Abstract
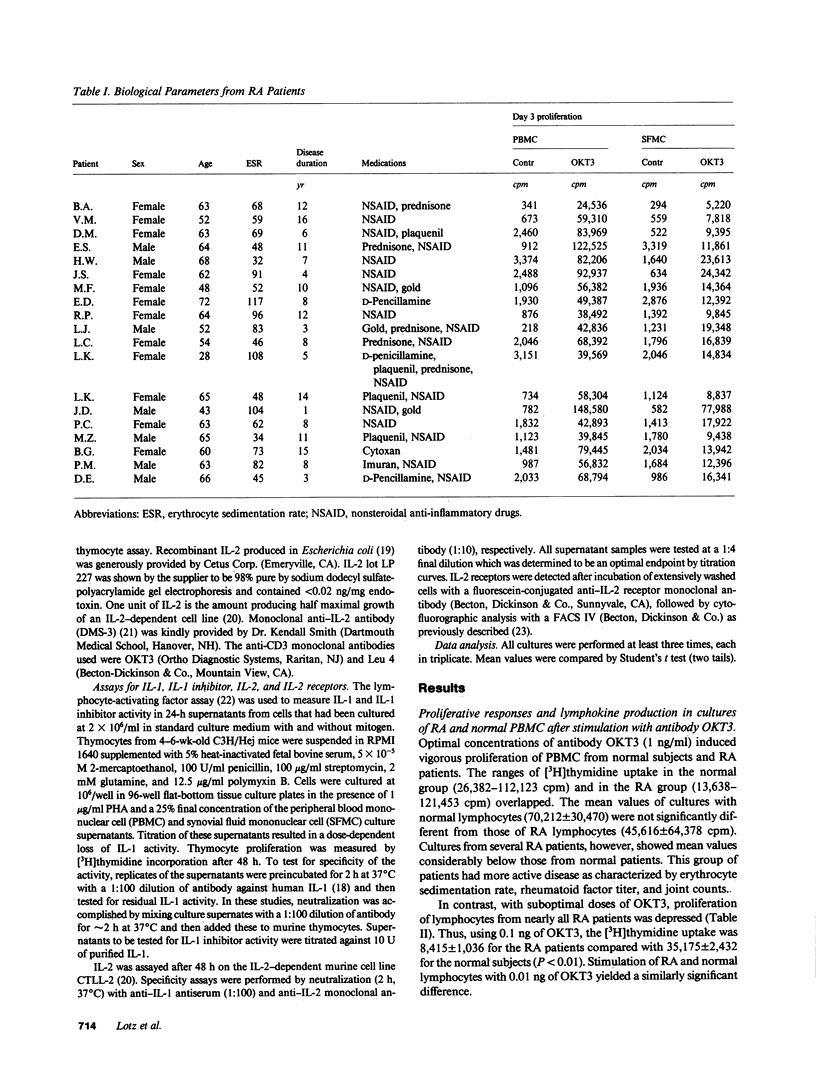
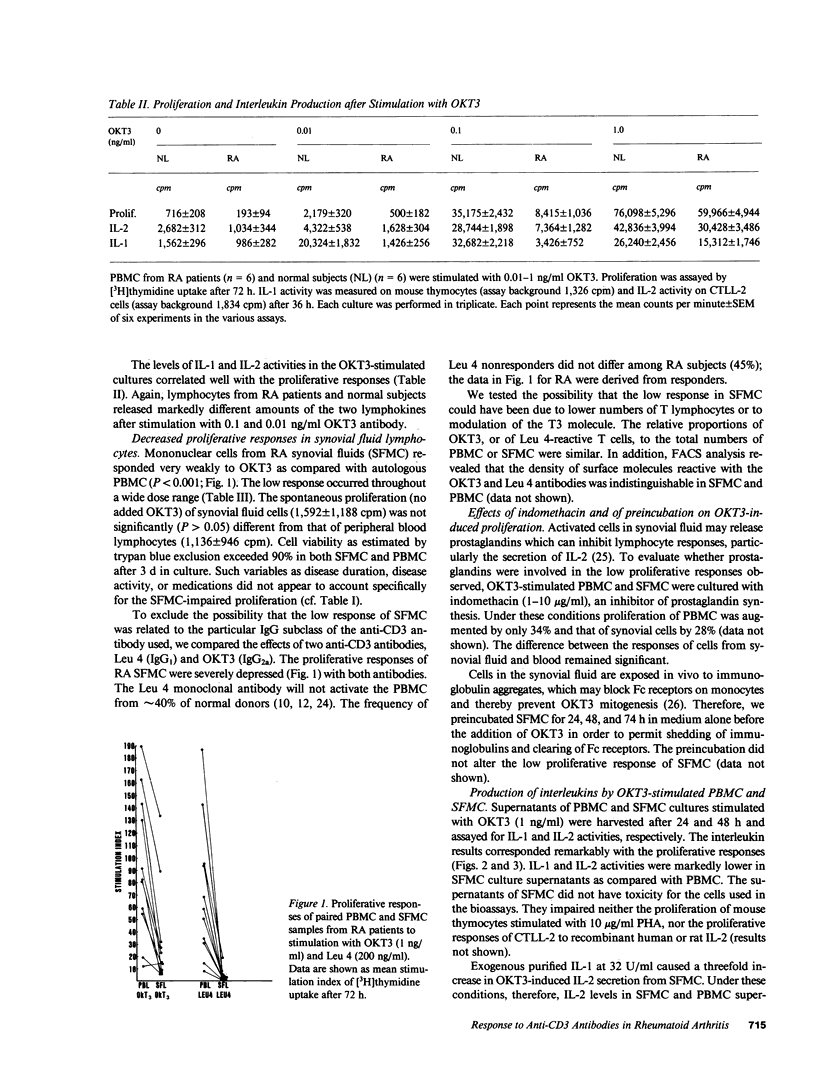
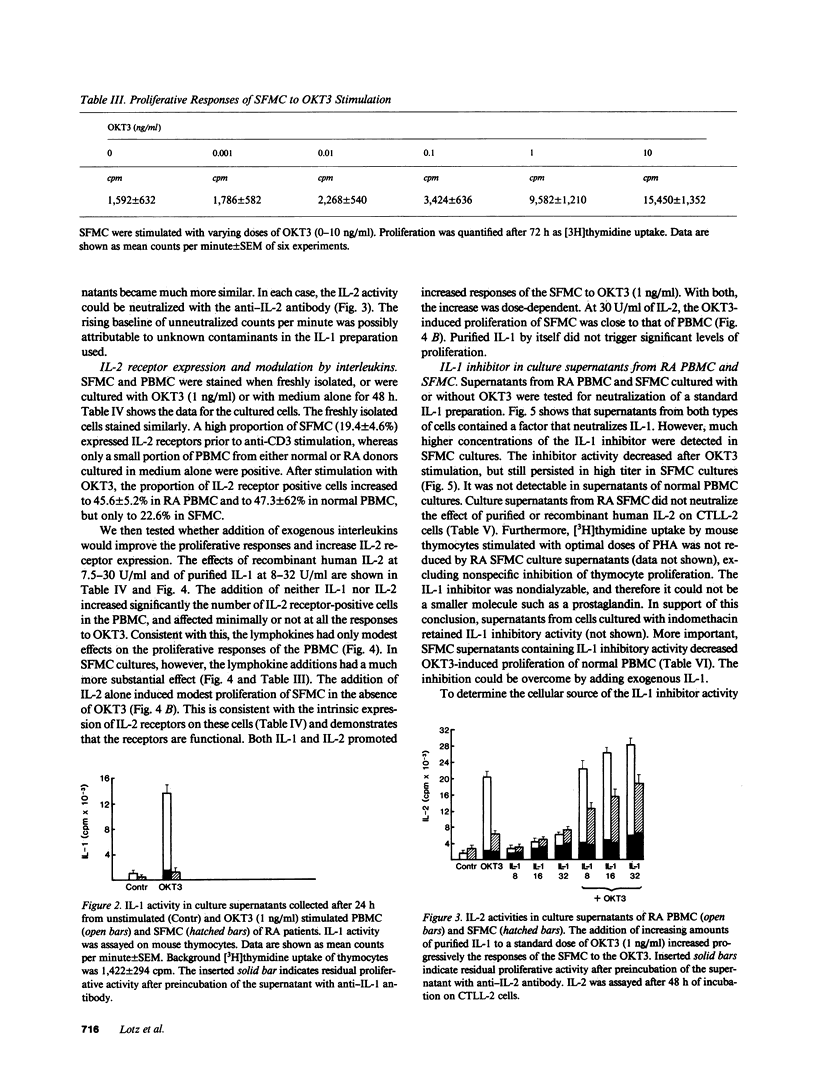
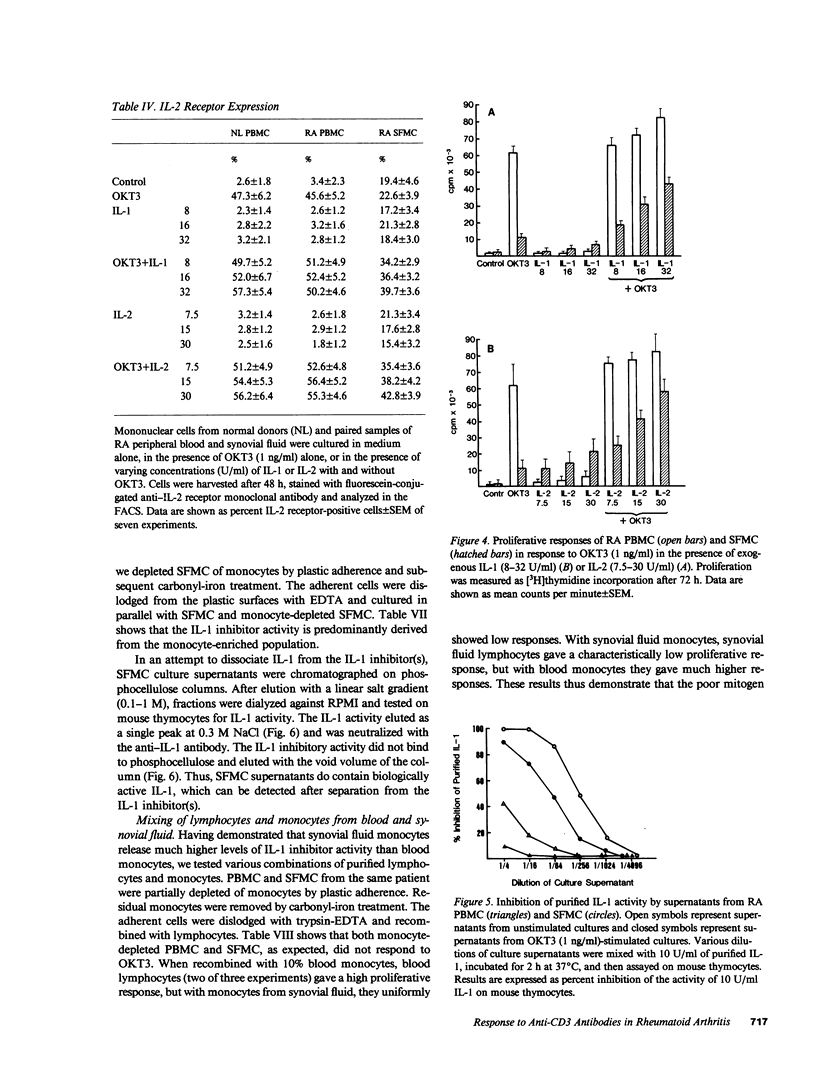
Synovial fluid mononuclear cells (SFMC) from patients with active rheumatoid arthritis characteristically respond poorly to mitogens. In this study, mitogenic antibodies reactive with the CD3(T3) antigen on human T lymphocytes were used to analyze the basis for the deficiency. OKT3-induced proliferation and release of interleukin 1 (IL-1) and interleukin 2 (IL-2) from SFMC were depressed in all patients. Purified IL-1 or recombinant IL-2 restored proliferative responses in SFMC and increased IL-2 receptor density. Exogenous IL-1 also enhanced IL-2 release. Fractionation of SFMC supernatants on phosphocellulose columns revealed the presence of IL-1 and a potent IL-1 inhibitor. The monocyte-derived IL-1 inhibitor blocked IL-1-dependent responses of normal peripheral blood lymphocytes to OKT3, but had no effect on IL-2-dependent events. These results suggest that IL-1 inhibitor(s) in SFMC impair(s) OKT3-induced mitogenesis by interfering with the effects of IL-1 on T lymphocytes. The net result is deficient IL-2 secretion, IL-2 receptor expression, and impaired cellular proliferation. This novel inhibitory circuit provides a rational explanation for the diminished function of synovial fluid T lymphocytes in rheumatoid arthritis patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Tilden A. B., Dunlap N. E. Analysis of the monocyte Fc receptors and antibody-mediated cellular interactions required for the induction of T cell proliferation by anti-T3 antibodies. J Immunol. 1985 Jul;135(1):165–171. [PubMed] [Google Scholar]
- Dinarello C. A., Renfer L., Wolff S. M. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Rosenwasser L. J., Wolff S. M. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J Immunol. 1981 Dec;127(6):2517–2519. [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Galili U., Rosenthal L., Galili N., Klein E. Activated T cells in the synovial fluid of arthritic patients: characterization and comparison with in vitro activated human and murine T cells in cooperation with monocytes in cytotoxicity. J Immunol. 1979 Mar;122(3):878–883. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A., Fisher R. I. Characterization of the lymphocyte surface receptors for Con A and PHA. J Immunol. 1975 Feb;114(2 Pt 1):710–714. [PubMed] [Google Scholar]
- Liao Z., Grimshaw R. S., Rosenstreich D. L. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med. 1984 Jan 1;159(1):126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Dinarello C. A., Carson D. A., Vaughan J. H. Release of lymphokines after infection with Epstein Barr virus in vitro. II. A monocyte-dependent inhibitor of interleukin 1 downregulates the production of interleukin 2 and interferon-gamma in rheumatoid arthritis. J Immunol. 1986 May 15;136(10):3643–3648. [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- Petersen J., Andersen V., Ingemann-Hansen T., Halkjaer-Kristensen J., Wiik A., Thyssen H. Synovial fluid and blood monocyte influence on lymphocyte proliferation in rheumatoid arthritis and traumatic synovitis. Scand J Rheumatol. 1983;12(3):299–304. doi: 10.3109/03009748309098553. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Rodgers B. C., Scott D. M., Mundin J., Sissons J. G. Monocyte-derived inhibitor of interleukin 1 induced by human cytomegalovirus. J Virol. 1985 Sep;55(3):527–532. doi: 10.1128/jvi.55.3.527-532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Kuang Y. D., Hall R. E., Muchmore A. V., Oppenheim J. J. Accessory cell function of human B cells. I. Production of both interleukin 1-like activity and an interleukin 1 inhibitory factor by an EBV-transformed human B cell line. J Exp Med. 1984 Jun 1;159(6):1637–1652. doi: 10.1084/jem.159.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Crow M. K., Russo C., Weksler M. E. Requirements for T cell activation by OKT3 monoclonal antibody: role of modulation of T3 molecules and interleukin 1. J Immunol. 1985 Sep;135(3):1714–1718. [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Stratton J. A., Peter J. B. The responses of peripheral blood and synovial fluid lymphocytes of patients with rheumatoid arthritis to in vitro stimulation with mitogens. Clin Immunol Immunopathol. 1978 Jun;10(2):233–241. doi: 10.1016/0090-1229(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. A comparison of PGE2 effects on human suppressor cell function and on interleukin 2 function. J Immunol. 1982 Dec;129(6):2469–2473. [PubMed] [Google Scholar]
- Tsoukas C. D., Lambris J., Lotz M., Valentine M. A., Vaughan J. H., Carson D. A. Lymphocyte mitogenesis induced by monoclonal antibodies to the T3 complex. Differential modulation by human IgG. Cell Immunol. 1984 Nov;89(1):66–74. doi: 10.1016/0008-8749(84)90198-9. [DOI] [PubMed] [Google Scholar]
- Valentine M. A., Tsoukas C. D., Rhodes G., Vaughan J. H., Carson D. A. Phytohemagglutinin binds to the 20-kDa molecule of the T3 complex. Eur J Immunol. 1985 Aug;15(8):851–854. doi: 10.1002/eji.1830150821. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Van Wauwe J. P., Goossens J. G. The mitogenic activity of OKT3 and anti-Leu 4 monoclonal antibodies: a comparative study. Cell Immunol. 1983 Apr 1;77(1):23–29. doi: 10.1016/0008-8749(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]



