Abstract
Background:
Patients with cancers of the upper aerodigestive tract (head and neck cancer (HNC)) tend to aspirate, either due to disease or treatment. The association of aspiration (documented on video fluorography (VFG)) with quality of life (QOL) and unexpected mortality was studied prospectively in patients treated with simultaneous integrated boost technique of intensity-modulated radiotherapy (SIB-IMRT).
Materials and Methods:
Moderately advanced (stage III/IV) HNC were treated by SIB-IMRT delivering 66 Gy/30 fr, 60 Gy/30 fr, and 54 Gy/30 fr to high, intermediate, and low risk volumes, respectively. They underwent serial VFG and QOL assessments (Quality of Life Questionnaire-Core 30 (QLQ-C30) and head and neck-35 (HN35) European Organisation for Research and Treatment of Cancer (EORTC) tools) at 0, 3, and 6 months. Pharyngeal musculature (PM) was additionally delineated on planning computed tomography (CT) scans as potential organs at risk (OARs).
Results:
Between November 2009 and May 2011, 20 HNC were treated as per protocol. All patients were fit (Karnofsky performance status (KPS) ≥ 80). Based on VFG findings, seven patients (4/9 oropharynx and 3/11 laryngopharynx) were grouped as aspirators (A) and remaining 13 as non-aspirators (NA). The QOL study showed that pretreatment coughing and swallowing difficulties were greater in group A versus NA and remained persistently higher. In group A, deaths attributable to aspiration were seen in 3/7 patients, while none occurred in the NA group (Fisher's exact P = 0.03). The mean PM dose was 60 Gy in both the groups and mean V60 was similar at 69 and 67% in A and NA groups, respectively.
Conclusions:
VFG helps identify patients who aspirate and are at risk of premature death due to its complications, alerting caregivers to direct attention appropriately.
Keywords: Aspiration, head and neck cancer, intensity-modulated radiotherapy, radiotherapy
Introduction
Patients with head and neck cancers (HNCs) aspirate oral contents into the airway, consequent to the loss of functional integrity of epiglottis and pharyngeal musculature (PM).[1] Apart from the disease, radiation-induced fibrosis of PM and dysfunction of cartilaginous and muscular laryngeal framework are believed for swallowing abnormalities and aspiration.[2] Aspiration may be silent and unrecognized or symptomatic, leading to recurrent pneumonitis, weight loss, failure to thrive, and even death.
Simultaneous integrated boost technique of intensity-modulated radiotherapy (SIB-IMRT) is a technique that can deliver moderately accelerated variable dose per fraction RT to clinical target volume (CTV) of different sites, including HNC.[3,4] Video fluorography (VFG) is a radiological tool to assess real time movement and coordination between swallowing structures by following the flow of radioopaque ingested bolus. In this study, VFG was used to categorize patients as aspirators (A) and non-aspirators (NA). The association of PM radiation dose on the probability of aspiration, QOL, and unexpected mortality between the groups were studied.
Materials and Methods
This study is approved by institutional review board.
Inclusion and exclusion criteria
Between November 2009 and May 2011, 20 patients with biopsy-proven stage III and IV (T3N0/T1-2 N1-2, lymph node ≤ 4 cm) oropharyngeal (including lingual surface of epiglottis), laryngeal (including laryngeal surface of epiglottis), or hypopharyngeal primaries received SIB-IMRT. Patients underwent complete clinical examination, hemogram, serum biochemistry, chest radiograph, contrast-enhanced CT (CECT) scan of face and neck, and dental prophylaxis. All patients were fit, that is, Karnofsky performance status (KPS) ≥ 80. A planned prospective collection of VFG data, swallowing- and cough-specific QOL (using Quality of Life Questionnaire-Core 30 (QLQ-C30) and head and neck-35 (HN35) European Organisation for Research and Treatment of Cancer (EORTC) tools) was undertaken at baseline (pretreatment) and at 3 and 6 months posttreatment.
Gross target volume (GTV) was outlined for each patient based on visible tumor and information from diagnostic CT images and endoscopic examination. The high risk clinical target volume (CTV_66) included GTV and involved lymph nodes along with minimum 5 mm margin to account for microscopic tumor spread, truncated at bone and air. The intermediate risk CTV, CTV_60, was defined as a margin around the CTV_66 that covered the entire compartment, likely to harbor the disease and adjacent nodal levels. The low risk CTV, CTV_54, encompassed nodal regions likely to have subclinical disease. Lymph node areas were defined according to the international guidelines.[5,6] PTVs were generated by adding a margin of 5 mm to CTVs. PTVs was edited to ensure that they did not come within 3 mm of the skin.
Besides the parotid glands and the spinal cord, the superior pharyngeal constrictor (SC) muscle, middle pharyngeal constrictor (MC) muscle, and inferior pharyngeal constrictor (IC) muscle were contoured as per the guidelines.[7]
Interventions
The Helios inverse planning module in Eclipse was used for all IMRT planning. A 5-7 field technique was used to cover the three PTV's in continuity. Treatment was delivered once daily, 5 fractions per week, over 6 weeks. The dose objectives to the high (PTV_66), intermediate (PTV_60), and low risk (PTV_54) volumes were 66 Gy/30 fr, 60 Gy/30 fr, and 54 Gy/30 fr, respectively. Following dose constraints were set on the organs at risk (OARs): Spinal cord-maximum dose 48 Gy, brain stem-maximum dose 55 Gy, and contralateral parotid-mean dose ≤ 26 Gy. Doses to PM (V50 and V60) were recorded, but not constrained by the IMRT process and recorded using dose-volume histograms (DVHs) for both PTV and OARs.
Subjective assessment of swallowing dysfunction consisted of the EORTC QLQ-HN35-item HNSW (consisting of four questions regarding swallowing of liquid, swallowing of pureed food, swallowing of solid food, or aspiration when swallowing) and objective evaluation of swallowing dysfunction was done by VFG at specified time points, while the assessment of cough and general quality of life (QOL) was done by using EORTC QLQ-HN35-item HNCO and EORTC QLQ-C30 (version 3) item global health status, respectively at specified time points.
Statistical analysis
The significance of mean differences of various parameters between the two groups (A and NA) was calculated using independent samples t-test. Spearman's rank correlation was used to explore the correlation between various parameters. The odds ratio of various parameters was calculated using Fisher's exact test. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS 2006; SPSS Inc, Chicago, IL) v 16.0.
Results
Patient and tumor characteristics are summarized in Table 1. There were 19 males, with a median age of 59 years (range, 38-75 years) at the time of diagnosis. The median follow-up was 8 months. All patients were fit (KPS ≥ 80). Patients were staged as per the American Joint Committee on Cancer (AJCC) TNM 2007 staging system.
Table 1.
Patient and tumor characteristics

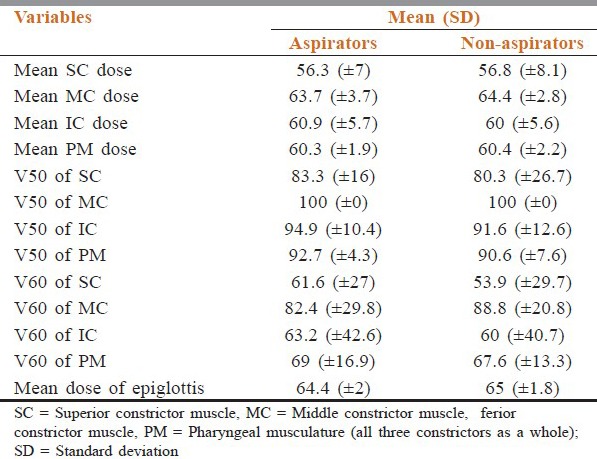
PTV coverage was adequate in all patients, that is, 99% of the PTV1 was covered by ≥ 90% of the prescribed isodose line (range 93-98%). Five patients (25%) needed nasogastric tube feeding during treatment. At baseline, six patients (three oropharynx and three larynx) were aspirating as detected by pretreatment VFG. In the posttreatment VFG at 3 months, one additional patient with oropharyngeal primary was documented as having aspiration (n = 7/20). Therefore, the site wise, 4/9 were from oropharynx and 3/11 was from larynx-hypopharynx (P = 0.64). No significant difference was observed between mean, V50, V60, or epiglottis doses between A and NA groups [Table 2].
Table 2.
Comparison of dose received
QOL scores are listed in Table 3. QOL study showed that baseline cough and swallowing difficulties were subjectively greater in the group A versus group NA. Though the mean differences were not statistically significant (P = 0.216 and 0.183, respectively). QOL assessment during follow-up showed that the symptoms of cough and swallowing difficulty were persistently higher in group A. No significant correlation was found between the mean dose and V60 of the PM with the swallowing problems and cough at 3 months posttreatment.
Table 3.
Comparison of QOL score
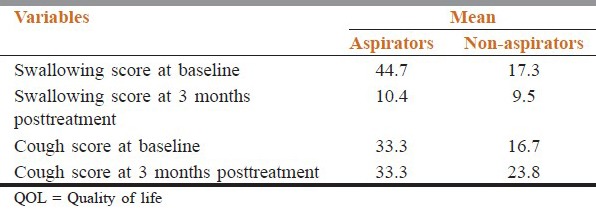
Assessment of general QOL by the QLQ-C30 item global health status showed that both at baseline and 3 months posttreatment, there is no significant difference between the groups A versus NA. Similarly, no significant correlation was found between the mean dose, V50, and V60 of the PM with the global health status at 3 months posttreatment.
Two of these seven died within 1 month due to aspiration pneumonia, while one died in an intensive care unit (ICU) elsewhere, reportedly due to sudden aspiration. One patient in the NA group died following brain metastasis within 3 months of treatment. Baseline diagnostic CECT scan revealed that nine (45%) patients had radiological evidence of epiglottis involvement, of which five had oropharyngeal and the rest had larynx-hypopharynx primary. Overall of these nine patients, six were documented as A in the VFG results.
Discussion
HNC are at a high risk of developing dysphagia and aspiration even prior to treatment. Aspiration was prevalent in one-third of cases at presentation. The incidence appears to be similar to what is reported by other studies.[7,8,9] The location and stage of tumor are considered to be important risk factors for aspiration.[8,9,10] Involvement of epiglottis (as seen on pretreatment CT scan) was probably the key structure in causation of aspiration in our series.
Documentation of swallowing abnormalities and aspiration by VFG were initially studied by Eisbruch et al.[1] We followed a similar protocol as described by them. Clinical prediction of aspiration has been shown to be notoriously inaccurate. In a prospective study by Rosen et al.,[7] it was observed that experienced clinicians such as otolaryngologist and speech pathologist could correctly predict aspiration in six out of the 11 patients who actually aspirated on VFG. Additionally, Langerman et al.,[11] reported that three-fourth patients who aspirated on VFG did not have any symptoms related to aspiration (silent aspirations, i.e., absence of cough reflex), further highlighting the importance of routine swallowing assessment in patients of HNC.
We had earlier reported our preliminary experience on VFG changes in HNC patients after RT.[12] Incomplete epiglottic closures resulting in silent aspirations were documented. In that study, patients were treated by chemo-RT (CTRT) and the aspirations (silent or otherwise) were seen in 69% cases during the follow-up period.
Persistent aspirations often lead to pulmonary compromise causing recurrent pneumonia leading to respiratory failure and even death. Langerman et al.,[11] reported 9% aspiration related mortality rate which was less as compared to ours (2/20, i.e., 15%). In the present study, we report two deaths due to aspiration pneumonitis, and the third death occurred elsewhere and appeared to be related to aspiration. Nguyen et al.,[9] reported in their study that five (8%) deaths occurred during or shortly following RT, occurred from aspiration pneumonia. They expressed lack of awareness about the fact that aspiration could lead to recurrent chest infections and then death. They therefore, at that point in time, did not stop oral feeding in A despite the presence of a percutaneous endoscopic gastrostomy (PEG) tube during the period of study. Once the risk of aspiration was recognized, all patients were kept on enteral feeding only until safety of oral feeding was ensured. Since then, no aspiration-related death was recorded. This highlights the need for adopting safer strategy for eating. Like Nguyen et al.,[9] we too, with the help of this study have begun to understand the mechanism of aspiration and related deaths. With this increasing awareness and understanding, we may be able to explain the high mortality rate in our earlier report.[12] In fact, one may conjecture that significant proportion of the deaths that were not explainable at that point of time could have been due to aspiration and better nutrition by enteral route may have prevented them. Extension of the same philosophy, along with an observation by Agarwal et al.,[10] who state that advanced presentations in the developing world may be responsible for higher aspiration rate in reports from this part of the world, probably explains the higher mortality rate as compared to that mentioned in western literature.
Pretreatment aspirations occur based on the location and the bulk of tumor. In the present study we observed that epiglottic involvement (as primary location or extension from oropharynx to lingual surface of epiglottis or larynx to the laryngeal surface) was the main cause of aspiration in this cohort. Unfortunately, it is difficult to glean this information from literature as usually workers have raised concern regarding aspiration following a therapeutic intervention, that is, ascribing it to the intervention. There are only a few reports that have looked into pretreatment aspirations.[7,8,9] These studies make it evident that aspiration starts early, that is, even before treatment.
The next question is, do patients who start to aspirate early during their course of illness, behave differently? In our experience we observed that baseline A tend to fare worse than NA. Since the number of patients who developed aspiration following treatment (one additional case, i.e., 5% excess) was less, a comparison between baseline and posttreatment aspirations could not be made. This observation is unlike other series who report excess by 33% after RT.[8] Dirix et al.,[13] in a multivariate analysis showed that pretreatment swallowing dysfunction was the only independent predictor for late dysphagia and aspiration. This observation highlights the importance of serial VFG assessment that includes baseline evaluation.
Dosimetric correlation between RT dose received by swallowing structures and evidence of aspiration has been shown by some workers.[14,15] Mean dose to epiglottis was similar and high in both the groups, in the current study, and therefore no correlation was possible. Michigan and Rotterdam[5] reported their combined experience, and found significant correlation between aspiration, and the dose-volume parameters for the superior and middle pharyngeal constrictors (SC and MC). V60 correlation was not seen in previous study.[15] As of present, no clear dose or volume cutoff can yet be proposed, and currently the best approach may be to try and keep mean dose and V50 of these structures as low as possible.[5] Regarding the QOL, unlike us Teguh et al.,[6] observed a significant correlation between dose to SC muscle and QOL. Regarding patient perception of dysphagia and aspiration, it has been reported that most patients showed deterioration of global and subdomain PSSHN scores during follow-up.[10] In the present study, although we did not find any correlation between dose to PM and QOL, but we did encounter steady deterioration of QOL in A, during follow-up.
This study has several limitations-it is a study of small sample size with a short follow-up which may imply that aspirations caused or aggravated by radiation may not have fully manifested at the time of reporting and similarly for the pulmonary compromise. Similarly dose correlation could not be found in this study due to small numbers.
On the other hand, the positive highlights of this study are that it is a prospective study where a pretreatment VFG was done, which provided baseline reference data for comparison with postoperative VFG findings and for ascertaining the prognostic implications of aspirations, especially at the time of presentation. Increase in aspiration rate during follow-up could directly be attributed to radiation, since no chemotherapy was administered in these patients. Unlike others, both subjective and objective assessment of aspiration was done in this study.
Conclusions
Aspiration is a consequence of disease-related distortion of anatomy or incoordination between different swallowing related structures. A proportion of HNC patients aspirate even before RT. Epiglottis involvement contributes significantly towards aspiration rate. Patients, who are aspirating at the time of diagnosis fare worse than their counterparts. This study therefore highlights the importance of baseline VFG assessment of HNC patients since that would in identifying high risk patients and monitor them closely, to avoid potentially fatal sequelae.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemo-radiotherapy for head and neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Purkey MT, Levine MS, Prendes B, Norman MF, Mirza N. Predictors of aspiration pneumonia following radiotherapy in head and neck cancer. Ann Otol Rhinol Laryngol. 2009;118:811–6. [PubMed] [Google Scholar]
- 3.Lauve A, Morris M, Schmidt-Ullrich R, Wu Q, Mohan R, Abayomi O, et al. Simultaneous integrated boost IMRT for head-and-neck carcinomas: II-clinical results. Int J Radiat Oncol Biol Phys. 2004;60:374–87. doi: 10.1016/j.ijrobp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A, Harris J, Garden AS, Chao CK, Straube W, Harari PM, et al. Multi-institutional trial of accelerated hypo fractionated Intensity-modulated radiation therapy for early stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76:1333–8. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisbruch A, Levendag PC, Feng FY, Teguh D, Lyden T, Schmitz PI, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemo-radiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 2007;69:S40–2. doi: 10.1016/j.ijrobp.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teguh DN, Levendag PC, Sewnaik A, Hakkesteegt MM, Noever I, Voet P, et al. Results of fiberoptic endoscopic evaluation of swallowing versus radiation dose in the swallowing muscles after radiotherapy of cancer in the oropharynx. Radiother Oncol. 2008;89:57–63. doi: 10.1016/j.radonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Rosen A, Rhee TH, Kaufman R. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2001;127:975–9. doi: 10.1001/archotol.127.8.975. [DOI] [PubMed] [Google Scholar]
- 8.Stenson KM, McCracken E, List M, Haraf DJ, Brockstein B, Weichselbaum R, et al. Swallowing function in patients with head and neck cancer prior to treatment. Arch Otolaryngol Head Neck Surg. 2000;126:371–7. doi: 10.1001/archotol.126.3.371. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, Bhamidipati PV, et al. Aspiration rate following chemoradiation for head and neck cancer: An under-reported occurrence. Radiother Oncol. 2006;80:302–6. doi: 10.1016/j.radonc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal J, Dutta D, Palwe V, Gupta T, Laskar SG, Budrukkar A, et al. Prospective subjective evaluation of swallowing function and dietary pattern in head and neck cancers treated with concomitant chemo-radiation. J Cancer Res Ther. 2010;6:15–21. doi: 10.4103/0973-1482.63563. [DOI] [PubMed] [Google Scholar]
- 11.Langerman A, Maccracken E, Kasza K, Haraf DJ, Vokes EE, Stenson KM. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1289–95. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]
- 12.Lal P, Tiwari A, Verma A, Das KJM, Baijal SS, Bajpai R, et al. Role of videofluorography in assessing functional abnormalities in patients of head and neck cancer treated with chemo-radiotherapy. Asia Pac J Clin Oncol. 2009;5:2649. [Google Scholar]
- 13.Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dyaphagia after chemoradiotherapy for head and neck squamous cell carcinoma: Dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75:385–92. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caglar HB, Tishler RB, Othus M, Burke E, Li Y, Goguen L, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–8. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]


