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggena P. Glutaraldehyde-fixation method for determining the permeability to water of the toad urinary bladder. Endocrinology. 1972 Jul;91(1):240–246. doi: 10.1210/endo-91-1-240. [DOI] [PubMed] [Google Scholar]
- Eggena P. Osmotic regulation of toad bladder responsiveness to neurohypophyseal hormones. J Gen Physiol. 1972 Dec;60(6):665–678. doi: 10.1085/jgp.60.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggena P., Walter R., Schwartz I. L. Relationship between hydro-osmotic flow and the inhibited response of the toad bladder to vasopressin. Life Sci. 1968 Jan 1;7(1):59–63. doi: 10.1016/0024-3205(68)90361-5. [DOI] [PubMed] [Google Scholar]
- Ellis S. J., Kachadorian W. A., DiScala V. A. Effect of osmotic gradient on ADH-induced intramembranous particle aggregates in toad bladder. J Membr Biol. 1980;52(2):181–184. doi: 10.1007/BF01869124. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gronowicz G., Masur S. K., Holtzman E. Quantitative analysis of exocytosis and endocytosis in the hydroosmotic response of toad bladder. J Membr Biol. 1980;52(3):221–235. doi: 10.1007/BF01869191. [DOI] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Bensinger R., Orloff J. Effect of adrenergic agents on toad bladder response to ADH, 3',5'-AMP, and theophylline. Am J Physiol. 1968 Nov;215(5):1024–1031. doi: 10.1152/ajplegacy.1968.215.5.1024. [DOI] [PubMed] [Google Scholar]
- Harmanci M. C., Stern P., Kachadorian W. A., Valtin H., DiScala V. A. Vasopressin and collecting duct intramembranous particle clusters: a dose-response relationship. Am J Physiol. 1980 Dec;239(6):F560–F564. doi: 10.1152/ajprenal.1980.239.6.F560. [DOI] [PubMed] [Google Scholar]
- Humbert F., Montesano R., Grosso A., de Sousa R. C., Orci L. Particle aggregates in plasma and intracellular membranes of toad bladder (granular cell). Experientia. 1977 Oct 15;33(10):1364–1367. doi: 10.1007/BF01920184. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Ellis S. J., Muller J. Possible roles for microtubules and microfilaments in ADH action on toad urinary bladder. Am J Physiol. 1979 Jan;236(1):F14–F20. doi: 10.1152/ajprenal.1979.236.1.F14. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Levine S. D. Effect of distension on ADH-induced osmotic water flow in toad urinary bladder. J Membr Biol. 1982;64(3):181–186. doi: 10.1007/BF01870884. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Muller J., Rudich S. W., DiScala V. A. Temperature dependence of ADH-induced water flow and intramembranous particle aggregates in toad bladder. Science. 1979 Aug 31;205(4409):910–913. doi: 10.1126/science.112678. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Muller J., Rudich S., DiScala V. A. Relation of ADH effects to altered membrane fluidity in toad urinary bladder. Am J Physiol. 1981 Jan;240(1):F63–F69. doi: 10.1152/ajprenal.1981.240.1.F63. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Sariban-Sohraby S., Spring K. R. Regulation of water permeability in toad urinary bladder at two barriers. Am J Physiol. 1985 Feb;248(2 Pt 2):F260–F265. doi: 10.1152/ajprenal.1985.248.2.F260. [DOI] [PubMed] [Google Scholar]
- Levine S. D., Jacoby M., Finkelstein A. The water permeability of toad urinary bladder. II. The value of Pf/Pd(w) for the antidiuretic hormone-induced water permeation pathway. J Gen Physiol. 1984 Apr;83(4):543–561. doi: 10.1085/jgp.83.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. D., Kachadorian W. A. Barriers to water flow in vasopressin-treated toad urinary bladder. J Membr Biol. 1981;61(2):135–139. doi: 10.1007/BF02007640. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Cooper S., Rubin M. S. Effect of an osmotic gradient on antidiuretic hormone-induced endocytosis and hydroosmosis in the toad urinary bladder. Am J Physiol. 1984 Aug;247(2 Pt 2):F370–F379. doi: 10.1152/ajprenal.1984.247.2.F370. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Holtzman E., Walter R. Hormone-stimulated exocytosis in the toad urinary bladder. Some possible implications for turnover of surface membranes. J Cell Biol. 1972 Jan;52(1):211–219. doi: 10.1083/jcb.52.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A. Aggregate-carrying membranes during ADH stimulation and washout in toad bladder. Am J Physiol. 1984 Jul;247(1 Pt 1):C90–C98. doi: 10.1152/ajpcell.1984.247.1.C90. [DOI] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A., DiScala V. A. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol. 1980 Apr;85(1):83–95. doi: 10.1083/jcb.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff J., Handler J. The role of adenosine 3',5'-phosphate in the action of antidiuretic hormone. Am J Med. 1967 May;42(5):757–768. doi: 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Parisi M., Bourguet J. Effects of cellular acidification on ADH-induced intramembrane particle aggregates. Am J Physiol. 1984 Jan;246(1 Pt 1):C157–C159. doi: 10.1152/ajpcell.1984.246.1.C157. [DOI] [PubMed] [Google Scholar]
- Parisi M., Bourguet J., Ripoche P., Chevalier J. Simultaneous minute by minute determination of unidirectional and net water fluxes in frog urinary bladder. A reexamination of the two barriers in series hypothesis. Biochim Biophys Acta. 1979 Oct 5;556(3):509–523. doi: 10.1016/0005-2736(79)90137-8. [DOI] [PubMed] [Google Scholar]
- Parisi M., Montoreano R., Chevalier J., Bourguet J. Cellular pH and water permeability control in frog urinary bladder. A possible action on the water pathway. Biochim Biophys Acta. 1981 Nov 6;648(2):267–274. doi: 10.1016/0005-2736(81)90043-2. [DOI] [PubMed] [Google Scholar]
- Parisi M., Ripoche P., Prevost G., Bourguet J. Regulation by ADH and cellular osmolarity of water permeability in frog urinary bladder: a time course study. Ann N Y Acad Sci. 1981;372:144–162. doi: 10.1111/j.1749-6632.1981.tb15467.x. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Kan F. W. Label-fracture: a method for high resolution labeling of cell surfaces. J Cell Biol. 1984 Sep;99(3):1156–1161. doi: 10.1083/jcb.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I. L., Walter R. Factors influencing the reactivity of the toad bladder to the hydro-osmotic action of vasopressin. Am J Med. 1967 May;42(5):769–776. doi: 10.1016/0002-9343(67)90094-0. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B. Membrane structural studies of the action of vasopressin. Fed Proc. 1985 Aug;44(11):2687–2692. [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]