Abstract
The study of the Ig variable region heavy chain (VH) genes used to encode antibodies specific for self-epitopes from murine hybridomas showed that three VH families are primarily utilized: VH J558, the largest family, and VH QPC52 and VH 7183, the families most proximal to the Ig joining region heavy chain genes. These monoclonal autoantibodies express cross-reactive idiotopes shared by rheumatoid factors and antibodies specific for Sm. The expression of these idiotypes is independent of major histocompatibility complex and Ig constant region heavy chain haplotypes, self-antigen specificity, and even the VH gene family utilized. Though the experiments described here are limited to murine autoantibodies, similarities exist between murine and human autoimmune diseases. Studies that aim to investigate the relationship between VH gene expression and the presence of cross-reactive idiotypes among human autoantibodies should enable us to better understand the mechanisms of autoimmunity and self-tolerance.
Full text
PDF

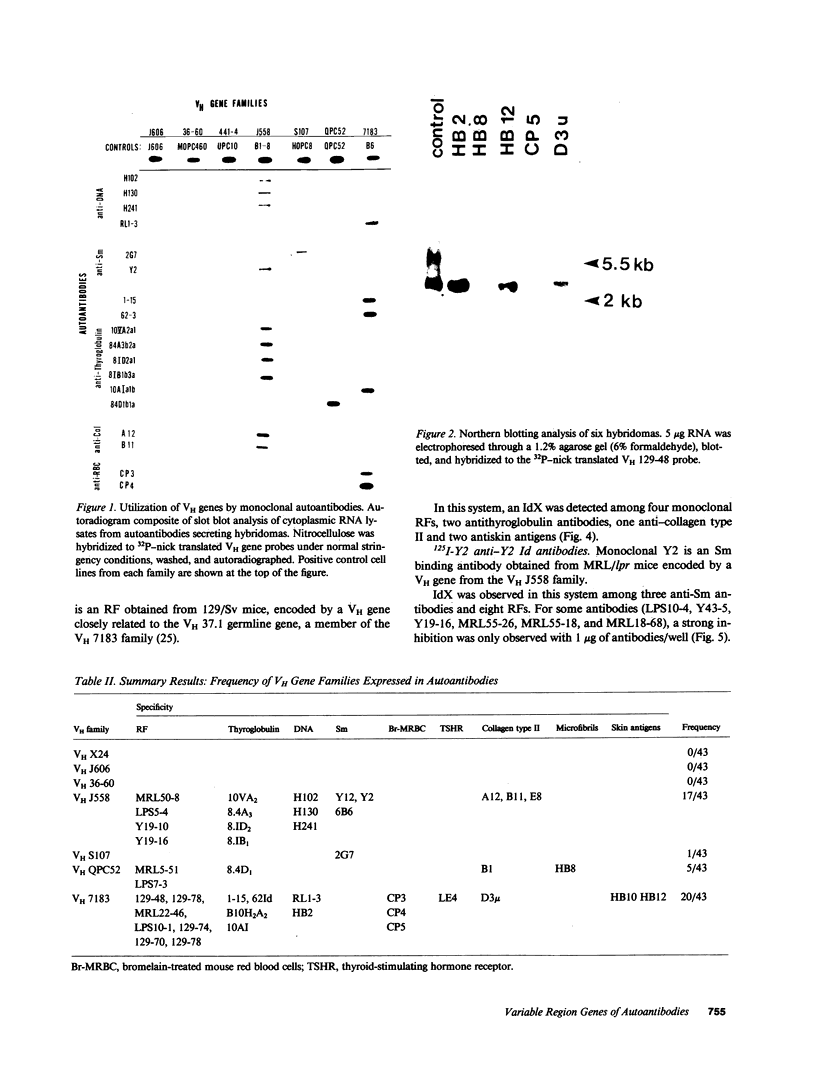
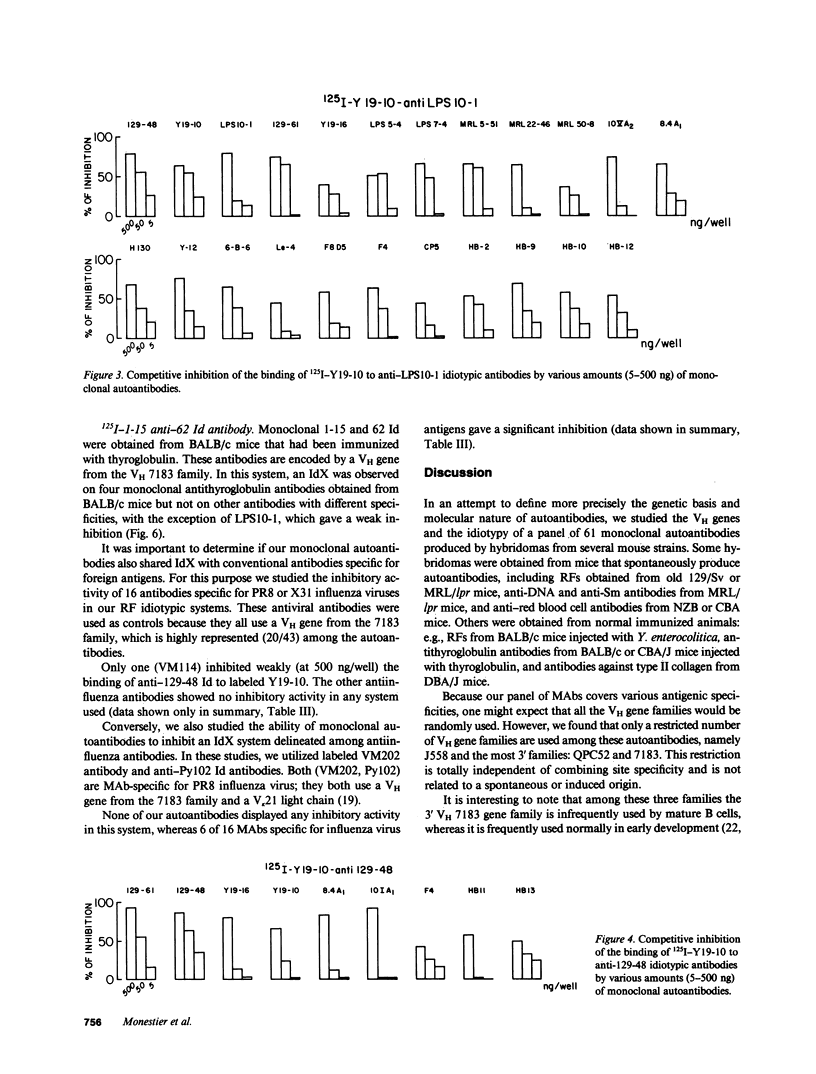
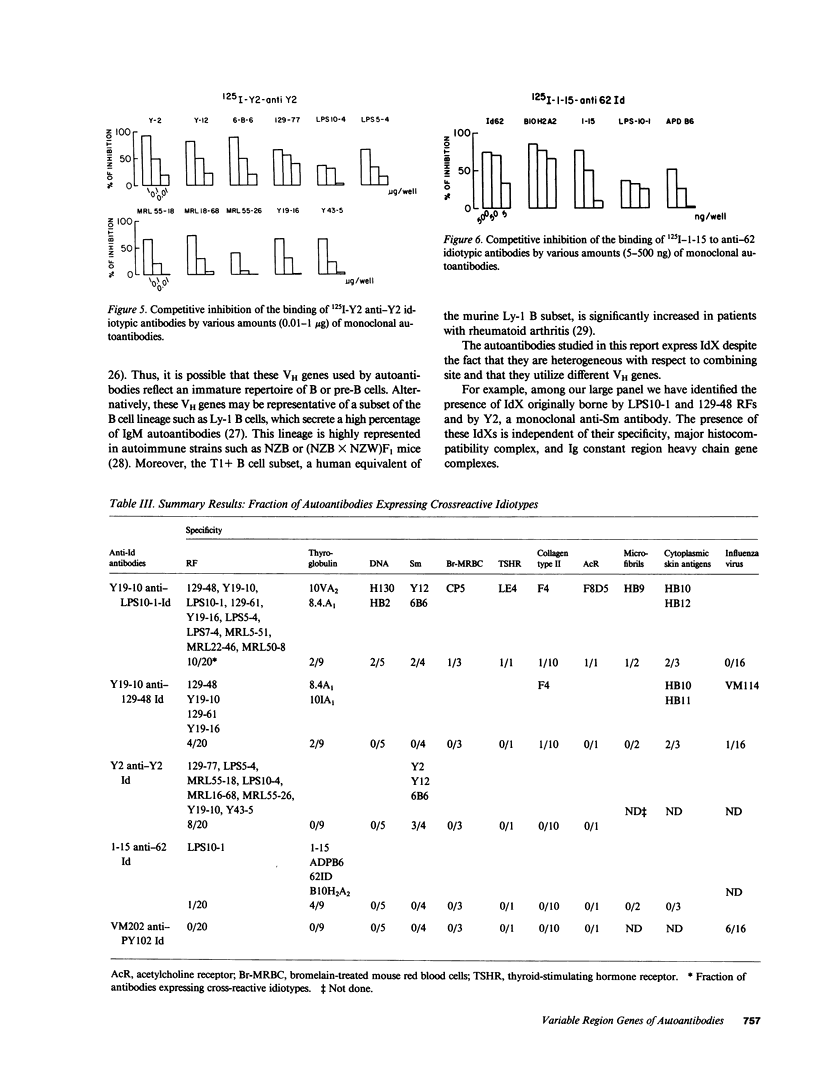


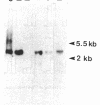
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. W., Capra J. D. Complete amino acid sequence of variable domains from two monoclonal human anti-gamma globulins of the Wa cross-idiotypic group: suggestion that the J segments are involved in the structural correlate of the idiotype. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3799–3803. doi: 10.1073/pnas.78.6.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Fong S., Normansell D., Houghten R. A., Karras J. G., Vaughan J. H., Carson D. A. Delineation of a cross-reactive idiotype on human autoantibodies with antibody against a synthetic peptide. J Exp Med. 1984 May 1;159(5):1502–1511. doi: 10.1084/jem.159.5.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland W. L., Wassermann N. H., Sarangarajan R., Penn A. S., Erlanger B. F. Monoclonal antibodies to the acetylcholine receptor by a normally functioning auto-anti-idiotypic mechanism. Nature. 1983 Sep 1;305(5929):56–57. doi: 10.1038/305056a0. [DOI] [PubMed] [Google Scholar]
- Dang H., Fischbach M., Talal N. Anti-idiotypic antiserum to monoclonal anti-Sm inhibits the autoantigen-induced proliferative response. J Immunol. 1985 Jun;134(6):3825–3830. [PubMed] [Google Scholar]
- Dawson J. F., Brochier J., Schmitt D., Saeland S., Thivolet J. Elastic fibres: histological correlation with orcein and a new monoclonal antibody, HB8. Br J Dermatol. 1984 May;110(5):539–546. doi: 10.1111/j.1365-2133.1984.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. S., Bradley R. J., Urquhart C. K., Kearney J. F. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature. 1983 Feb 17;301(5901):611–614. doi: 10.1038/301611a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. A public idiotypic determinant is present on spontaneous cationic IgG antibodies to DNA from mice of unrelated lupus-prone strains. J Immunol. 1984 Dec;133(6):3015–3019. [PubMed] [Google Scholar]
- Halpern R., Davidson A., Lazo A., Solomon G., Lahita R., Diamond B. Familial systemic lupus erythematosus. Presence of a cross-reactive idiotype in healthy family members. J Clin Invest. 1985 Aug;76(2):731–736. doi: 10.1172/JCI112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Leo O., Slaoui M., Marvel J., Milner E. C., Hiernaux J., Moser M., Capra J. D., Urbain J. Idiotypic analysis of polyclonal and monoclonal anti-p-azophenylarsonate antibodies of BALB/c mice expressing the major cross-reactive idiotype of the A/J strain. J Immunol. 1985 Mar;134(3):1734–1739. [PubMed] [Google Scholar]
- Lymberi P., Dighiero G., Ternynck T., Avrameas S. A high incidence of cross-reactive idiotypes among murine natural autoantibodies. Eur J Immunol. 1985 Jul;15(7):702–707. doi: 10.1002/eji.1830150712. [DOI] [PubMed] [Google Scholar]
- McCoy J. P., Jr, Michaelson J. H., Bigazzi P. E. Anti-idiotypic immunity and autoimmunity: III. Investigations in human autoimmune thyroiditis. Life Sci. 1983 Jan 3;32(1-2):109–118. doi: 10.1016/0024-3205(83)90178-9. [DOI] [PubMed] [Google Scholar]
- Monier J. C., Brochier J., Moreira A., Sault C., Roux B. Generation of hybridoma antibodies to double-stranded DNA from non-autoimmune BALB/c strain: studies on anti-idiotype. Immunol Lett. 1984;8(2):61–68. doi: 10.1016/0165-2478(84)90051-8. [DOI] [PubMed] [Google Scholar]
- Moran T. M., Reale M. A., Monestier M., Mayer R., Schulman J. L., Bona C. A. Idiotypy of anti-influenza virus immune responses. Concepts Immunopathol. 1986;3:233–252. [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Lerner E. A. Idiotypic analysis of a monoclonal anti-Sm antibody. J Immunol. 1982 Oct;129(4):1489–1492. [PubMed] [Google Scholar]
- Plater-Zyberk C., Maini R. N., Lam K., Kennedy T. D., Janossy G. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 1985 Sep;28(9):971–976. doi: 10.1002/art.1780280903. [DOI] [PubMed] [Google Scholar]
- Poncet P., Kocher H. P., Pages J., Jaton J. C., Bussard A. E. Monoclonal autoantibodies against mouse red blood cells: a family of structurally restricted molecules. Mol Immunol. 1985 May;22(5):541–551. doi: 10.1016/0161-5890(85)90177-4. [DOI] [PubMed] [Google Scholar]
- Rauch J., Murphy E., Roths J. B., Stollar B. D., Schwartz R. S. A high frequency idiotypic marker of anti-DNA autoantibodies in MRL-Ipr/Ipr mice. J Immunol. 1982 Jul;129(1):236–241. [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Schwartz R. S. Immunologic and genetic factors in autoimmune diseases. N Engl J Med. 1984 Oct 18;311(16):1019–1029. doi: 10.1056/NEJM198410183111605. [DOI] [PubMed] [Google Scholar]
- Talal N. Autoimmunity and the immunologic network. Arthritis Rheum. 1978 Sep-Oct;21(7):853–861. doi: 10.1002/art.1780210719. [DOI] [PubMed] [Google Scholar]
- Victor-Kobrin C., Manser T., Moran T. M., Imanishi-Kari T., Gefter M., Bona C. A. Shared idiotopes among antibodies encoded by heavy-chain variable region (VH) gene members of the J558 VH family as basis for cross-reactive regulation of clones with different antigen specificity. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7696–7700. doi: 10.1073/pnas.82.22.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Zanetti M., De Baets M., Rogers J. High degree of idiotypic cross-reactivity among murine monoclonal antibodies to thyroglobulin. J Immunol. 1983 Nov;131(5):2452–2457. [PubMed] [Google Scholar]