Abstract
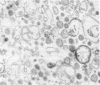
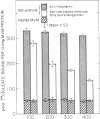
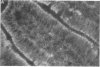
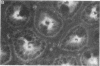
The mechanisms by which FFA are absorbed by the gut are unclear. To examine these processes, binding of [14C]oleate to isolated rat jejunal microvillous membranes (MVM) was studied in vitro. When [14C]oleate alone or compounded with bovine serum albumin at various molar ratios was incubated with MVM aliquots, binding was time- and temperature-dependent, inhibitable by addition of excess cold oleate, and decreased by heat denaturation or trypsin digestion of the membranes. When [14C]oleate binding to heat denatured MVM, which increased continuously as a function of the free oleate concentration and was taken as a measure of nonspecific binding, was subtracted from total binding to native MVM, a curve suggestive of saturable specific binding was observed. In contrast to fatty acids, there was no specific binding of [14C]taurocholate or [35S]sulfobromophthalein to jejunal MVM. After MVM solubilization with 1% Triton X-100, affinity chromatography over oleate-agarose and elution with 7 M urea yielded a single 40,000-mol-wt protein. This Sudan Black/periodic acid-Schiff-stain-negative protein co-chromatographed on Sephadex G-100 with [14C]oleate, [14C]palmitate, [14C]arachidonate, and [14C]linoleate, but not with the [14C]oleate ester of cholesterol, [14C]phosphatidylcholine, [14C]taurocholate, or [35S]sulfobromophthalein. A rabbit antibody to the previously reported hepatic membrane fatty acid binding protein (FABP) gave a single line of immunologic identity between the FABPs of rat jejunum and rat liver membrane. It inhibited the binding of [14C]oleate to native MVM but not heat denatured MVM, and, in immunohistochemical studies, demonstrated the presence of the FABP in the apical and lateral portions of the brush border cells of the jejunum, but not on the luminal surface of esophagus or colon. These data are compatible with the hypothesis that a specific FABP plays a role in fatty acid absorption from the gut.
Full text
PDF

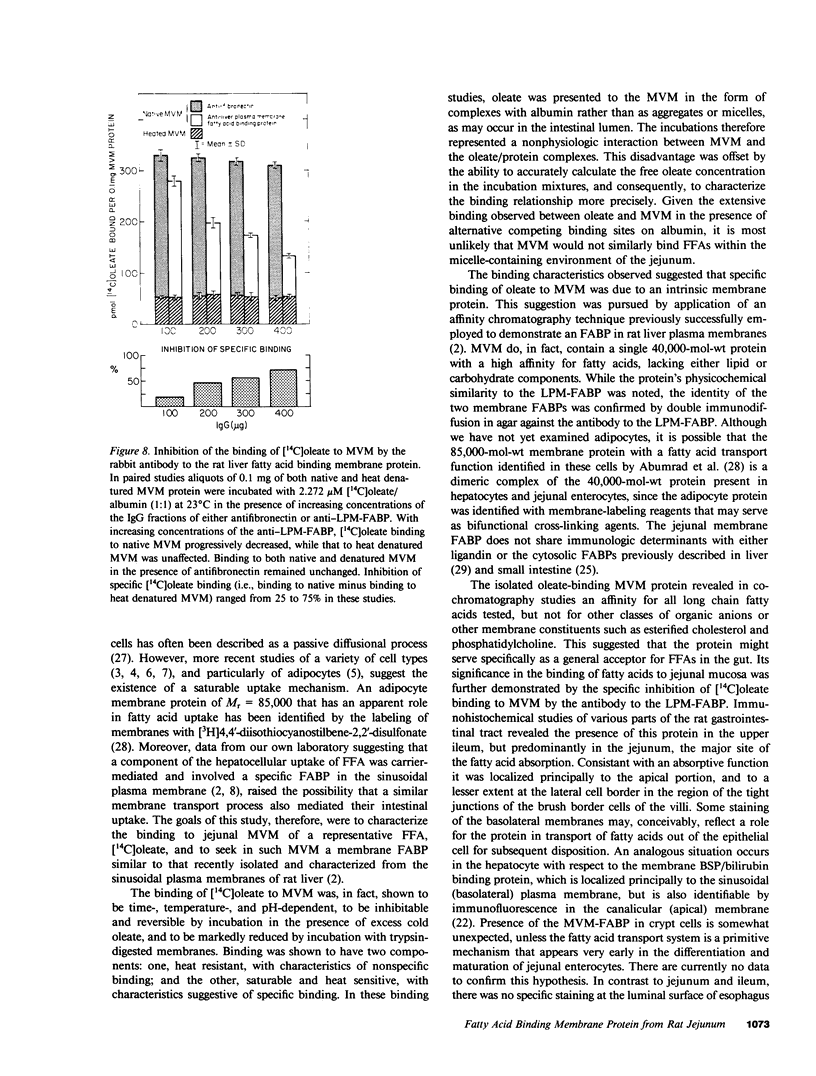
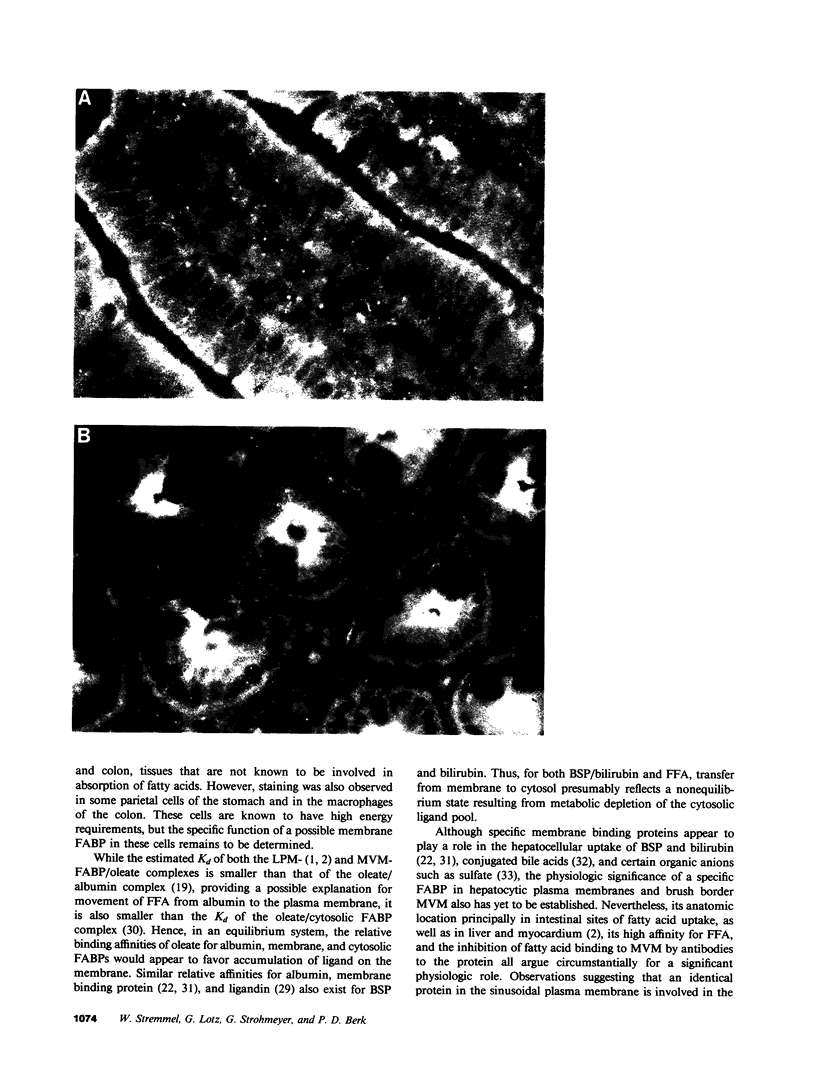


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Baudhuin P., Evrard P., Berthet J. Electron microscopic examination of subcellular fractions. I. The preparation of representative samples from suspensions of particles. J Cell Biol. 1967 Jan;32(1):181–191. doi: 10.1083/jcb.32.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Dewald B., Dulaney J. T., Touster O. Solubilization and polyacrylamide gel electrophoresis of membrane enzymes with detergents. Methods Enzymol. 1974;32:82–91. doi: 10.1016/0076-6879(74)32011-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Matsui H., Nagano K., Nakao M. Asymmetric distribution of ouabain-sensitive ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1971 Apr 13;233(2):404–408. doi: 10.1016/0005-2736(71)90337-3. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Sauer F. Effect of trypsin, phospholipases, and membrane-impermeable reagents on the uptake of palmitic acid by isolated rat liver cells. Arch Biochem Biophys. 1974 Sep;164(1):185–193. doi: 10.1016/0003-9861(74)90021-6. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Samuel D., Romey G., Ailhaud G. Uptake of fatty acids by cultured cardiac cells from chick embryo: evidence for a facilitation process without energy dependence. Biochimie. 1979;61(3):361–367. doi: 10.1016/s0300-9084(79)80129-7. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Taniuchi H., Anfinsen C. B., Jr Affinity chromatography of serum albumin with fatty acids immobilized on agarose. J Biol Chem. 1973 Apr 10;248(7):2447–2451. [PubMed] [Google Scholar]
- Reichen J., Blitzer B. L., Berk P. D. Binding of unconjugated and conjugated sulfobromophthalein to rat liver plasma membrane fractions in vitro. Biochim Biophys Acta. 1981 Jan 8;640(1):298–312. doi: 10.1016/0005-2736(81)90554-x. [DOI] [PubMed] [Google Scholar]
- Samuel D., Paris S., Ailhaud G. Uptake and metabolism of fatty acids and analogues by cultured cardiac cells from chick embryo. Eur J Biochem. 1976 May 1;64(2):583–595. doi: 10.1111/j.1432-1033.1976.tb10338.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Gerber M. A., Glezerov V., Thung S. N., Kochwa S., Berk P. D. Physicochemical and immunohistological studies of a sulfobromophthalein- and bilirubin-binding protein from rat liver plasma membranes. J Clin Invest. 1983 Jun;71(6):1796–1805. doi: 10.1172/JCI110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Kochwa S., Berk P. D. Studies of oleate binding to rat liver plasma membranes. Biochem Biophys Res Commun. 1983 Apr 15;112(1):88–95. doi: 10.1016/0006-291x(83)91801-6. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Potter B. J., Berk P. D. Studies of albumin binding to rat liver plasma membranes. Implications for the albumin receptor hypothesis. Biochim Biophys Acta. 1983 Mar 15;756(1):20–27. doi: 10.1016/0304-4165(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosilait W. D., Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed. 1976 Oct;6(3):142–148. doi: 10.1016/0010-468x(76)90020-9. [DOI] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Characterization of the bile acid transport system in normal and transformed hepatocytes. Photoaffinity labeling of the taurocholate carrier protein. J Biol Chem. 1983 Jul 25;258(14):8896–8901. [PubMed] [Google Scholar]