Abstract
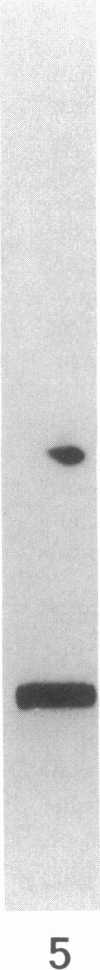
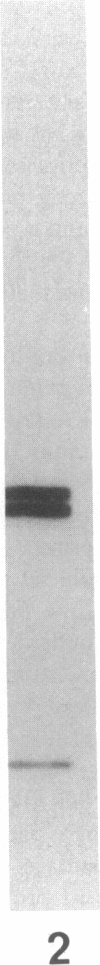
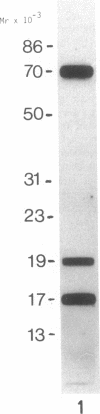
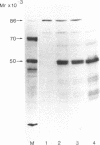
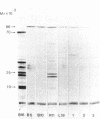
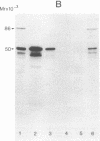
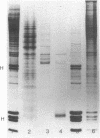
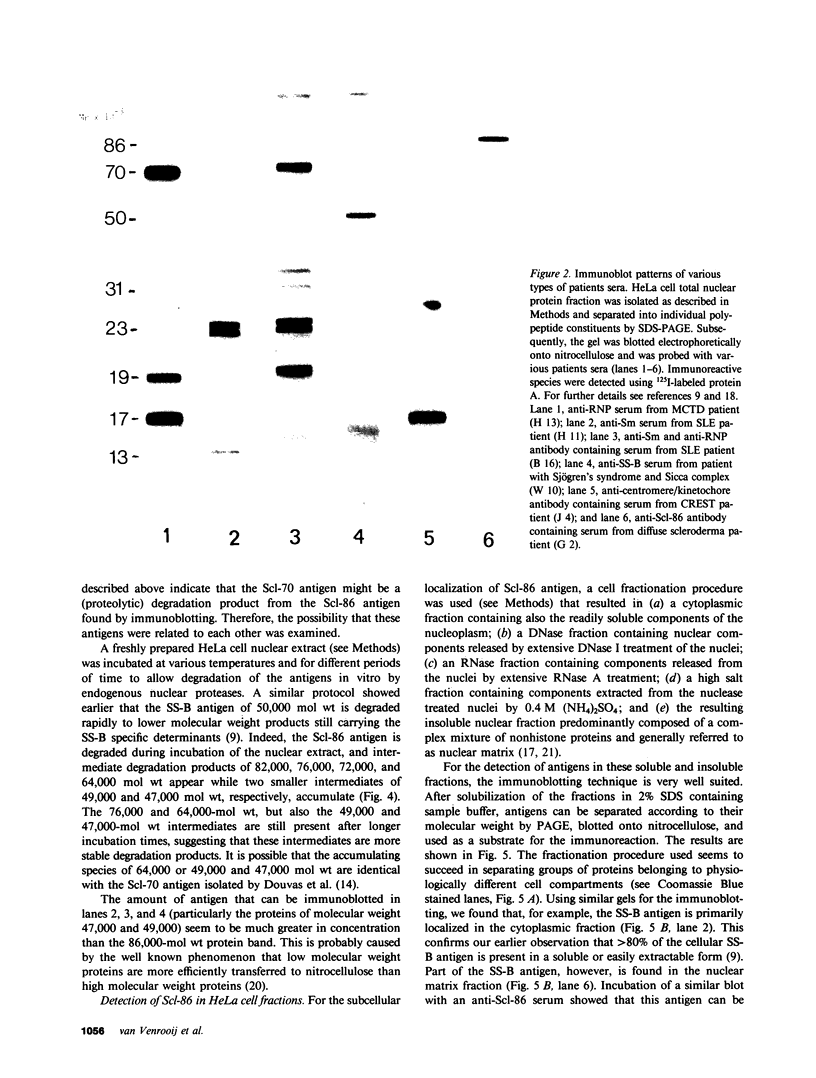
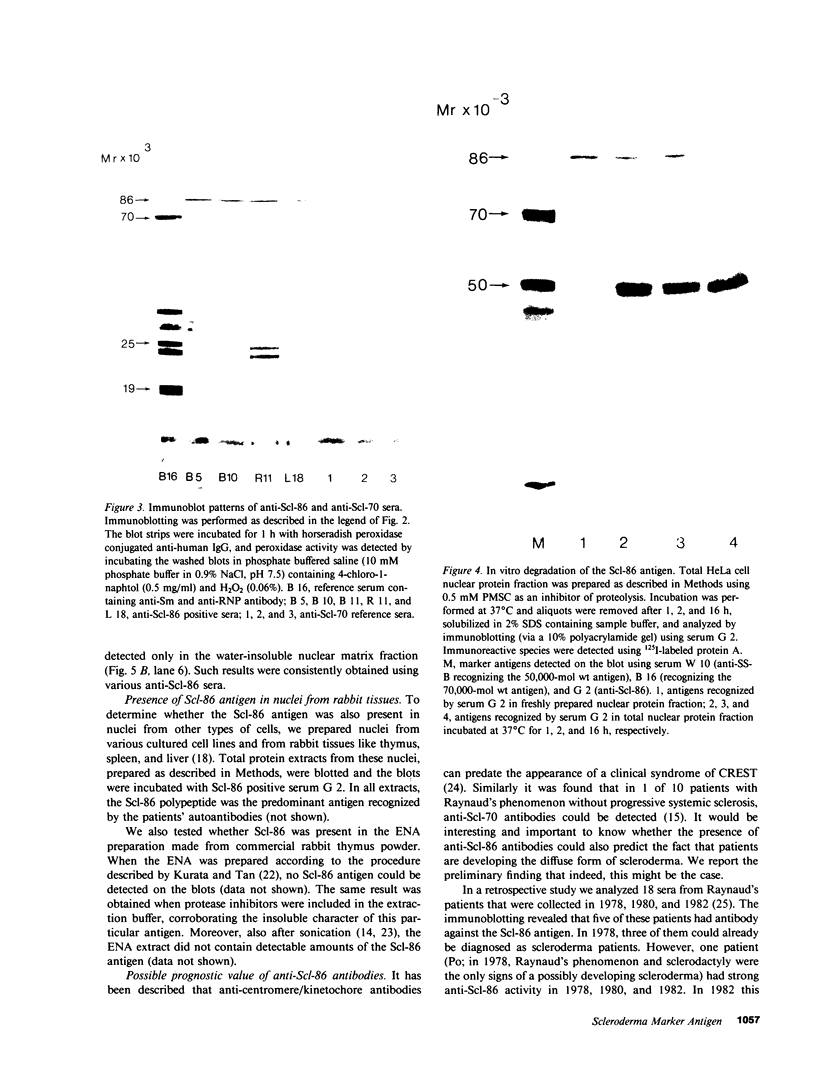
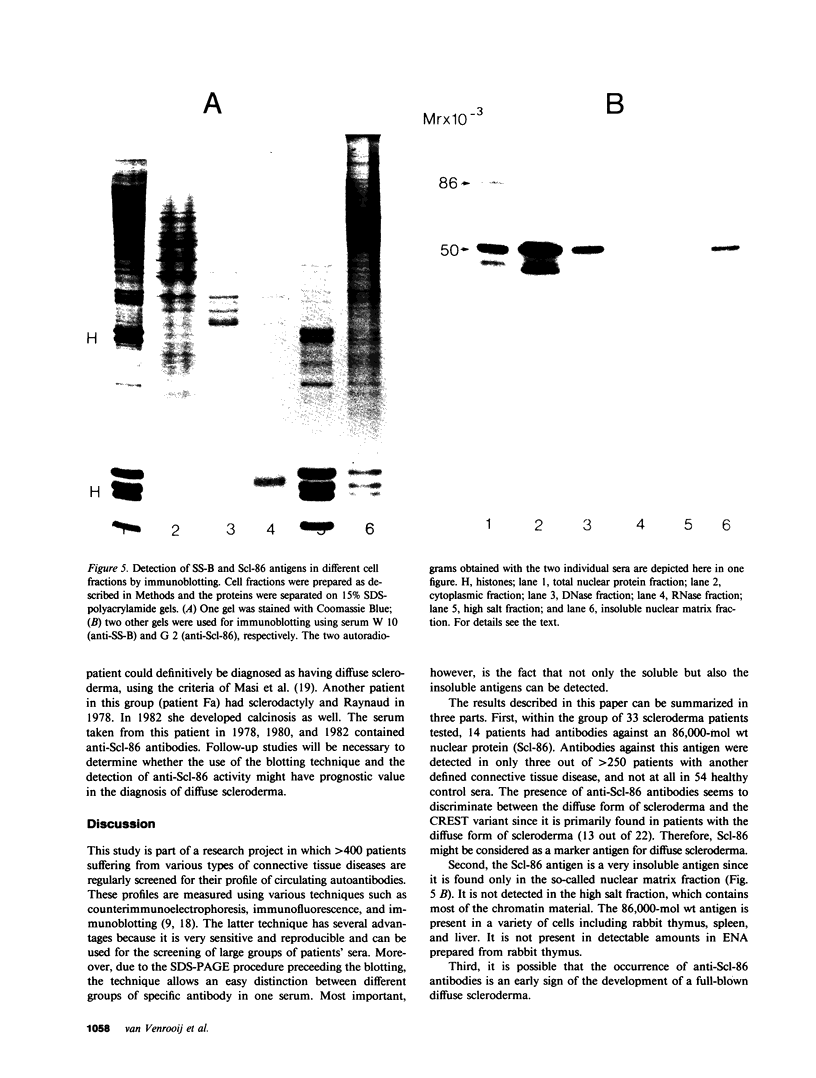
More than 300 sera from patients with a connective tissue disease were analyzed with the immunoblotting technique. The presence of autoantibodies against an 86,000-mol wt marker antigen for diffuse scleroderma (Scl-86) was found in 14 out of 33 patients with scleroderma. The presence of anti-Scl-86 antibodies seemed to correlate with the diagnosis of diffuse scleroderma since they were found in 13 out of 22 diffuse scleroderma patients and in only one out of 11 patients with limited scleroderma. All scleroderma sera (33 patients' sera and 13 reference sera) were also tested for the presence of anti-Scl-70 antibodies. It was found that all anti-Scl-70 positive sera (n = 25) contained anti-Scl-86 antibody as well, suggesting a relationship between these two antigens. However, the Scl-86 antigen was shown to be an extremely insoluble nonchromosomal protein, resistant to boiling in sodium dodecyl sulfate. This contrasts with the Scl-70 antigen, which has been described as a thermolabile, soluble antigen present in the chromatin fraction. Together, our results are consistent with the idea that Scl-70 is a degradation product of Scl-86. The Scl-86 antigen is present in freshly prepared rabbit thymus, spleen, and liver nuclei as well as in nuclei from various cultured cell lines, but is not detectable in extractable nuclear antigen from rabbit thymus. In a limited retrospective study, the anti-Scl-86 antibodies were found in two sera from patients with Raynaud's phenomenon before the development of diffuse scleroderma. Therefore, it is possible that screening of patients' serum for this antibody might predict the development of diffuse scleroderma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agutter P. S., Richardson J. C. Nuclear non-chromatin proteinaceous structures: their role in the organization and function of the interphase nucleus. J Cell Sci. 1980 Aug;44:395–435. doi: 10.1242/jcs.44.1.395. [DOI] [PubMed] [Google Scholar]
- Akizuki M., Powers R., Holman H. R. A soluble acidic protein of the cell nucleus which reacts with serum from patients with systemic lupus erythermatosus and Sjögren's syndrome. J Clin Invest. 1977 Feb;59(2):264–272. doi: 10.1172/JCI108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Steigerwald J. C., Tan E. M. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982 Apr;48(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O. Isolation of intact Sm/RNP antigens from rabbit thymus. J Immunol. 1983 Jul;131(1):347–351. [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975 Feb 20;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Achten M., Tan E. M. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem. 1979 Oct 25;254(20):10514–10522. [PubMed] [Google Scholar]
- Fritzler M. J., Kinsella T. D. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980 Oct;69(4):520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Habets W. J., de Rooij D. J., Salden M. H., Verhagen A. P., van Eekelen C. A., van de Putte L. B., van Venrooij W. J. Antibodies against distinct nuclear matrix proteins are characteristic for mixed connective tissue disease. Clin Exp Immunol. 1983 Oct;54(1):265–276. [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., den Brok J. H., Boerbooms A. M., van de Putte L. B., van Venrooij W. J. Characterization of the SS-B (La) antigen in adenovirus-infected and uninfected HeLa cells. EMBO J. 1983;2(10):1625–1631. doi: 10.1002/j.1460-2075.1983.tb01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., Wouda A. A., The T. H. Systemic involvement and immunologic findings in patients presenting with Raynaud's phenomenon. Am J Med. 1980 Nov;69(5):675–680. doi: 10.1016/0002-9343(80)90417-9. [DOI] [PubMed] [Google Scholar]
- Kurata N., Tan E. M. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 1976 May-Jun;19(3):574–580. doi: 10.1002/art.1780190309. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter L., Schopfer K., Wilhelm J. A., Nyffenegger T., Parisot R. F., De Robertis E. M. Molecular characterization of ribonucleoprotein antigens bound by antinuclear antibodies. A diagnostic evaluation. Arthritis Rheum. 1982 Nov;25(11):1278–1283. doi: 10.1002/art.1780251102. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Tuffanelli D. L., Fukuyama K., Kirschner M. W. Autoimmune response directed against conserved determinants of nuclear envelope proteins in a patient with linear scleroderma. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4374–4378. doi: 10.1073/pnas.80.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Chused T. M., Mann D. L., Klippel J. H., Fauci A. S., Frank M. M., Lawley T. J., Hamburger M. I. Sjögren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980 Feb;92(2 Pt 1):212–226. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Peebles C. L., Tan E. M. Microhemagglutination test for detection of antibodies to nuclear Sm and ribonucleoprotein antigens in systemic lupus erythematosus and related diseases. Am J Clin Pathol. 1978 Nov;70(5):800–807. doi: 10.1093/ajcp/70.5.800. [DOI] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Bull Rheum Dis. 1981;31(1):1–6. [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., May C. M., Holman H. R., McDuffie F. C., Hess E. V., Schmid F. R. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976 Nov 18;295(21):1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- Takano M., Agris P. F., Sharp G. C. Purification and biochemical characterization of nuclear ribonucleoprotein antigen using purified antibody from serum of a patient with mixed connective tissue disease. J Clin Invest. 1980 Jun;65(6):1449–1456. doi: 10.1172/JCI109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Rodnan G. P., Garcia I., Moroi Y., Fritzler M. J., Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis. Anti-centromere antibody and its relationship to CREST syndrome. Arthritis Rheum. 1980 Jun;23(6):617–625. doi: 10.1002/art.1780230602. [DOI] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., Salden M. H., Habets W. J., van de Putte L. B., van Venrooij W. J. On the existence of an internal nuclear protein structure in HeLa cells. Exp Cell Res. 1982 Sep;141(1):181–190. doi: 10.1016/0014-4827(82)90080-5. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Groeneveld A. A., Bloemendal H., Benedetti E. L. Cultured calf lens epithelium. I. Methods of cultivation and characteristics of the cultures. Exp Eye Res. 1974 Jun;18(6):517–526. doi: 10.1016/0014-4835(74)90058-x. [DOI] [PubMed] [Google Scholar]