Abstract
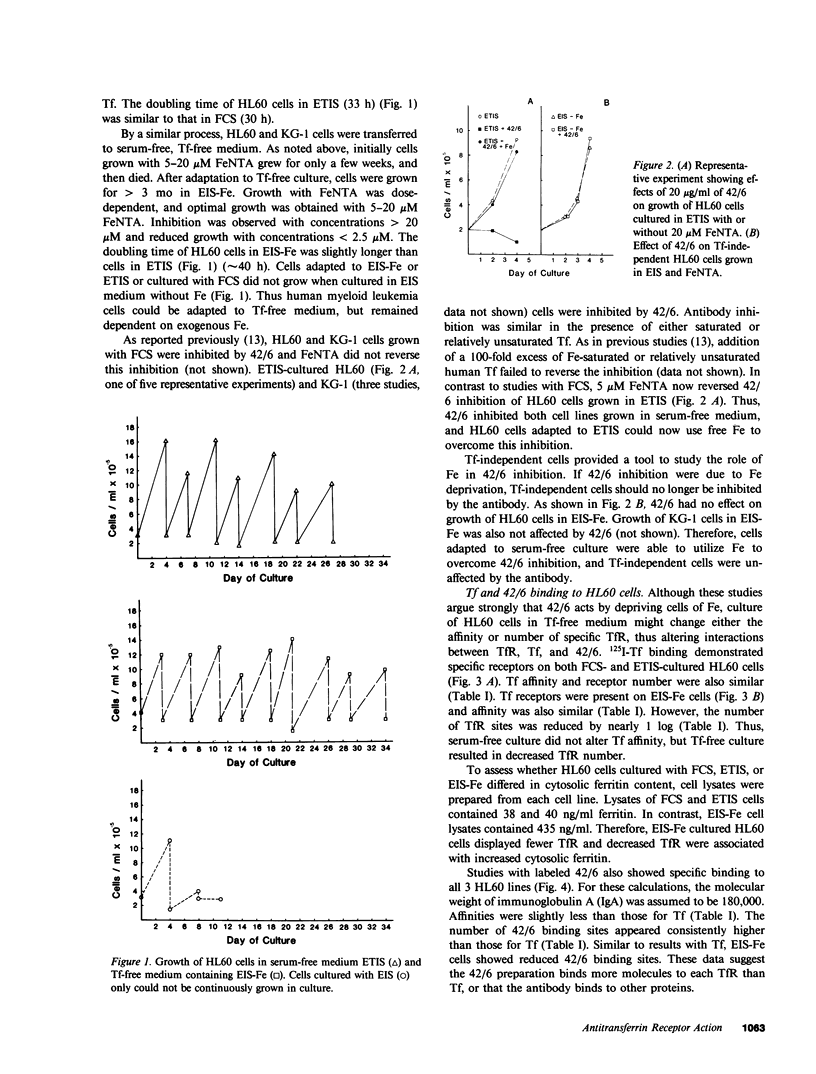
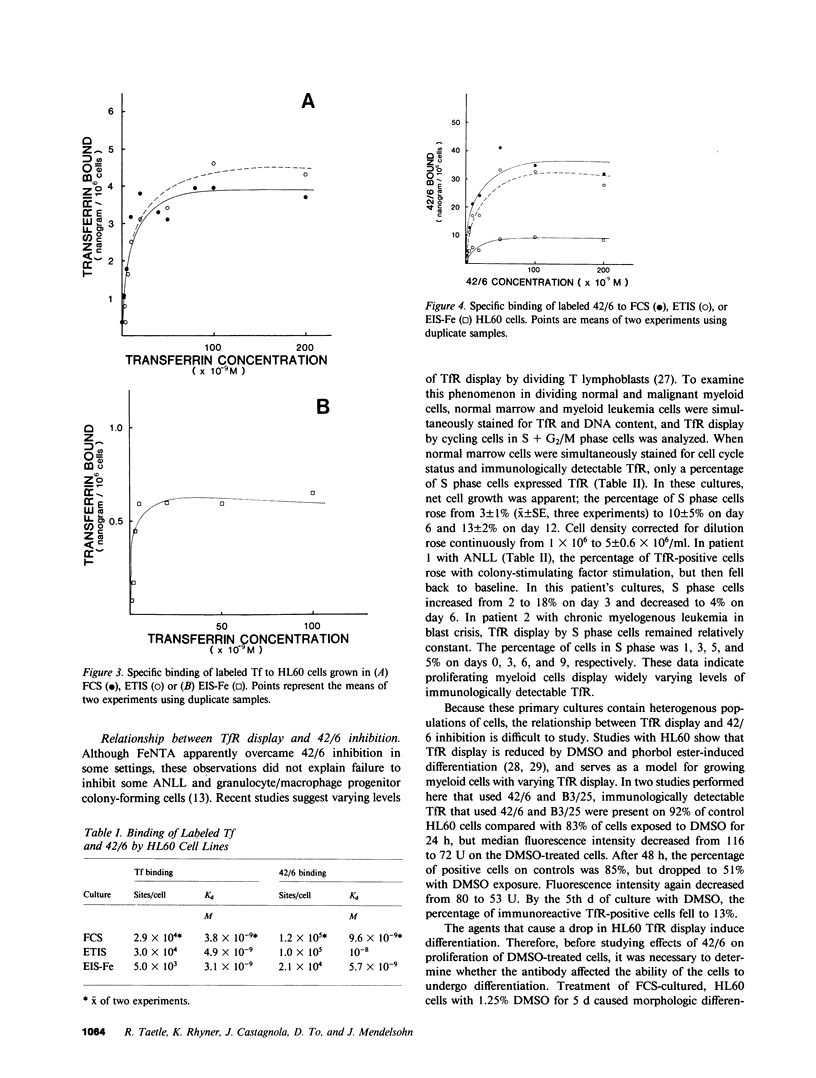
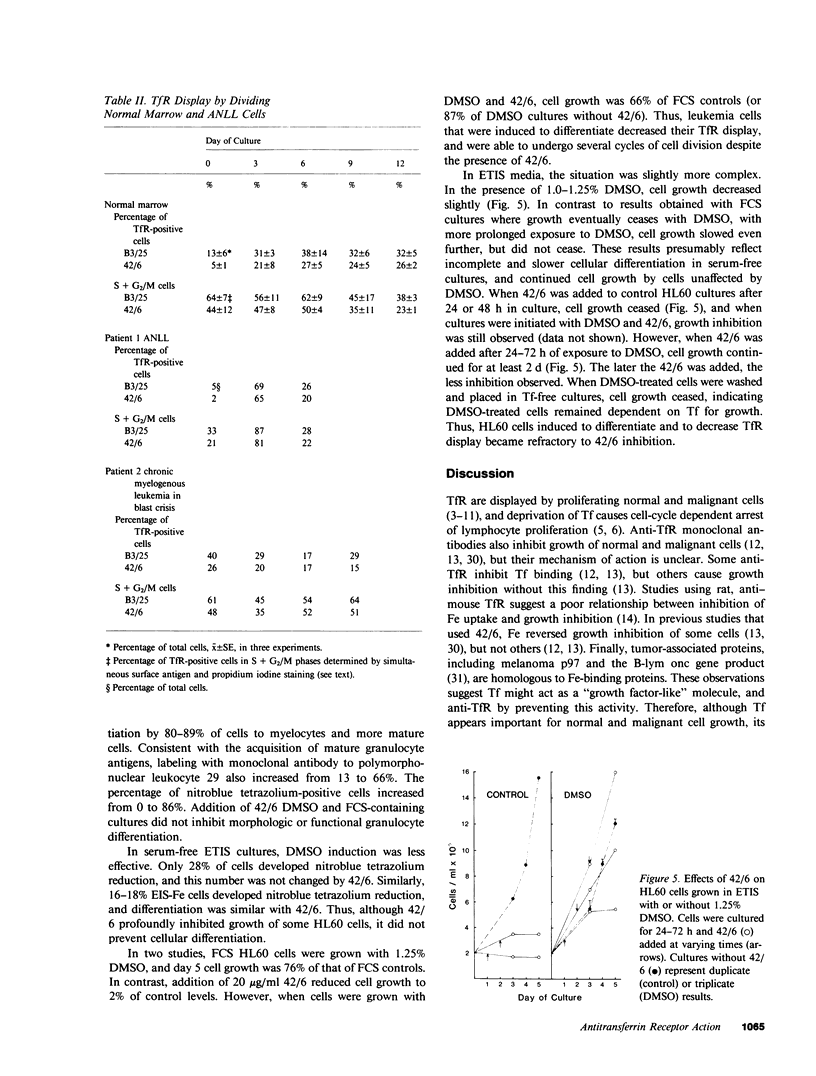
In previous studies, antitransferrin receptor antibody 42/6 inhibited growth of normal granulocyte/macrophage progenitors and some malignant myeloid cells. In these studies, leukemia cell lines cultured without serum and fresh leukemia cells were used to investigate the roles of Fe, transferrin receptors, and transferrin in leukemia cell growth, and mechanisms of 42/6 inhibition and resistance. HL60 and KG-1 leukemia cells grown in serum-free medium were inhibited by 42/6. In contrast to results in fetal calf serum (FCS), soluble Fe (ferric nitriloacetate) reversed 42/6 growth inhibition of serum-free HL60 cells. When HL60 cells were adapted for growth in serum-free, transferrin-free medium, they became refractory to 42/6 growth inhibition. By using radiolabeled transferrin and 42/6, HL60 cells cultured in FCS and transferrin displayed similar quantities of transferrin receptors (29,000-30,000/cell) and similar Kd's (3.8-4.9 X 10(-9) M). Cells grown in transferrin-free medium showed a similar Kd (3.1 X 10(-9) M), but fewer transferrin binding sites (5,000/cell). Transferrin-independent cells contained a log higher concentration of intracellular ferritin. For both FCS and serum-free HL60 cells, calculated affinities for 42/6 were lower (5.7-10.0 X 10(-9) M), but the number of binding sites was three- to fourfold higher. To investigate further the relationship between receptor display and antibody inhibition in proliferating normal and malignant myeloid cells, simultaneous immunofluorescence was used to determine the cell cycle status of transferrin receptor-positive cells. Malignant cells in S + G2/M displayed approximately 50% of the amount of transferrin receptors detected in normal dividing colony-stimulating factor-stimulated marrow cells. Receptor display by dividing cells from two patients with acute nonlymphocytic leukemia was variable. When HL60 cells were exposed to dimethyl sulfoxide, transferrin receptor display decreased, and 42/6 growth inhibition was abrogated or greatly diminished. The presence of 42/6 did not prevent dimethyl sulfoxide-induced HL60 differentiation in serum-containing or serum-free cultures. We conclude that human leukemia cells require Fe for growth and that 42/6 inhibits transferrin-dependent cells by Fe deprivation. Some dividing normal and differentiating malignant cells display reduced transferrin receptors, and can also escape antibody inhibition. The increased ferritin levels and decreased transferrin receptors in transferrin-independent HL60 cells confirm the inverse relationship between cell ferritin content and transferrin receptor display. These studies indicate a critical role for Fe in leukemia cell growth and possible roles in cellular differentiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R., Massey E. J., Seligman P. A. Regulation of transferrin receptor expression on human leukemic cells during proliferation and induction of differentiation. Effects of gallium and dimethylsulfoxide. J Clin Invest. 1983 Oct;72(4):1314–1325. doi: 10.1172/JCI111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Cooper G. M., Ritz J., Lane M. A. Identification and molecular cloning of the human Blym transforming gene activated in Burkitt's lymphomas. Nature. 1983 Sep 8;305(5930):112–116. doi: 10.1038/305112a0. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Thesleff I., Lehto V. P., Virtanen I. Distribution of the transferrin receptor in normal human fibroblasts and fibrosarcoma cells. Int J Cancer. 1983 Jan 15;31(1):111–117. doi: 10.1002/ijc.2910310118. [DOI] [PubMed] [Google Scholar]
- Frazier J. L., Caskey J. H., Yoffe M., Seligman P. A. Studies of the transferrin receptor on both human reticulocytes and nucleated human cells in culture: comparison of factors regulating receptor density. J Clin Invest. 1982 Apr;69(4):853–865. doi: 10.1172/JCI110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Human myeloid leukemia cell lines: a review. Blood. 1980 Sep;56(3):344–350. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman H. M., Cohen A., Lee J. W., Freedman M. H., Gelfand E. W. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984 Sep;64(3):748–753. [PubMed] [Google Scholar]
- Matsui T., Nakao Y., Kobayashi N., Kishihara M., Ishizuka S., Watanabe S., Fujita T. Phenotypic differentiation-linked growth inhibition in human leukemia cells by active vitamin D3 analogues. Int J Cancer. 1984 Feb 15;33(2):193–202. doi: 10.1002/ijc.2910330207. [DOI] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Mendelsohn J., Trowbridge I., Castagnola J. Inhibition of human lymphocyte proliferation by monoclonal antibody to transferrin receptor. Blood. 1983 Oct;62(4):821–826. [PubMed] [Google Scholar]
- Musgrove E., Rugg C., Taylor I., Hedley D. Transferrin receptor expression during exponential and plateau phase growth of human tumour cells in culture. J Cell Physiol. 1984 Jan;118(1):6–12. doi: 10.1002/jcp.1041180103. [DOI] [PubMed] [Google Scholar]
- Okabe T., Fujisawa M., Takaku F. Long-term cultivation and differentiation of human erythroleukemia cells in a protein-free chemically defined medium. Proc Natl Acad Sci U S A. 1984 Jan;81(2):453–455. doi: 10.1073/pnas.81.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R. Acidic isoferritins (leukemia-associated inhibitory activity) fail to inhibit blast proliferation in acute myelogenous leukemia. Blood. 1981 Sep;58(3):653–657. [PubMed] [Google Scholar]
- Taetle R., Caviles A., Koziol J. Response of human myeloid leukemia cells to various sources of colony-stimulating activity and phytohemagglutinin-conditioned medium. Cancer Res. 1983 May;43(5):2350–2357. [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M., Trowbridge I. Effects of anti-transferrin receptor antibodies on growth of normal and malignant myeloid cells. Int J Cancer. 1983 Sep 15;32(3):343–349. doi: 10.1002/ijc.2910320314. [DOI] [PubMed] [Google Scholar]
- Taetle R., To D., Caviles A., Norby S. W., Mendelsohn J. Characterization of normal peripheral blood lymphocyte colony-forming cells: cell cycle status, surface markers, and cellular growth requirements. Blood. 1983 Mar;61(3):548–555. [PubMed] [Google Scholar]
- Tormey D. C., Imrie R. C., Mueller G. C. Identification of transferrin as a lymphocyte growth promoter in human serum. Exp Cell Res. 1972 Sep;74(1):163–169. doi: 10.1016/0014-4827(72)90492-2. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. Biological effects of transferrin on human lymphocytes in vitro. Exp Cell Res. 1972 Sep;74(1):220–226. doi: 10.1016/0014-4827(72)90500-9. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R. Murine cell surface transferrin receptor: studies with an anti-receptor monoclonal antibody. J Cell Physiol. 1982 Sep;112(3):403–410. doi: 10.1002/jcp.1041120314. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]



