Abstract
Background
Determining the background incidence of intussusception is important in countries implementing rotavirus immunization. Rotavirus immunization was introduced into the routine infant immunization program in Israel during late 2010. Incidence and risk factors for intussusception were examined in children aged less than five years between 1992 and 2009.
Methods
Data were collected from medical records of children hospitalized due to intussusception (N = 190), and from control children (N = 295), at Carmel and Hillel Yaffe hospitals in northern Israel.
Results
The average annual incidence of intussusception in Jewish and Arab children aged less than five years was estimated at 36.1 (95% CI 17.0-76.5) vs. 23.2 per 100,000 (95% CI 9.3-57.9); for infants less than 12 months of age- 128.1 (95% CI 53.0-309.6) vs. 80.1 (95% CI 29.1-242.6) per 100,000. The risk of intussusception was higher in infants aged 3–5 months: OR 5.30 (95% CI 2.11-13.31) and 6–11 months: OR 2.53 (95% CI 1.13-5.62) when compared to infants aged less than 3 months; in those living in low vs high socioeconomic communities: OR 2.81 (95% CI 1.45-5.43), and in children with recent gastroenteritis: OR 19.90 (95% CI 2.35-168.32) vs children without recent gastroenteritis. Surgical reduction was required in 23.2%. The likelihood of surgery was significantly increased in patients presenting with bloody stool, in Arabs and those who were admitted to Hillel Yaffe Hospital.
Conclusions
The incidence of intussusception prior to universal rotavirus immunization was documented in northern Israel. Despite the lower incidence, Arab patients underwent surgery more often, suggesting delayed hospital admission of Arab as opposed to Jewish patients.
Keywords: Intussusception, Risk factors, Surgery, Ethnicity, Pediatrics
Background
Intussusception is among the most common abdominal emergencies among young children [1-4]. Symptoms include sudden onset of vomiting, abdominal pain, intermittent lethargy and irritability, and rectal bleeding that has been described as “currant jelly” [3-6]. Reduction is usually accomplished by air or barium enema, and in some cases by surgery, with or without bowel resection [3,4,6]. Intussusception primarily affects young children [3,5], with highest incidence in infants aged 4–10 months [3-5]. Reported yearly estimates of intussusception vary among populations and regions from 20 to 100 per 100,000 infants [3,7-9], but a higher incidence has also been reported [10].
The causes of intussusception are not fully understood, yet, there is evidence linking recent episodes of gastroenteritis and increased risk of intussusception [11,12]. Adenovirus was repeatedly recovered in higher proportions from fecal samples of patients with intussusception compared with control children [13-15], however no association has been found between natural rotavirus infection and intussusception [15-17].
In 1998 the reassortant rhesus human tetravalent oral rotavirus vaccine (RotaShield, Wyeth-Lederle, Pearl River, NY) was licensed in the United States. Shortly after its introduction into the routine childhood vaccination schedule, an excess risk for intussusception was found within 2 weeks after immunization with the first dose [1 intussusception case per 10,000 vaccinees [18-20]]. Consequently the vaccine was withdrawn from the market in 1999. Large clinical trials with two recent oral rotavirus vaccines (RotaTeq (Merck) [21] and Rotarix (GSK) [22]), and early post-marketing studies [23,24] showed no significant increase in post-immunization intussusception. However, later studies showed that in some settings e.g., Australia, and Mexico, there is an increased risk of intussusception during the first week post vaccination with the first dose of either rotavirus vaccine [25-27]. At present, this rare adverse event is estimated at about 1–2 intussusception cases per 100,000 vaccine recipients [28,29], nonetheless the vaccine’s benefits clearly exceed this small risk, thus rotavirus vaccines are recommended for use worldwide [29]. It is important to establish the baseline incidence of intussusception to assess the safety of rotavirus vaccines [3,29,30] in countries considering the introduction of rotavirus vaccination.
In Israel, rotavirus was found to be the most common pathogen causing acute gastroenteritis, and was detected in 39% of children less than 5 years of age hospitalized for diarrhea [31], leading to more than 4000 hospitalizations countrywide annually [31]. Both Rotarix and RotaTeq were licensed in Israel in mid-2007, but it was only in December 2010 that RotaTeq was included in the national immunization program. The aims of this study were to examine the incidence, clinical characteristics and potential correlates of intussusception among children less than five years of age from January 1st, 1992 to December 31st, 2009, before the introduction of rotavirus vaccine into the national immunization program.
Methods
The study was conducted in two hospitals in northern Israel: Carmel in Haifa and Hillel Yaffe in Hadera. The population residing in the catchment area of the two hospitals includes representation of the two major ethnic groups of the Israeli population, Jews and Arabs. It is estimated that 20% and 90% of children aged 0–4 years in Haifa and Hadera sub-districts, respectively, receive inpatient services at these facilities. Based on this information and on publications of the Israel Central Bureau of Statistics the estimated number of children less than five years of age residing in the study area ranged from 29,000 in 1992 to 40,700 in 2009 (annual average 35,600).
We identified children less than five years of age who were hospitalized with intussusception (n = 190) at the study hospitals between January 1st, 1992 and December 31st, 2009 by searching for the ICD-9 diagnosis code for intussusception (560.0) in discharge records. All records with this code were retrieved regardless of its being a primary or secondary diagnosis. Also the word “intussusception” was searched in text regardless of diagnosis coding. In both hospitals, the diagnosis of intussusception was based on radiological findings, usually ultrasound. In order to examine the correlates of intussusception, we retrieved records of control children (N = 295) hospitalized for reasons other than intussusception. The primary diagnoses of the control children were trauma (50.3%), otitis media (23.8%), local infection (17%) (e.g., cellulitis, abscess, mastoiditis, urinary tract infection), fever (4.1%), and elective procedures/other (4.8%). From the archives of each hospital, we retrieved lists of potential consecutive control children with these diagnoses. Intussusception cases and control children were frequently matched by hospital, sex, season/date of admission (±2 months). We did not strictly match cases and controls by age; however knowing that the majority of cases were children under one year of age we restricted the age of controls to 24 months or less. Both case and control groups consisted of generally healthy children; 95% and 94% respectively had no underlying significant health problems. A control child with multiple hospitalizations was included only once.
Using a standardized form, demographic and clinical information was collected including age, sex, hospitalization date (month and year), maternal and paternal age, birth weight, birth week, history of gastroenteritis prior to hospitalization, breastfeeding, and significant medical problems. For cases, data were also obtained on clinical symptoms and treatment modality (e.g. air enema, barium enema or surgery). Socioeconomic rank of place of residence according to the Israel Bureau of Statistics [32] was used as a proxy measure of socioeconomic status; ranks 1–4, 5–6 and 7–10 were grouped as low, intermediate and high socioeconomic status, respectively.
The study protocol was approved by the institutional review board of Carmel medical center (Protocol number 0099-09-CMC) and Hillel Yaffe medical center (Protocol number HYMC-0051-09), which allowed access to medical records. Data abstraction was done by one person (S.E.), and the identity of the patients was kept confidential and was retained in the study hospitals.
Sample size and power calculation
Assuming that the yearly incidence of intussusception in children less than five years of age is 35 per 100,000 children, with 95% confidence intervals (CIs) and maximum acceptable difference of 20 per 100,000 between the assumed and true incidence, then the required sample size was estimated at 33,602. The catchment area of the study hospitals had on average 35,600 children less than five years of age. Assuming a 30% prevalence of breastfeeding (i.e., 70% not breastfed), and an odds ratio (OR) for intussusception of 0.5 for children who are not breastfed, with the available 190 cases and 295 controls, we had 87% statistical power to detect a significant difference with two sided test.
Statistical analysis
The average annual incidence (per 100,000) and 95% CIs of intussusception were calculated. Possible risk factors for intussusception and those for surgery were examined in intussusception patients, using Student t test for continuous variables and chi square test for categorical variables, and multivariable stepwise logistic regression models. The OR and 95% CI were obtained. The variables included in the analysis at step 1 were age, hospital, socioeconomic rank of place of residence, recent gastroenteritis episode, and breastfeeding. Two sided P < 0.05 was considered statistically significant. Data were analyzed with SPSS version 19.0. Imputation of missing values was not performed. Our research adhered to the STROBE guidelines (Additional file 1).
Results
One hundred and ninety four hospitalizations for intussusception in 190 children between 01.01.1992 and 12.31.2009 were identified (4 children had recurrent episodes). Mean age of intussusception patients was 10 [Standard deviation (SD) 7] months; 65.3% were infants aged 3–11 months; 119 (62.6%) were boys. The most common symptom of intussusception was vomiting (77.3%), followed by irritability (69.1%), parental reporting of abdominal pain (47.8%), bloody stool (38.7%), lethargy (30.5%) and diarrhea (19.6%). Bloody stool was more common in infants 0–11 months of age than in children 12–59 months of age, 46.3% versus 19.6%, respectively, P < 0.001. Air enema, barium enema and surgery were performed in 62.4%, 33.5%, and 23.2% of the episodes, respectively. Median duration of hospital stay was 2 days (range 1 to 12).
The overall annual incidence of intussusception was estimated at 29.5 (95% CI 16.4-53.1) per 100,000; 36.1 (95% CI 17.0-76.5) and 23.2 per 100,000 (95% CI 9.3-57.9) for Jewish and Arab children less than five years of age, respectively. The corresponding estimates for infants less than 12 months of age were 105.6 (95% CI 52.9-210.7) per 100,000; 128.1 (95% CI 53.0-309.6) and 80.1 (95% CI 29.1-242.6) per 100,000 in Jewish and Arab infants, respectively. There were year-to-year fluctuations in the incidence of intussusception, with a general trend of decline over the past few years (Figure 1). No clear evidence of seasonality was found (Figure 2). Children with intussusception and controls were similar in terms of sex, hospital, ethnic group and year and season of admission (Table 1). The percentages of children aged 3–5 and 6–11 months, those living in low socioeconomic settings, and children with history of gastroenteritis prior to hospitalization were significantly higher in cases than in controls (Table 2). Toddlers aged 1–4 years who were not breasted had a lesser likelihood of intussusception compared with breastfed children (Table 2). In multivariable analysis, associations with age, socioeconomic rank of town of residence, and recent gastroenteritis remained significant (Table 3). Since rotavirus vaccines were licensed and became available in Israel by mid-2007, an additional case–control analysis was performed including only children admitted prior to 2007. The results of both the bivariate and multivariable analyses were similar to the full dataset analysis presented.
Figure 1.
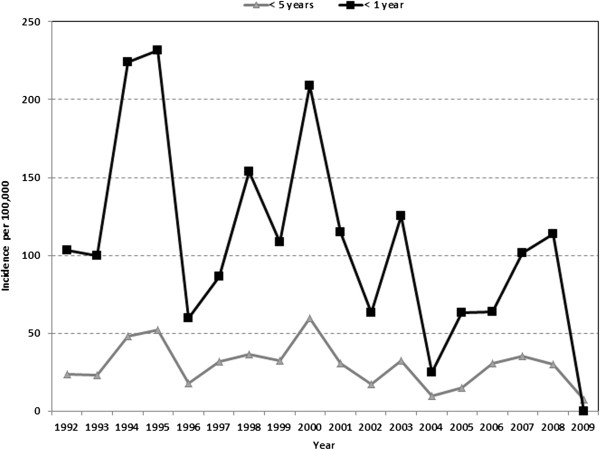
The incidence of intussusception (per 100,000) by age, in a northern region of Israel 1992–2009.
Figure 2.
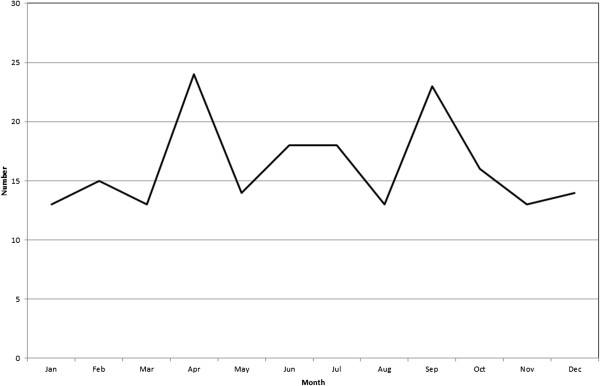
The number of intussusception episodes by month of admission in a northern region of Israel 1992–2009.
Table 1.
Distribution of cases and controls by year, season and sex
| |
Cases, n (%) |
Controls, n (%) |
Pv |
|---|---|---|---|
| (N = 190) | (N = 295) | ||
|
Year |
|
|
|
| 1992 |
7 (3.7) |
17 (5.8) |
0.45 |
| 1993 |
7 (3.7) |
9 (3.1) |
|
| 1994 |
15 (7.9) |
24 (8.1) |
|
| 1995 |
17 (8.9) |
17 (5.8) |
|
| 1996 |
6 (3.2) |
15 (5.1) |
|
| 1997 |
11 (5.8) |
15 (5.1) |
|
| 1998 |
12 (6.3) |
15 (5.1) |
|
| 1999 |
12 (6.3) |
25 (6.1) |
|
| 2000 |
22 (11.6) |
36 (12.2) |
|
| 2001 |
12 (6.3) |
20 (6.8) |
|
| 2002 |
7 (3.7) |
7 (2.4) |
|
| 2003 |
11 (5.8) |
24 (8.1) |
|
| 2004 |
4 (2.1) |
11 (3.7) |
|
| 2005 |
6 (3.2) |
14 (4.7) |
|
| 2006 |
12 (6.3) |
16 (5.4) |
|
| 2007 |
14 (7.4) |
19 (6.4) |
|
| 2008 |
12 (6.3) |
4 (1.4) |
|
| 2009 |
3 (1.6) |
4 (1.4) |
|
|
Season |
|
|
|
| Winter |
46 (24.2) |
74 (25.1) |
0.93 |
| Spring |
48 (25.3) |
73 (24.7) |
|
| Summer |
48 (25.3) |
68 (23.1) |
|
| Fall |
48 (25.3) |
80 (27.1) |
|
|
Sex |
|
|
|
| Males |
119 (62.6) |
176 (59.7) |
0.54 |
| Females |
71 (37.4) |
118 40.1) |
|
|
Ethnic group by hospital |
|
|
|
| Hillel Yaffe Medical Center |
|
|
|
| Arabs |
49 (49.0) |
56 (40.3) |
|
| Jews |
51 (51.0) |
83 (59.7) |
0.24 |
| Carmel Medical Center |
|
|
|
| Arabs |
27 (30.0) |
36 (23.2) |
0.18 |
| Jews | 63 (70.0) | 119 (76.8) |
Table 2.
Bivariate analysis of the risk factors for intussusception
| Cases | Controls | OR (95% CI) | Pv | |
|---|---|---|---|---|
|
Age (months), n (%) |
|
|
|
|
| < 3 |
10 (5.3) |
33 (11.2) |
Reference |
<0.001 |
| 3-5 |
48 (25.3) |
21 (7.1) |
7.54 (3.14-18.07) |
|
| 6-11 |
76 (40.0) |
88 (29.8) |
2.85 (1.31-6.16) |
0.008 |
| 12-23 |
44 (24.4) |
136 (46.1) |
1.06 (0.48-2.34) |
0.87 |
| 24-59 |
12 (6.3) |
17 (5.8) |
2.32 (0.83-6.48) |
0.10 |
| Paternal age (yrs), mean (SD)a |
34.7 (5.8) |
33.3 (5.6) |
1.04 (0.99-1.09) |
0.078 |
| Maternal age (yrs), mean (SD)a |
31.0 (5.1) |
29.6 (5.1) |
1.05 (1.00-1.11) |
0.045 |
| SES of residence town, n (%)a |
|
|
|
|
| Low |
66 (42.9) |
73 (29.4) |
2.29 (1.32-3.97) |
0.003 |
| Intermediate |
60 (39.0) |
104 (41.9) |
1.46 (0.85-2.51) |
0.16 |
| High |
28 (18.2) |
71 (28.6) |
Reference |
|
| Birth weight, n (%) |
|
|
|
|
| Birth weight < 2500 gra |
6 (4.2) |
14 (6.0) |
Reference |
0.44 |
| Birth weight ≥ 2500 gr |
138 (95.8) |
221 (94.0) |
1.45 (0.54-3.88) |
|
| Birth week, n (%) |
|
|
|
|
| Less than 37 weeks |
6 (4.3) |
10 (4.6) |
Reference |
0.86 |
| ≥ 37 weeks |
135 (95.7) |
206 (95.4) |
1.09 (0.38-3.07) |
|
| Gastroenteritis before admission, n (%) |
|
|
|
|
| Yes |
12 (6.3) |
1 (0.3) |
19.82 (2.55-153.72) |
<0.001 |
| No |
178 (93.7) |
294 (99.7) |
Reference |
|
| Breastfeeding < 1 yr, n (%) |
|
|
|
|
| Breastfed aged < 1 yr |
50 (37.3) |
40 (28.2) |
Reference |
0.10 |
| Not breasted children aged <1 yr |
84 (62.7) |
102 (71.8) |
0.65 (0.39-1.09) |
|
| Breastfeeding 1–4 yrs, n (%) |
|
|
|
|
| Breastfed children age 1-4 yrs |
9 (16.1) |
8 (5.2) |
Reference |
|
| Not breasted children age 1-4 yrs | 47 (83.9) | 145 (94.8) | 0.28 (0.10-0.78) | 0.011 |
aData on maternal and paternal age were available for 93 (47%) cases and 143 (48%) controls. Information on birth week was available for 141 (74%) cases and 216 (73%) controls, and on birth weight it was available for 144 (76%) cases and 235 (80%) controls. Information on SES of place of residence was available for 154 (81%) cases and 248 (84%) controls.
Table 3.
Multivariable logistic regression model of the risk factors for intussusception in children less than 5 years of age
| Adjusted OR (95% CI)* | Pv | |
|---|---|---|
|
Age (months) |
|
|
| <3 |
Reference |
|
| 3-5 |
5.30 (2.11-13.31) |
<0.001 |
| 6-11 |
2.53 (1.13-5.62) |
0.023 |
| 12-23 |
0.73 (0.32-1.65) |
0.4 |
| 24-59 |
2.01 (0.73-5.99) |
0.1 |
|
Socioeconomic rank of place of residence |
|
|
| Low |
2.81 (1.45-5.43) |
0.002 |
| Intermediate |
1.66 (0.87-3.16) |
0.1 |
| High |
Reference |
0.12 |
|
Gastroenteritis prior to hospitalization |
|
|
| No |
Reference |
|
| Yes | 19.90 (2.35-168.32) | 0.006 |
*Variables entered to the model at step 1: age, socioeconomic rank of place of residence, history of gastroenteritis, hospital, and breastfeeding. Data presented in the table are the final model which included the variables in the table and hospital.
Among patients with intussusception, the percentage of those who underwent surgery was significantly higher in infants, Arabs, residents of low socioeconomic status settings, in patients who had bloody stool and those admitted to Hillel Yaffe Medical Center (Table 4). In multivariable analysis the odds for surgery remained significantly 2-fold higher in patients who presented with bloody stool, in Arabs and in those who were admitted to Hillel Yaffe. Children who presented with diarrhea had lower odds for requiring surgery (Table 5).
Table 4.
Correlates of surgery among cases with intussusception
| Total | Surgery, n (%) | Pv | |
|---|---|---|---|
| Age |
|
|
|
| < 1 year |
134 |
36 (26.9) |
0.11 |
| 1-4 years |
56 |
9 (16.1) |
|
| Sex |
|
|
|
| Males |
119 |
33 (27.7) |
0.089 |
| Females |
71 |
12 (16.9) |
|
| Ethnic group |
|
|
|
| Arabs |
76 |
25 (32.9) |
0.015 |
| Jews |
114 |
20 (17.5) |
|
| Socioeconomic rank of residence place |
|
|
|
| Low |
66 |
21 (31.8) |
0.006 |
| Intermediate |
60 |
11 (18.3) |
|
| High |
28 |
2 (7.1) |
|
| Hospital |
|
|
|
| Hillel Yaffe Medical Center |
100 |
33 (33.0) |
0.001 |
| Carmel Medical Center |
90 |
12 (13.3) |
|
| Breastfeeding |
|
|
|
| Breastfed |
59 |
17 (28.8) |
0.26 |
| Not breasted |
131 |
28 (21.4) |
|
| Blood in the stool |
|
|
|
| No |
117 |
22 (18.8) |
0.045 |
| Yes |
73 |
23 (31.5) |
|
| Vomiting |
|
|
|
| No |
44 |
6 (13.6) |
0.074 |
| Yes |
146 |
39 (26.7) |
|
| Diarrhea |
|
|
|
| No |
154 |
41 (26.6) |
0.052 |
| Yes |
36 |
4 (11.1) |
|
| Irritability |
|
|
|
| No |
59 |
17 (28.8) |
0.26 |
| Yes | 131 | 28 (21.4) |
Table 5.
Multivariable analysis of factors associated with surgery among patients with intussusception
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | Pv | |
|---|---|---|---|
| Hillel Yaffe Medical Center vs. Carmel Medical Center |
3.20 (1.53-6.69) |
4.42 (1.70-11.46) |
0.002 |
| Arabs vs. Jews |
2.30 (1.16-4.54) |
2.32 (0.99-5.45) |
0.05 |
| Blood in the stool (yes vs. no) |
1.98 (1.00-3.91) |
2.78 (1.17-6.60) |
0.021 |
| Diarrhea (yes vs. no) | 0.34 (0.11-1.03) | 0.22 (0.05-1.1) | 0.06 |
*In addition to the variables in the table, the variables socioeconomic status of place of residence, age, sex, and interaction between ethnicity and hospital were added to the analysis. The adjusted ORs and Pv presented in the table were obtained from the final model.
Discussion
We estimated the incidence and correlates of intussusception in northern Israel over 18 year period from 1992–2009, prior the introduction of universal rotavirus immunization in Israel.
The estimated mean annual incidence of intussusception among infants, 105.6 (95% CI 52.9-210.7) per 100,000 in this study, is slightly higher than the reported incidence in Europe [33-35], and about 2–3 fold higher than rates reported in the United States [7,36]. The incidence of intussusception in infants in this region of northern Israel decreased over time, in concert with previous studies [7,34,36,37].
The incidence of intussusception was higher among Jewish than Arab children, in agreement with an earlier study in southern Israel [10], where the incidence among Jewish and Bedouin children less than five year of age was estimated at 49.3 and 18.9 per 100,000, respectively, and for infants 199.6 and 66.8 per 100,000, respectively [10]. Ethnic differences in the incidence of intussusception have been described before [7,9,36-38]. Higher risk of intussusception has been noted among black and Hispanic children vs white, non-Hispanic children in the United States [7,36,38]. In Australia and New Zealand lower incidence of intussusception was observed in indigenous infants compared to non-indigenous infants [37], and specifically, among Maori compared with European infants, respectively [9].
About two thirds of intussusception cases occurred in infants 3–11 months of age; the risk of intussusception increased substantially by 5 fold in children aged 3–5 months compared to younger infants while children aged 6–11 months had about 2-fold increased risk of intussusception compared to the youngest age group (<3 months). Increased risk of intussusception was found more among children who lived in low socioeconomic communities than among those who lived high socioeconomic settings. A previous study from the United States showed that infants enrolled in Medicaid, used as a marker for low socioeconomic status, had 1.5 fold increased risk of intussusception [39]. It is not clear what underlying mechanisms might explain the association of intussusception and socioeconomic strata, but it is possible that genetic, environmental and cultural exposures including exposure to enteric pathogens and child nutritional practices [3] may play a role. In this study, recent history of gastroenteritis was associated with increased the risk of intussusception, and similar findings have been shown in other studies [11,12]. However, it is possible that our findings overestimate such an association, since physicians may have questioned parents of control children less intensively than intussusception patients’ parents on a recent history of gastroenteritis.
The common clinical symptoms of intussusception were similar to those reported previously; it is worth mentioning that visible (macroscopic) blood in stool was documented in only 39% of cases, and it appeared more than twofold in infants compared with toddlers. Irritability was also common, reported in 69%. This is probably due to the fact that infants and young children lack the ability to express pain verbally. These findings suggest that suspecting intussusception in children presenting with “atypical” symptoms is warranted.
The median hospital stay was 2 days, but reached 12 days in some cases. Usually, reduction with conservative treatment like air or barium enema was successful, but surgery was required in about 1/4 of intussusception patients. The percentage of intussusception patients undergoing surgery varied widely in previous studies - from 12% to 88% [3]. In our study, children who presented with bloody stool, Arabs, and those who were admitted to Hillel Yaffe Medical Center were more likely to undergo surgery. Despite the lower incidence of intussusception among Arab children, they underwent surgery about twice as often as did Jewish children. Interestingly a previous study from southern Israel also showed that Bedouin children with intussusception were more likely to undergo surgery than their Jewish peers [10]. However, in the southern Israel, about 50% of the Bedouin population lives in remote villages so that limited access to primary health care might explain why Bedouin children require surgery more often. This is not the case in northern Israel. In the study area, despite the fact that Arab residents live mostly in separate towns and villages, all have a basic infrastructure similar to that of Jewish communities, including on-site primary care clinics run by the main health maintenance organizations. Furthermore, in 1995 the National Health Insurance law was implemented in Israel, resulting in near uniform access to health care, preventive, ambulatory and inpatient services, thus minimizing disparities between Arab and Jewish populations. In the United States bowel resection was significantly increased in patients who had the symptoms for 2 days or more before admission compared to those who were admitted earlier [38]. Therefore, taking into account the characteristics of the study communities we postulate that admission may be delayed in some Arab patients with intussusception. This may be due to different referral behaviors of community physicians in Arab towns, parental perception of intussusception symptoms as non-serious (e.g., mistakenly confused with gastroenteritis), or both. If this hypothesis is proven true, the potential exists to reduce the need for intussusception surgery, especially in Arab children, by educating parents on when to seek medical care for young children with possible intussusception, and when pediatric caregivers in community practice should refer children to hospital for suspected intussusception. This finding may be relevant to countries with multiple ethnicities as well. Since mostly Arab physicians work in clinics in the Arab towns and villages, there is a possibility of a combined doctor-patient ethnic effect upon the decision whether or not to refer patients to hospital for further evaluation. Since the incidence of intussusception in Arab children is lower than that found among Jewish children, seeking medical attention might be delayed for intussusception with mild symptoms which might, in some cases, resolve spontaneously. This could lead to higher risk estimates for surgery among Arab vs. Jewish children. Therefore, the average incidence of intussusception associated with surgery was calculated, and was found to be higher among Arab vs. Jewish children (7.50 vs. 6.83 per 100,000 children less five years of age, but this difference was not significant).
The review of hospital records over an 18 year period from 2 hospitals in northern Israel yielded robust estimates of the incidence of intussusception, its clinical symptoms and treatment strategies prior the introduction of universal rotavirus immunization in Israel. The diagnosis of intussusception relied on radiological and/or sonographic findings throughout the study period in both medical centers. A case–control design was utilized to obtain insight in to the correlates of intussusception. These can be regarded as strengths of the study. Yet the study has some notable weaknesses: variability in obtaining clinical history probably occurred over time and among pediatricians. Hospital controls may not be the optimal control group, yet these groups were from the same source population and were comparable in terms of sex, study period, geographic region and ethnicity.
Conclusions
We documented a relatively low incidence of pediatric intussusception prior the introduction of universal rotavirus immunization in Israel, but higher than that found in European and US children. Although incidence was lower among Arabs than Jews, the former group was more likely to undergo surgery, suggesting the possibility of delayed admission of Arab patients to hospital resulting from specific referral patterns of physicians and/or health care seeking behaviors of parents. These findings have public health and clinical implications.
Abbreviations
CI: Confidence intervals; ICD-9: International classification of disease -9th edition; SD: Standard deviation; OR: Odds ratio.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KM, ME, EK and DC contributed to the conception and design of the study. SE, SG, EK and ME made substantial contribution in data acquisition and analysis, and together with KM and DC they interpreted the study findings. KM and EK wrote the first draft of the manuscript, and DC and ME have been involved in significantly in critical revision of the article. All authors approved the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
STROBE Statement—Checklist of items that should be included in reports of case-control studies.
Contributor Information
Khitam Muhsen, Email: khitam15@yahoo.com.
Eias Kassem, Email: eias.kassem@gmail.com.
Sigalit Efraim, Email: sigalefr@walla.com.
Sophy Goren, Email: sophyg@post.tau.ac.il.
Dani Cohen, Email: dancohen@post.tau.ac.il.
Moshe Ephros, Email: mefrat@tx.technion.ac.il.
Acknowledgement
We thank the secretaries at the archives departments at Hillel Yaffe and Carmel medical centres for the assistance in retrieving the medical records.
References
- Bines J, Ivanoff B. Acute Intussusception in Infants and Children: Incidence, Clinical Presentation and Management: A Global Perspective. Geneva: World Health Organization; 2002. [Google Scholar]
- Pepper VK, Stanfill AB, Pearl RH. Diagnosis and management of pediatric appendicitis, intussusception, and Meckel diverticulum. Surg Clin North Am. 2012;92:505–526. doi: 10.1016/j.suc.2012.03.011. vii. [DOI] [PubMed] [Google Scholar]
- Bines JE, Patel M, Parashar U. Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis. 2009;200(Suppl 1):S282–S290. doi: 10.1086/605051. [DOI] [PubMed] [Google Scholar]
- Huppertz HI, Soriano-Gabarro M, Grimprel E, Franco E, Mezner Z, Desselberger U, Smit Y, Wolleswinkel van den Bosch J, De Vos B, Giaquinto C. Intussusception among young children in Europe. Pediatr Infect Dis J. 2006;25:S22–S29. doi: 10.1097/01.inf.0000197713.32880.46. [DOI] [PubMed] [Google Scholar]
- Mandeville K, Chien M, Willyerd FA, Mandell G, Hostetler MA, Bulloch B. Intussusception: clinical presentations and imaging characteristics. Pediatr Emerg Care. 2012;28:842–844. doi: 10.1097/PEC.0b013e318267a75e. [DOI] [PubMed] [Google Scholar]
- Samad L, Marven S, El Bashir H, Sutcliffe AG, Cameron JC, Lynn R, Taylor B. Prospective surveillance study of the management of intussusception in UK and Irish infants. British J Surg. 2012;99:411–415. doi: 10.1002/bjs.7821. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Holman RC, Cummings KC, Staggs NW, Curns AT, Zimmerman CM, Kaufman SF, Lewis JE, Vugia DJ, Powell KE, Glass RI. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics. 2000;106:1413–1421. doi: 10.1542/peds.106.6.1413. [DOI] [PubMed] [Google Scholar]
- O’Ryan M, Lucero Y, Pena A, Valenzuela MT. Two year review of intestinal intussusception in six large public hospitals of Santiago, Chile. Pediatr Infect Dis J. 2003;22:717–721. doi: 10.1097/01.inf.0000078374.82903.e8. [DOI] [PubMed] [Google Scholar]
- Chen YE, Beasley S, Grimwood K. New Zealand Rotavirus Study Group. Intussusception and rotavirus associated hospitalisation in New Zealand. Arch Dis Childhood. 2005;90:1077–1081. doi: 10.1136/adc.2005.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D, Givon-Lavi N, Newman N, Wheeler J, Cohen Z, Dagan R. Intussusception in children in Southern Israel: disparity between 2 populations. Pediatr Infect Dis J. 2008;27:236–240. doi: 10.1097/INF.0b013e31815bb6b1. [DOI] [PubMed] [Google Scholar]
- Mansour AM, ElKoutby M, El Barbary MM, Mohamed W, Shehata S, El Mohammady H, Mostafa M, Riddle MS, Sebeny PJ, Young SYN, Abdel-Messih I. Enteric viral infections as potential risk factors for intussusception. J Infect Dev Ctries. 2013;7:28–35. doi: 10.3855/jidc.2321. [DOI] [PubMed] [Google Scholar]
- Nylund CM, Denson LA, Noel JML. Bacterial enteritis as a risk factor for childhood. Intussusception: a retrospective cohort study (vol 156, pg 761, 2010) J Pediatric. 2010;157:696–696. doi: 10.1016/j.jpeds.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Lappalainen S, Ylitalo S, Arola A, Halkosalo A, Rasanen S, Vesikari T. Simultaneous presence of human herpesvirus 6 and adenovirus infections in intestinal intussusception of young children. Acta Paediatrica. 2012;101:663–670. doi: 10.1111/j.1651-2227.2012.02616.x. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Kao CL, Huang LM, Ni YH, Lai HS, Lin FY, Chang MH. Viral etiology of intussusception in Taiwanese childhood. Pediatr Infect Dis J. 1998;17:893–898. doi: 10.1097/00006454-199810000-00009. [DOI] [PubMed] [Google Scholar]
- Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, De Campo M, Barnett P, Bishop RF, Robins-Browne R, Carlin JB. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatric. 2006;149:452–460. doi: 10.1016/j.jpeds.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Luna G, Cedillo R, Torres J, Munoz O. Natural rotavirus infection is not associated to intussusception in Mexican children. Pediatr Infect Dis J. 2004;23:S173–S178. doi: 10.1097/01.inf.0000142467.50724.de. [DOI] [PubMed] [Google Scholar]
- Chang EJ, Zangwill KM, Lee H, Ward JI. Lack of association between rotavirus infection and intussusception: implications for use of attenuated rotavirus vaccines. Pediatr Infect Dis J. 2002;21:97–102. doi: 10.1097/00006454-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. JAMA. 1999;282:2113–2114. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X. et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, Cortese M, Braun MM, Belongia EA, Miller E, Ball R, Iskander J, Parashar UD. et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008;121:1206–1212. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, Hambidge SJ, Glanz JM, Klein NP, Weintraub E. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012;307:598–604. doi: 10.1001/jama.2012.97. [DOI] [PubMed] [Google Scholar]
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE, Grp PAS. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29:3061–3066. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Marquez AB, Flannery B, Esparza-Aguilar M, Renoiner EIM, Luna-Cruz ME, Sato HK, Hernández-Hernández Ldel C, Toledo-Cortina G, Cerón-Rodríguez M, Osnaya-Romero N, Martínez-Alcazar M, Aguinaga-Villasenor RG, Plascencia-Hernández A, Fojaco-González F, Hernández-Peredo Rezk G, Gutierrez-Ramírez SF, Dorame-Castillo R, Tinajero-Pizano R, Mercado-Villegas B, Barbosa MR, Maluf EM, Ferreira LB, De Carvalho FM, Dos Santos AR, Cesar ED, De Oliveira ME. et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. New Eng J Med. 2011;364:2283–2292. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, Breuer T, Verstraeten T. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31:736–744. doi: 10.1097/INF.0b013e318253add3. [DOI] [PubMed] [Google Scholar]
- Tate JE, Steele AD, Bines JE, Zuber PLF, Parashar UD. Research priorities regarding rotavirus vaccine and intussusception: a meeting summary. Vaccine. 2012;30:A179–A184. doi: 10.1016/j.vaccine.2011.08.110. [DOI] [PubMed] [Google Scholar]
- Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- Vesikari T, Van Damme P, Giaquinto C, Gray J, Mrukowicz J, Dagan R, Guarino A, Szajewska H, Usonis V. Expert Working Group. European Society for Paediatric Infectious Diseases/European Society for Paediatric Gastroenterology, Hepatology, and Nutrition evidence-based recommendations for rotavirus vaccination in Europe: executive summary. J Pediatr Gastroenterol Nutr. 2008;46:615–618. doi: 10.1097/MPG.0b013e31816e213a. [DOI] [PubMed] [Google Scholar]
- Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D. TAU-HCLV Rota Study Group. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children <5 years of age, 2007–2008. J Infect Dis. 2009;200(Suppl 1):S254–S263. doi: 10.1086/605425. [DOI] [PubMed] [Google Scholar]
- Israel Central Bureau of Statistics. Characterization and Classification of Local Authorities by the Socio-Economic Level of the Population 2001. Jerusalem: Israel; 2004. [Google Scholar]
- Gay N, Ramsay M, Waight P. Rotavirus vaccination and intussusception. Lancet. 1999;354:956–956. doi: 10.1016/S0140-6736(05)75710-X. [DOI] [PubMed] [Google Scholar]
- Fischer TK, Bihrmann K, Perch M, Koch A, Wohlfahrt J, Kare M, Melbye M. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics. 2004;114:782–785. doi: 10.1542/peds.2004-0390. [DOI] [PubMed] [Google Scholar]
- Kohl LJ, Streng A, Grote V, Koletzko S, Liese JG. Intussusception-associated hospitalisations in Southern Germany. Eur J Pediatric. 2010;169:1487–1493. doi: 10.1007/s00431-010-1248-x. [DOI] [PubMed] [Google Scholar]
- Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, Parashar UD. Trends in intussusception hospitalizations among US infants, 1993–2004: Implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121:E1125–E1132. doi: 10.1542/peds.2007-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. J Paediatr Child Health. 2005;41:475–478. doi: 10.1111/j.1440-1754.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- Johnson B, Gargiullo P, Murphy TV, Parashar UD, Patel MM. Factors associated with bowel resection among infants with intussusception in the United States. Pediatr Emerg Care. 2012;28:529–532. doi: 10.1097/PEC.0b013e3182587d12. [DOI] [PubMed] [Google Scholar]
- Johnson B, Gargiullo P, Murphy TV, Parashar UD, Patel MM. Sociodemographic and dietary risk factors for natural infant intussusception in the United States. J Pediatr Gastroenterol Nutr. 2010;51:458–463. doi: 10.1097/MPG.0b013e3181d3273f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE Statement—Checklist of items that should be included in reports of case-control studies.


